Abstract
Alcohol use disorder commonly occurs in patients with schizophrenia and contributes greatly to its morbidity. Unfortunately, the neural and behavioral underpinnings of alcohol drinking in these patients are not well understood. In order to begin to understand the cognitive and reward-related changes that may contribute to alcohol drinking, this study was designed to address: 1) latent inhibition; 2) conditioning; and 3) extinction of autoshaping in a neurodevelopmental rat model with relevance to co-occurring schizophrenia and alcohol use disorders, the neonatal ventral hippocampal lesioned (NVHL) rat. NVHL lesions (or sham surgeries) were performed on post-natal day 7 (PND7) and animals were given brief exposure to alcohol during adolescent (PND28–42). Latent inhibition of autoshaping, conditioning and extinction were assessed between PND72–90. On PND90 animals were given alcohol again and allowed to establish stable drinking. Latent inhibition of autoshaping was found to be prolonged in the NVHL rats; the NVHL rats pre-exposed to the lever stimulus were slower to acquire autoshaping than sham pre-exposed rats. NVHL rats that were not pre-exposed to the lever stimulus did not differ during conditioning, but were slower to extinguish conditioned responding compared to sham controls. Finally, the NVHL rats from both groups drank significantly more alcohol than sham rats, and the extent of latent inhibition predicted future alcohol intake in the pre-exposed animals. These findings suggest that the latent inhibition of autoshaping procedure can be used to model cognitive- and reward- related dysfunctions in schizophrenia, and these dysfunctions may contribute to the development of co-occurring alcohol use.
Keywords: Dual Diagnosis, NVHL, Latent Inhibition, Autoshaping, Sign-Tracking, Ethanol
Introduction
Substance and alcohol use disorders (AUD) commonly occur in patients with schizophrenia; 30% of patients drink regularly, and this use significantly worsens the course of schizophrenia (Regier et al., 1990). AUD in these patients is associated with poor treatment response, treatment non-compliance (Owen et al., 1996), relapse (Drake and Mueser, 1996; Gupta et al., 1996), violence (Bartels et al., 1991; Swanson et al., 1990) and suicide (Allebeck et al., 1987; Harkavy-Friedman and Nelson, 1997). Unfortunately, there is a lack of studies describing the neural and behavioral underpinnings of AUD in patients with schizophrenia. While a variety of hypotheses have been presented regarding the prevalence of substance use in schizophrenia (Chambers et al., 2001; Green et al., 1999; Ng et al., 2013), there is a great need for further understanding the cognitive and reward-related underpinnings of this substance use (Duijkers et al., 2016).
In this regard, we employed a neurodevelopmental model of schizophrenia and co-occurring substance use disorder, namely the neonatal ventral hippocampal lesioned (NVHL) rat. The NVHL rat is one of the best-described animals models of SCZ (Lipska and Weinberger, 2002) and displays behavioral and neurobiological dysfunctions analogous to those seen in patients with schizophrenia. The NVHL rat, experimentally created by producing an excitotoxic lesion in the ventral hippocampus in a 7-day old rat pup (analogous to a third trimester insult), has strong construct, face, and predictive validity for schizophrenia (Tseng et al., 2009). For example, schizophrenia is a neurodevelopmental disorder involving a prefrontal-limbic network disconnection, which is modeled in the NVHL rat (i.e., construct validity). Further, as adults, NVHL rats exhibit a variety of behavioral, neurochemical, and molecular alterations associated with the clinical symptoms of schizophrenia (i.e., face validity (Tseng et al., 2009)). Finally, in the NVHL rat, many of the behavioral and biological analogs of schizophrenia can be reversed by antipsychotic medications (i.e., predictive validity (Tseng et al., 2009)).
Modeling the rates of substance and alcohol use disorders in schizophrenia (Volkow, 2009), NVHL rats display enhanced sensitivity to, and increased use of, cocaine, nicotine, and methamphetamine (Berg et al., 2014; Brady et al., 2008; Chambers and Self, 2002; Chambers and Taylor, 2004; Karlsson et al., 2013), as well as altered reactivity to the rewarding effects of cannabinoids (Gallo et al., 2014a; Gallo et al., 2014b). Importantly, a recent investigation demonstrated that brief exposure of NVHL rats to alcohol in adolescence (between post-natal days [PND] 28–42) produced a loss of control over alcohol drinking in adulthood, potentially suggesting the NVHL rat as a valid model of AUD and schizophrenia (Jeanblanc et al., 2015). These animals also lever-pressed more for alcohol, required more sessions to extinguish behavior, and showed greater relapse than shams.
To further assess the underlying cognitive and reward-related dysfunctions underlying alcohol drinking in this rat model of AUD in schizophrenia, the current study assessed the performance of NVHL rats on latent inhibition, conditioning and extinction of appetitive autoshaping. We were interested in a behavioral assay that would capture multiple facets of psychopathology of schizophrenia including both cognitive (captured by latent inhibition (Almey et al., 2013; Weiner, 2003)) and reward-related deficits (captured by the conditioning and extinction of autoshaping (Day and Carelli, 2007)). Latent inhibition refers to the observation that the rate of association between a conditioned stimulus (CS) and an unconditioned stimulus (US) is decreased if the subject is first exposed to the to-be-conditioned CS in the absence of the US (i.e., stimulus takes longer to acquire meaning). Autoshaping refers to a procedure in which a cue (CS) repeatedly paired with a reward (US) elicits a conditioned response to the cue itself (“sign-tracking”), even if the reward is not contingent upon the action toward the cue. Thus, the current study was designed to address: 1) latent inhibition, 2) conditioning (learning of cue-reward association) and 3) extinction (cue responding in absence of reward) of autoshaping, in the NVHL rat model of schizophrenia and co-occurring alcohol use disorder (Jeanblanc et al., 2015).
While latent inhibition of autoshaping procedures have not been tested in the NVHL rat, previous studies have suggested that NVHL rats displayed lower sign-tracking compared to NVHL rats (Lopez et al., 2015). Moreover, both reduced as well as no changes in latent inhibition have been observed in these animals compared to sham animals depending on the behavioral assays tested (Angst et al., 2007; Grecksch et al., 1999). Despite these variable findings, we hypothesized that, consistent with clinical investigations of latent inhibition in patients with schizophrenia (Yogev et al., 2004), NVHL rats would show decreased latent inhibition compared to sham animals.
We also hypothesized that these deficits would be present prior to the development of excessive alcohol drinking. Furthermore, by correlating measures from this task with future alcohol drinking, we assessed the utility of these measures to predict alcohol drinking as a potential biomarker that can then be targeted for the development of therapeutic strategies to decrease (or prevent [if captured prior to development of AUD]) alcohol use in schizophrenia. A recent study used a similar approach to suggest that impairments in radial arm maze performance could predict nicotine seeking in the NVHL rat (Rao et al., 2016), further supporting the utility of translational behavioral measures as biomarkers.
Methods
Subjects
Timed pregnant Sprague-Dawley dams were ordered from Charles River (Wilmington, MA) to arrive at gestational day 13 and were singly housed with ad libitum access to food and water. Male rat pups used in the study were individually housed in a colony room maintained on a 14:10 hr light-dark cycle. Experimentation took place during the light period of the cycle. Two weeks before the beginning of test procedures the rats were placed on a food deprivation schedule to maintain their weights between 80% and 85% of their free feeding weights. They were monitored and cared for in compliance with the Association for Assessment and Accreditation of Laboratory Care guidelines and the IACUC of Dartmouth College.
NVHL Preparation and Surgery
Male Sprague-Dawley rat pups (n = 40) on post-natal day 7 (PND 7, 15–20 g) were anesthetized using hypothermia and then placed on a Styrofoam platform attached to a stereotactic apparatus (Kopf Instruments, Tujunga). Half of the pups (NVHL) were bilaterally injected with excitotoxic ibotenic acid (3.0 μg ibotenic acid [Tocris, Minneapolis] dissolved in 0.3 μl of artificial cerebrospinal fluid (aCSF); n = 20) into their ventral hippocampi (AP −3.0 mm, ML ± 3.5 mm, VD ± 5.0 mm relative to bregma). The remaining pups (n = 20) were injected with aCSF at the same co-ordinates (Sham, unlesioned). After the surgery, wounds were closed using tissue glue, and when activity level had returned to normal, pups were returned to their dams. Rats were weaned on PND 21 and housed individually for the duration of the study.
Alcohol drinking in adolescence and adulthood
We followed the protocol of Jeanblanc et al. (2014), in which the rats were given access to alcohol in a free-access 2-bottle (water and 10% alcohol) design between PND 28 and 42. This was done to ensure that the NVHL rats would consume alcohol preferentially in adulthood; NVHL rats not exposed to alcohol during adolescence do not display increased drinking in adulthood (Jeanblanc et al., 2015). At the end of this period, the alcohol bottle was removed, and the animals only had access to water until adulthood. Alcohol, water and food intake as well as body weight were measured daily during PND 28–42 and then again in adulthood upon resuming alcohol drinking. All animals went through the latent inhibition of autoshaping procedure (described below) between PND 56 and PND 90. Alcohol was then reintroduced to the rats in adulthood (PND 90) in a continuous-access 2-bottle choice (water and 20% alcohol) design. The position of the two bottles were switched daily to prevent positional preference, consistent with our previous investigations (Khokhar and Green, 2016). The animals’ performance on the behavioral assay was correlated with their alcohol drinking between PND 90 and PND 150 to assess the predictive ability of these measures toward future alcohol drinking.
Behavioral Apparatus
Latent inhibition, conditioining and extinction of autoshaping were carried out in eight identical standard conditioning chambers (24 × 30.5 × 29 cm: Med Associates) enclosed in sound-attenuating chambers (62 × 56 × 56 cm) with background noise (68 dB SPL) provided by an exhaust fan. The conditioning chambers (Med Associates, ENV-007) consisted of aluminum front and back walls and clear acrylic sides and top. The grid floor was stainless steel rods (5 mm diameter) spaced 1.5 cm apart (center to center). The chambers were illuminated by one 6-W bulb, with a red cover, mounted on the ceiling of the sound attenuation chamber. Each chamber contained a food cup, recessed in the center of the front wall, mid-way between two retractable levers (Med Associates model: ENV-112CM). A 10 second insertion of the left lever served as the CS throughout the experiment. (The right lever was not used and remained retracted throughout the experiment.) Lever presses were counted throughout the session, and a photocell recorded head entries into the food cup. The apparatus was controlled by computer equipment located in an adjacent room. The reinforcer was a 45-mg grain-based rodent food pellet (Bioserv).
Behavioral Procedures
Prior to the start of the pre-exposure (PE) phase, each rat was fed 1 g of the 45-mg reward pellets in its home cage for two consecutive days. On the following day each rat was assigned to one chamber and received a 45-min adaptation session with no levers or pellets.
Pre-exposure
During the pre-exposure phase (to assess latent inhibition), 12 rats from each lesion condition were pre-exposed (PE) to the lever-stimulus. Each 45-minute session consisted of 20 trials of pre-exposure to the CS (i.e., the 10-s insertion of the left lever). The time between trials (intertrial interval [ITI]) was variable, with an average of 2 minutes (± 25%). The remaining rats (n=8) from both sham and NVHL groups received only a single lever-insertion trial each session, and were deemed non pre-exposed (NPE) groups. In total, the NPE rats received 3 pre-exposure trials, while rats in the PE groups received 40 pre-exposure trials.
Conditioning
For the next 12 days, all rats received the following conditioning procedure. In each daily 45-min conditioning session, each rat received 20, 10-s lever insertion CS trials with the same trial spacing used for the PE group in the pre-exposure sessions. Two food pellets were delivered into the food cup at the end of the CS (i.e., lever retraction) on each trial independent of any response.
Extinction
Six days of extinction began the day following the last conditioning session. Extinction sessions were identical to the conditioning sessions except without the food pellets.
Behavioral Observations and Data Analysis
We report three primary response measures: 1) the rate of lever pressing per minute; 2) the percentage of trials with at least 1 lever press response; and 3) the percentage of time spent in the food cup during CS presentations. Since prior studies have demonstrated that both autoshaped lever-pressing and food cup responding peaks during the last 5-s interval of the CS (Chang et al., 2012a, 2012b; Holland et al., 2013), our analyses focused on this last interval. We report food cup responding as an elevation score (percent time spent in the food cup during the CS minus percent time spent in the food cup during the 5 s interval just prior to the CS). In addition, we report the percentage of time spent in the food cup during the pre-CS (5 s interval prior to CS onset).
Lesion Verification and Analysis
Upon euthanasia, brains were removed and flash frozen. Lesions were verified by sectioning (40 mm) the dorsal and ventral hippocampus using a freezing microtome. Sections were mounted on glass slides and Nissl stained. The hippocampus was examined microscopically for evidence of bilateral damage, which typically includes cell loss, thinning, cellular disorganization, and ventricular enlargement. Animals with unilateral or extra-hippocampal lesions were excluded from the analysis.
Results
Histology
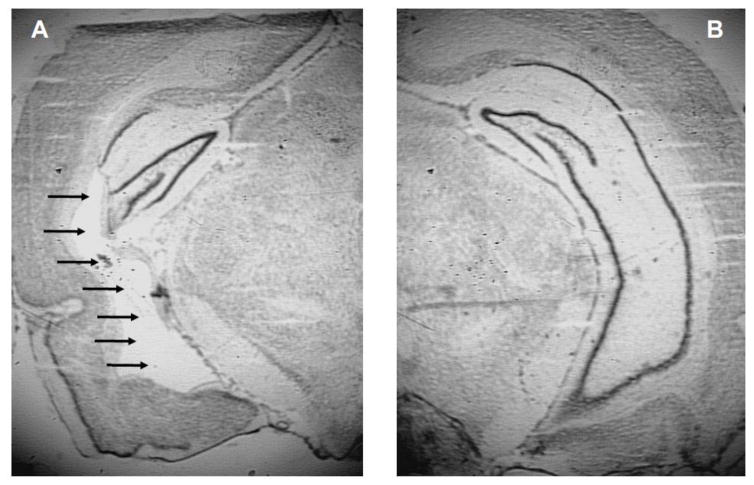
One rat was removed from NPE-NVHL group due to a lack of bilateral lesioning of the ventral hippocampus and one rat was removed from the PE-NVHL group due to extra-hippocampal damage. Representative photomicrographs depicting lesion location and extent are presented in Figure 1.
Figure 1.
Representative photomicrographs depicting lesion extent and location. Arrows point to lesion in a representative NVHL rat hippocampus (A) compared to a sham rat hippocampus (B).
Behavior
Pre-exposure
There was virtually no unconditioned responding to the lever stimulus during the pre-exposure phase. During the first stimulus presentation of the first pre-exposure session, the mean rate of lever pressing (presses per minute) for each group was: 6.0 (NPE-Sham; SEM = 4.5), 1.68 (NPE-NVHL; SEM = 1.6), 1.0 (PE-Sham; SEM = 1.0), 1.0 (PE-NVHL; SEM = 1.1). Neither the main effect of condition (NPE vs. PE) nor lesion (Sham vs. NVHL) was significant (ps > .2). For groups PE-Sham and PE-NVHL, the average rate of responding for all 20 pre-exposure trials was 2.0 (PE-Sham; SEM = 0.92) and 1.7 (PE-NVHL; SEM = .45). There were no differences in the percentage of trials with a response, elevation in percent time spent in the food cup during lever presentations, or percent time spent in the food cup during the pre-CS period.
During the first stimulus presentation of the second pre-exposure session, the mean rate of lever pressing for each group was: 3.0 (NPE-Sham; SEM = 3.0), 5.0 (NPE-NVHL; SEM = 3.5), 1.0 (PE-Sham; SEM = 1.0), 1.0 (PE-NVHL; SEM = 1.0). Neither the main effect of condition nor the main effect of lesion were significant (ps > .16). Averaged over the entire second pre-exposure session, there was significantly more responding by the PE-Sham rats compared to the PE-NVHL rats (p < .05), although the numerical difference was relatively small (PE-Sham = 1.6; SEM = 0.4, PE-NVHL = 0.2; SEM = 0.1). There were no differences in the percent measure, percent change in time in food cup, or percent time in the food cup during the pre-CS interval. As described, due to a procedural error the NPE groups received a third pre-exposure session in which they received a single lever presentation. The groups did not differ significantly, and responding averaged 1.4 (NPE-Sham; SEM = 1.5) and 5.0 (NPE-NVHL; SEM = 3.33).
Conditioning
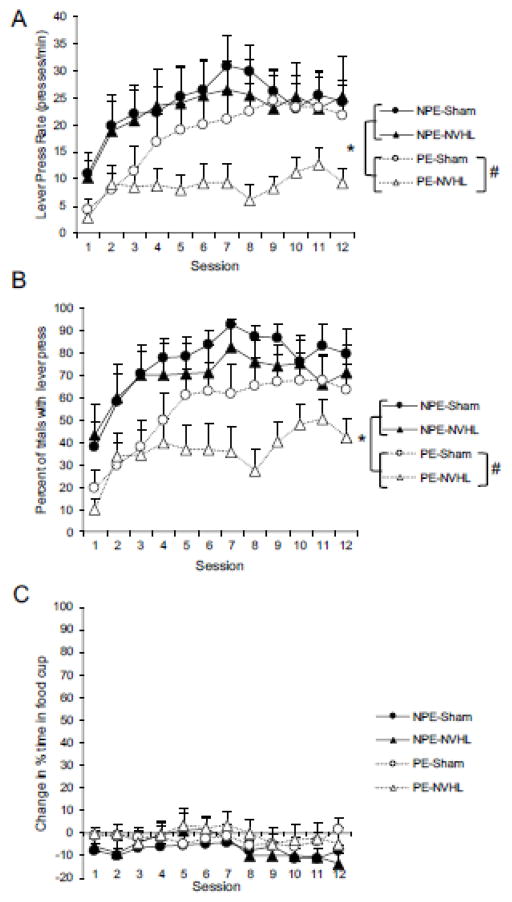
As shown in Figure 2A (average rate of lever pressing), latent inhibition was observed. Early in conditioning, both NPE groups responded more than both PE groups, and there was no influence of lesion type (i.e., Sham vs. NVHL). Separate 2 (Condition: PE vs. NPE) × 2 (Lesion: Sham vs. NVHL) ANOVAs on sessions 1 and 2 revealed a main effect of condition for both session 1, F(1, 34) = 8.82, p < .01, and session 2, F(1, 34) < 7.60, p < .01, indicating that NPE rats responded significantly more than PE rats. Neither the main effect of lesion nor the interaction between lesion and condition were significant for either session, Fs < 1. As training progressed, an effect of the NVHL lesion emerged that depended upon the pre-exposure condition. Groups that were not pre-exposed (NPE-Sham and NPE-NVHL) responded at a similar rate over the course of conditioning. However, conditioning was especially delayed for the PE-NVHL group compared to PE-Sham group. For the NPE groups, a 2 (Lesion: Sham vs. NVHL) × 12 (Session) ANOVA revealed a main effect of session, F(11, 143) = 2.81, p < .01. Neither the main effect of lesion nor the lesion by session interaction were significant (ps > .80). For the PE groups, the same analysis revealed a main effect of session, F(11, 231) = 7.62, p < .001. In contrast to the NPE group, although the main effect of lesion was not significant, F(1, 21) = 3.01, p = .09, there was a significant interaction between lesion and session, F(11, 231) = 3.25, p < .001. As shown in Figure 2, responding for group PE-Sham rose quickly and to a higher level compared to group PE-NVHL. An identical analysis on the percentage of trials with at least 1 lever press data (Figure 2B) showed consistent findings.
Figure 2.
Pre-exposed animals displayed latent inhibition, with the NVHL animals displaying an abnormally persistent latent inhibition phenotype. (A) mean lever presses per minute, (B) mean percent trials with at least 1 lever press, (C) mean percent change in time spent in food cup (CS minus pre-CS). NPE = non pre-exposed, PE = pre-exposed. Error bars represent +1 SEM. * p<0.05 main effect of condition (PE vs NPE) on sessions 1 and 2; # p<0.05 lesion (Sham vs. NVHL) x session interaction.
Figure 2C presents the mean change in percent time in the food cup, over the 12 sessions of conditioning. In general, there was very little food cup activity during lever presentations. A 2 (Condition: PE vs. NPE) × 2 (Lesion: Sham vs. NVHL) × 12 (Session) ANOVA revealed a main effect of session, F(11, 374) = 1.91, p = .04. No other main effects or interactions were significant (ps > .24). There were also no group differences in the amount of time spent in the food cup during the pre-CS interval (i.e., the 5 s period just prior to CS onset). A 2 (Condition: PE vs. NPE) × 2 (Lesion: Sham vs. NVHL) × 12 (Session) revealed a significant main effect of session, F(11, 374) = 2.34, p < .01. There were no other significant main effects or interactions (ps > .27). Averaged over the course of conditioning, the percent time spent in the food cup during the pre-CS period was 10.11 (NPE-Sham; SEM = 1.5), 12.85 (NPE-NVHL; SEM = 4.3), 13.23 (PE-Sham; SEM = 2.2) and 17.70 (PE-NVHL; SEM = 4.5).
In summary, pre-exposure to the lever stimulus latently inhibited autoshaped lever pressing during the conditioning phase. Specifically, groups that received only 3 pre-exposure trials responded more to the lever stimulus when it was later paired with food, relative to groups that received 40 pre-exposure trials. This was true for both the rate measure (Figure 2A), and the percent measure (Figure 2B). More so, although lesion status had no impact on conditioning for NPE groups, the effect of pre-exposure was greater for PE-NVHL compared to PE-Sham rats. Thus, neonatal lesions of the ventral hippocampus appear to exacerbate the effect of stimulus pre-exposure.
Extinction
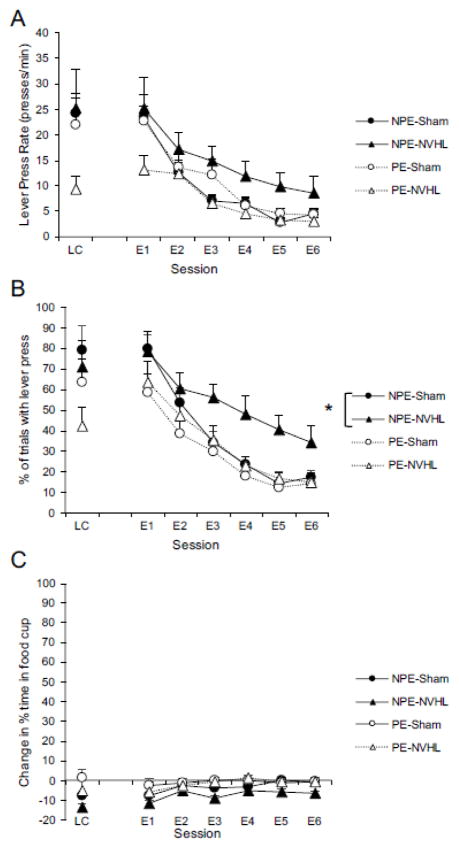
The results of the extinction phase are presented in Figure 3. The left-most data point in each panel represent the responding during the last session of the conditioning phase (“LC”). The difference between groups PE-NVHL and PE-Sham approached significance (p = .067; Figure 2A). Nevertheless, it is clear that the PE-NVHL group did not reach the same level on the performance scale as the other three groups, making it difficult to assess the rate of extinction. Therefore, although we present the data for the PE groups, our statistical analysis focused on the NPE groups, which did not differ over the course of conditioning. As shown in Figure 3A and B, extinction (both rate of lever pressing and percent of trials with a response) was slowed for the NPE-NVHL group, relative to the NPE-Sham group.
Figure 3.
Non pre-exposed NVHL rats displayed impaired extinction of autoshaping behavior. (A) mean lever presses per minute. (B) mean percent trials with at least 1 lever press. (C) mean percent change in time spent in food cup (CS minus pre-CS). NPE = non pre-exposed, PE = pre-exposed. LC = last session of conditioning. E1 E6 = extinction sessions 1 to 6. Error bars represent +1 SEM. * p<0.05 main effect of lesion (Sham vs. NVHL).
For the rate of lever-pressing in the NPE groups (Figure 3A), a 2 (Lesion: Sham vs. NVHL) × 6 (Session) ANOVA revealed a main effect of Session, F(5, 65) = 38.71, p < .001. The interaction between session and lesion was not significant (p = .76), and the main effect of lesion approached significance, F(1, 13) = 3.34, p = .089. Nevertheless, as shown in Figure 3A, NPE-NVHL rats trended toward extinguishing slower than NPE-Sham rats.
Figure 3B depicts the mean percent of trials with at least 1 lever press over the course of extinction training. Once again, our analysis focused on the lesion effect within the NPE groups since these groups did not differ over the course of conditioning. A 2 (Lesion: Sham vs. NVHL) × 6 (Session) ANOVA revealed a significant main effect of Session, F(5, 65) = 46.89, p < .001. In addition, both the main effect of lesion, F(1, 13) = 5.31, p = .038, and the interaction between lesion and session was significant, F(5, 65) = 2.65, p = .03. Thus, the NPE-NVHL group responded more in extinction than the NPE-Sham group, supporting the trend seen in Figure 3A. For the change in percent time spent in the food cup in NPE groups (Figure 3C), a 2 (Lesion: Sham vs. NVHL) × 6 (Session) ANOVA revealed a significant main effect of session, F(5, 65) = 4.34, p < .01. Neither the main effect of lesion, F(1, 13) = 3.35, p = .09, nor the interaction between lesion and session were significant, F < 1. Averaged over the entire extinction phase, the mean change in percent time spent in the food cup was −2.7 (SEM = .41) and −6.3 (SEM = 2.1) for NPE-Sham and NPE-NVHL, respectively. For the extinction phase, pre-CS responding was analyzed with a 2 (Lesion: Sham vs. NVHL) × 6 (Session) ANOVA. This revealed a main effect of lesion, F(1, 13) = 5.6, p = .034. Neither the main effect of session nor the interaction between session and lesion were significant (ps > .23). Averaged over the entire extinction phase, the mean percent time spent in the food cup was during the pre-CS period was 4.16 (SEM = 0.45) and 9.5 (SEM = 2.3) for NPE-Sham and NPE-NVHL, respectively.
In summary, with both the rate and percent measure (i.e., Figure 3A and 3B, respectively), group NPE-NVHL responded more during the extinction phase than group NPE-Sham. Thus, neonatal ventral hippocampal lesions impair the extinction of autoshaping. Unexpectedly, group NPE-NVHL spent more time in the food cup during the pre-CS period than group NPE-Sham. This difference in baseline behavior makes it difficult to interpret the change in percent time in the food cup during lever presentations. Nevertheless, it is clear that neither group substantially increased time spent in the food cup during lever presentations as compared to the pre-CS period (see Figure 3C). Furthermore, the mean percent time spent in the food cup during lever presentations (without subtracting out pre-CS responding) was not significantly different (NPE-Sham = 1.4 (SEM = .39), NPE-NVHL = 3.1 (SEM = 1.2), p = .20). The fact that the percent time spent in the food cup during lever CS presentations did not differ is important, in that it rules out the possibility that extinction progressed at different rates between groups due to response competition between the food cup and the lever.
Latent Inhibition Estimates Future Alcohol Drinking
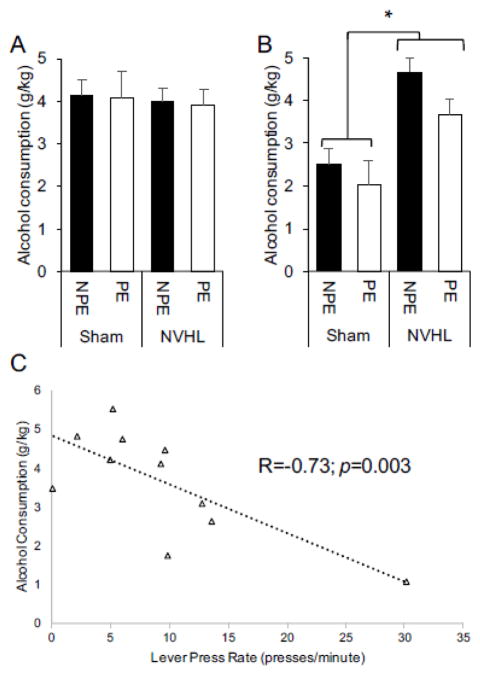
Confirming the findings of Jeanblanc et al., we found that there were no differences in alcohol drinking (10% v/v in a two-bottle [water and alcohol] continuous access paradigm) between NVHL and sham animals during adolescence (Figure 4A). Upon completion of the behavioral studies, rats were given access to alcohol (20% v/v in a two-bottle [water and alcohol] continuous access paradigm) and allowed to establish stable drinking over a period of 60 days. Mean alcohol consumption over the 60 days (Figure 4B) was found to be higher in the NVHL rats (both PE and NPE) compared to sham animals. A 2 (Condition: PE vs. NPE) × 2 (Lesion: Sham vs. NVHL) revealed a significant main effect of Lesion, F(1, 32) = 17.463, p < .001. Neither the main effect of condition, nor the interaction between condition and lesions was significant, suggesting that the behavioral experiment did not affect the differences in drinking between NVHL and sham animals.
Figure 4.
NVHL shams display increased alcohol drinking in adulthood, but not in adolescence; this drinking correlates with latent inhibition behavior. (A) Mean alcohol drinking in NVHL and sham animals in adolescence; and (B) mean alcohol drinking in NVHL and sham animals in adulthood. (C) correlation between lever press rate during conditioning and future alcohol drinking in PE-NVHL animals. NPE = non pre-exposed, PE = pre-exposed. Error bars represent +1 SEM.* p<0.05 main effect of lesion (Sham vs. NVHL).
Mean alcohol consumption was then correlated with average lever pressing rate during each of the conditioning and extinction phases (Table 1). The PE-NVHL rats showed a strong negative correlation between lever pressing rate and alcohol consumption (R=−0.73, p=0.003, Figure 4D). While no other significant correlations were observed between lever pressing during conditioning and during extinction with future alcohol drinking, there were some trends toward significant correlations: (i) lever pressing during conditioning trended toward negatively correlating with future alcohol drinking in the PE-sham animals (R=−0.53, p=0.051); lever pressing during extinction trended toward positively correlating with future alcohol drinking in the NPE-NVHL animals (R=0.59, p=0.074).
Table 1.
Correlations between lever pressing during conditioning and during extinction with future alcohol consumption
| Condition | Lesion | Conditioning | Extinction | ||
|---|---|---|---|---|---|
| Pearson’s R | p-value | Pearson’s R | p-value | ||
| NPE | Sham | 0.24 | 0.52 | −0.30 | 0.40 |
| NVHL | 0.21 | 0.47 | 0.59 | 0.074 | |
| PE | Sham | −0.53 | 0.051 | −0.26 | 0.38 |
| NVHL | −0.73 | 0.003* | −0.52 | 0.055 | |
Discussion
To the best of our knowledge, this is the first study to assess latent inhibition of autoshaping in an animal model of schizophrenia. There were four main findings from this study. First, latent inhibition of autoshaping was abnormally persistent in the NVHL rats. Specifically, NVHL rats that were pre-exposed to the lever stimulus were slower to condition than Sham pre-exposed rats. Secondly, NVHL rats that were not pre-exposed to the lever stimulus were slower to extinguish conditioned responding compared to Sham controls. Finally, the extent of latent inhibition predicted future alcohol intake in the pre-exposed animals.
Cognitive deficits in selective attention and information-processing are hallmarks of schizophrenia and one method of assessing these in humans and rodents employs the testing of latent inhibition (Moser et al., 2000). In the present study, latent inhibition was abnormally persistent in adolescent alcohol exposed NVHL rats. This finding contrasts with two previous studies (cf. Grecksch et al., 1999; Ouhaz et al., 2014) which found disrupted (i.e., less) latent inhibition in the NVHL rat. However, those studies examined latent inhibition in a different paradigm (conditioned reaction in a two-way shuttle box), and used NVHL rats that were not exposed to alcohol during adolescence. Nevertheless, the present study is consistent with several other studies that have reported prolonged latent inhibition in other rodent models of schizophrenia including: NMDA receptor antagonism (Gaisler-Salomon and Weiner, 2003); glycine co-agonist site inhibition (Labrie et al., 2008); or the or mutation of Grin1 and Dao1 (Labrie et al., 2010). Latent inhibition perseveration has been suggested to indicate an impaired switching ability, as the switch from ignoring an irrelevant stimulus to responding in an appropriate manner is reduced (Weiner, 1990). Clinical studies have also supported this prolonged latent inhibition phenomenon in patients with schizophrenia (Cohen et al., 2004; Rascle et al., 2001), consistent with a behavioral inflexibility in these patients as measured using the Wisconsin Card Sorting Task (Berman et al., 1997). The inconsistencies in the impact of different schizophrenia models on latent inhibition is consistent with the disparate patterns of latent inhibition alterations observed in patients with schizophrenia, ranging from impairments to elevations depending on the symptom profile or chronicity of the patients tested (Swerdlow, 2010), further supporting increased investigation into potential mechanisms and consequences of this heterogeneity.
The impaired extinction (in the % of trials with a response measure) observed in the non pre-exposed NVHL rats is consistent with previous investigations showing a resistance to extinction phenotype for lever-pressing for alcohol, cocaine and nicotine (Berg et al., 2014; Jeanblanc et al., 2015; Karlsson et al., 2013). This resistance to extinction of autoshaped lever pressing is an entirely novel finding that further supports the utility of this assay to study both cognitive- and reward-related behaviors, and supports previous assertions that this model may have an increased vulnerability to substance use and decreased ability to extinguish appetitive behaviors (Jeanblanc et al., 2015; Karlsson et al., 2013). Interestingly, there were no differences in conditioning between the NPE NVHL and sham animals. This contrasts with a previous study that found decreased sign-tracking in NVHL rats, but only in NVHL rats that tended to approach the lever (sign-trackers), compared to rats that tended to approach the food cup (goal-trackers) (Lopez et al., 2015). There are, however, a number of procedural differences between the present study and the study by Lopez et al. (2015) that might account for the contrasting results. For example, in the current study rats were exposed to alcohol as adolescents, prior to any behavioral training.
The strong negative correlation between lever pressing during conditioning in the PE NVHL rats with future alcohol drinking suggests that the prolonged latent inhibition in these animals may be indicative of underlying behavioral processes that may contribute to the development of alcohol drinking. Furthermore, the trends toward significant correlations in the extinction phase in both the PE (negative correlation; same as above) and NPE (positive correlation; increased lever pressing during extinction related to increased drinking) NVHL rats further supports this contention, since the direction of these correlations reflects consistent effects of impairments in behavioral switching on addiction vulnerability. In fact, patients with co-occurring schizophrenia and substance use disorders often display shifting and inhibition behaviors (Duijkers et al., 2016); specifically these patients display impaired cognitive set-shifting and task switching in the Iowa Gambling Tasks and the Trail Making Task (Benaiges et al., 2013; Schnell et al., 2009; Sevy et al., 2007). While both the prolonged latent inhibition and the impaired extinction are suggestive of switching impairments in the NVHL rat model, the impairments in extinction may also represent impaired behavioral inhibition, which has also been observed in these patients (Jockers-Scherubl et al., 2007; Rodriguez-Jimenez et al., 2010). These findings suggest that impairments in cognitive functioning may, in fact, contribute to the addiction vulnerability in this animal model, and possibly in patients. Lastly, since previous studies have also shown that the persistence of latent inhibition can be reversed by pharmacological intervention (Gaisler-Salomon and Weiner, 2003; Labrie et al., 2010), future studies may be able to assess the effects of ameliorating switching deficits on the development or maintenance of alcohol drinking, to begin to assess the causal contribution of these deficits to substance use in schizophrenia.
Conclusion
This study is the first study to assess latent inhibition of autoshaping in an animal model of schizophrenia, and, consistent with previous investigations, showed prolonged latent inhibition in the NVHL rat model of alcohol use disorder in schizophrenia. These animals also displayed impaired extinction of autoshaping in the non pre-exposed animals, suggesting that this assay may also capture some of the reward-related dysfunctions observed in patients with schizophrenia. Lastly, the animals’ lever pressing in both the conditioning and extinction phases of the study may have potential utility as an early estimator of future alcohol drinking. Future studies in this line of work have the potential of uncovering a potential behavioral biomarker that could be used to test and establish prevention strategies (i.e., pharmacological and behavioral) to reduce the morbidity associated with substance use in patients with schizophrenia.
Acknowledgments
This work was supported by a Canadian Institute of Health Research Fellowship award (JYK) and a pilot grant from the Hitchcock Foundation (JYK) as well as NIMH F32MH105125 (TPT). The authors thank Dr. Alan I. Green for valuable feedback during manuscript preparation.
Footnotes
Contributors
Both Drs. Khokhar and Todd were involved the design, implementation, data analysis for this study as well as the manuscript preparation.
Conflicts of Interest
Neither of the authors have any conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allebeck P, Varla A, Kristjansson E, Wistedt B. Risk factors for suicide among patients with schizophrenia. Acta Psychiatr Scand. 1987;76(4):414–419. doi: 10.1111/j.1600-0447.1987.tb05626.x. [DOI] [PubMed] [Google Scholar]
- Almey A, Hafez NM, Hantson A, Brake WG. Deficits in latent inhibition induced by estradiol replacement are ameliorated by haloperidol treatment. Frontiers in behavioral neuroscience. 2013;7:136. doi: 10.3389/fnbeh.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MJ, Macedo CE, Guiberteau T, Sandner G. Alteration of conditioned emotional response and conditioned taste aversion after neonatal ventral hippocampus lesions in rats. Brain Res. 2007;1143:183–192. doi: 10.1016/j.brainres.2007.01.093. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Drake RE, Wallach MA, Freeman DH. Characteristic hostility in schizophrenic outpatients. Schizophr Bull. 1991;17(1):163–171. doi: 10.1093/schbul/17.1.163. [DOI] [PubMed] [Google Scholar]
- Benaiges I, Serra-Grabulosa JM, Prat G, Adan A. Executive functioning in individuals with schizophrenia and/or cocaine dependence. Human psychopharmacology. 2013;28(1):29–39. doi: 10.1002/hup.2279. [DOI] [PubMed] [Google Scholar]
- Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol. 2014 Nov;19(6):1020–1031. doi: 10.1111/adb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25(1):1–10. doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O’Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 2008;200(2):205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27(6):889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56(5):308–316. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sereni N, Kaplan O, Weizman A, Kikinzon L, Weiner I, Lubow RE. The relation between latent inhibition and symptom-types in young schizophrenics. Behav Brain Res. 2004;149(2):113–122. doi: 10.1016/s0166-4328(03)00221-3. [DOI] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13(2):148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Mueser KT. Alcohol-use disorder and severe mental illness. Alcohol Health Res World. 1996;40:87–93. [PMC free article] [PubMed] [Google Scholar]
- Duijkers JC, Vissers CT, Egger JI. Unraveling Executive Functioning in Dual Diagnosis. Frontiers in psychology. 2016;7:979. doi: 10.3389/fpsyg.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Weiner I. Systemic administration of MK-801 produces an abnormally persistent latent inhibition which is reversed by clozapine but not haloperidol. Psychopharmacology (Berl) 2003;166(4):333–342. doi: 10.1007/s00213-002-1311-z. [DOI] [PubMed] [Google Scholar]
- Gallo A, Bouchard C, Fortier E, Ducrot C, Rompre PP. Cannabinoids reward sensitivity in a neurodevelopmental animal model of schizophrenia: A brain stimulation reward study. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2014a Sep;24(9):1534–1545. doi: 10.1016/j.euroneuro.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Gallo A, Bouchard C, Rompre PP. Animals with a schizophrenia-like phenotype are differentially sensitive to the motivational effects of cannabinoid agonists in conditioned place preference. Behav Brain Res. 2014b;268:202–212. doi: 10.1016/j.bbr.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bernstein HG, Becker A, Hollt V, Bogerts B. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology. 1999;20(6):525–532. doi: 10.1016/S0893-133X(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harv Rev Psychiatry. 1999;6(6):287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hendricks S, Kenkel AM, Bhatia SC, Haffke EA. Relapse in schizophrenia: is there a relationship to substance abuse? Schizophr Res. 1996;20(1–2):153–156. doi: 10.1016/0920-9964(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Harkavy-Friedman JM, Nelson E. Management of the suicidal patient with schizophrenia. Psychiatr Clin North Am. 1997;20(3):625–640. doi: 10.1016/s0193-953x(05)70334-8. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Balguerie K, Jeanblanc V, Coune F, Legastelois R, Naassila M. Light alcohol intake during adolescence induces alcohol addiction in a neurodevelopmental model of schizophrenia. Addict Biol. 2015 May;20(3):490–499. doi: 10.1111/adb.12146. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, Gomez-Carrillo de Castro A, Kuhl KP. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1054–1063. doi: 10.1016/j.pnpbp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Kircher DM, Shaham Y, O’Donnell P. Exaggerated cue-induced reinstatement of cocaine seeking but not incubation of cocaine craving in a developmental rat model of schizophrenia. Psychopharmacology (Berl) 2013;226(1):45–51. doi: 10.1007/s00213-012-2882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar JY, Green AI. Effects of iloperidone, combined with desipramine, on alcohol drinking in the Syrian golden hamster. Neuropharmacology. 2016;105:25–34. doi: 10.1016/j.neuropharm.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2008;200(2):217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Labrie V, Wang W, Barger SW, Baker GB, Roder JC. Genetic loss of D-amino acid oxidase activity reverses schizophrenia-like phenotypes in mice. Genes Brain Behav. 2010;9(1):11–25. doi: 10.1111/j.1601-183X.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. A neurodevelopmental model of schizophrenia: neonatal disconnection of the hippocampus. Neurotox Res. 2002;4(5–6):469–475. doi: 10.1080/1029842021000022089. [DOI] [PubMed] [Google Scholar]
- Lopez JC, Karlsson RM, O’Donnell P. Dopamine D2 Modulation of Sign and Goal Tracking in Rats. Neuropsychopharmacology. 2015;40(9):2096–2102. doi: 10.1038/npp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser PC, Hitchcock JM, Lister S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Brain Res Rev. 2000;33(2–3):275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Ng E, McGirr A, Wong AH, Roder JC. Using rodents to model schizophrenia and substance use comorbidity. Neuroscience and biobehavioral reviews. 2013;37(5):896–910. doi: 10.1016/j.neubiorev.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Owen RR, Fischer EP, Booth BM, Cuffel BJ. Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatr Serv. 1996;47(8):853–858. doi: 10.1176/ps.47.8.853. [DOI] [PubMed] [Google Scholar]
- Rao KN, Sentir AM, Engleman EA, Bell RL, Hulvershorn LA, Breier A, Chambers RA. Toward early estimation and treatment of addiction vulnerability: radial arm maze and N-acetyl cysteine before cocaine sensitization or nicotine self-administration in neonatal ventral hippocampal lesion rats. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, Thomas P. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51(2–3):149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Rodriguez-Jimenez R, Bagney A, Martinez-Gras I, Ponce G, Sanchez-Morla EM, Aragues M, Rubio G, Jimenez-Arriero MA, Santos JL, Palomo T, Parg Executive function in schizophrenia: influence of substance use disorder history. Schizophr Res. 2010;118(1–3):34–40. doi: 10.1016/j.schres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology (Berl) 2009;205(1):45–52. doi: 10.1007/s00213-009-1512-9. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, Bechara A. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92(1–3):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JW, Holzer CE, 3rd, Ganju VK, Jono RT. Violence and psychiatric disorder in the community: evidence from the Epidemiologic Catchment Area surveys. Hosp Community Psychiatry. 1990;41(7):761–770. doi: 10.1176/ps.41.7.761. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR. A cautionary note about latent inhibitino in schizophrenia: are we ignoring relevant information? In: Lubow RE, Weiner I, editors. Latent Inhibition: Cognition, Neurosicence and Applications to Schizophrenia. Cambridge University Press; 2010. pp. 448–456. [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioural brain research. 2009;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND. Substance use disorders in schizophrenia--clinical implications of comorbidity. Schizophr Bull. 2009;35(3):469–472. doi: 10.1093/schbul/sbp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: the switching model. Psychol Bull. 1990;108(3):442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169(3–4):257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Yogev H, Sirota P, Gutman Y, Hadar U. Latent inhibition and overswitching in schizophrenia. Schizophr Bull. 2004;30(4):713–726. doi: 10.1093/oxfordjournals.schbul.a007125. [DOI] [PubMed] [Google Scholar]