Abstract
IMPORTANCE
Given recently published results of a 750-patient adjuvant sunitinib trial showing improved disease-free survival (DFS), the appropriate strategy for treating high-risk patients is unclear. We sought to determine whether there is improved disease-free survival benefit to taking the active drug in patients with high-risk (pT3, pT4, node-positive) clear cell renal cancer (ccRCC) in the ASSURE trial (adjuvant sunitinib or sorafenib vs placebo in resected unfavorable renal cell carcinoma [RCC]), the largest adjuvant trial published to date.
OBJECTIVE
To evaluate DFS and overall survival (OS) in ccRCC high-risk patients randomized to sunitinib or sorafenib vs placebo among patients with stages comparable to other high-risk adjuvant trials.
DESIGN, SETTING, AND PARTICIPANTS
The DFS and OS at 10 years postactivation were calculated for 1069 patients in US and Canadian cooperative groups with high-risk patients who had ccRCC histology and pT3, pT4, or node-positive disease accrued between 2006 and 2010 to the double-blind randomized placebo-controlled phase 3 trial. Outcome analyses by dose quartiles of these patients receiving sunitinib or sorafenib were also performed.
INTERVENTIONS
Patients received 1 year of adjuvant sunitinib (50 mg), sorafenib (800 mg) daily, or equivalent placebo. The study was amended for patient intolerance to sunitinib (37.5 mg), sorafenib (400 mg) daily, or equivalent placebo with mandatory dose escalation if no serious adverse effects were experienced.
MAIN OUTCOMES AND MEASURES
Disease-free survival, defined as time from randomization to recurrence, second primary cancer, or death.
RESULTS
Of 1069 patients, 358 (243 [67.9%] men, 115 [32.1%] women) received sunitinib, 355 (248 [69.9%] men, 107 [30.1%] women) received sorafenib, and 356 (254 [71.3%] men, 102 [28.7%] women) received placebo as adjuvant therapy. The mean (SD) age for each group was 58.3 (10.6) years, 56.8 (10.3) years, and 57.5 (10.4) years, respectively. Five-year DFS rates were 47.7%, 49.9%, and 50.0%, respectively for sunitinib, sorafenib, and placebo (HR, 0.94 for sunitinib vs placebo; and HR, 0.90; 97.5%CI, 0.71–1.14 for sorafenib vs placebo), with 5-year OS of 75.2%, 80.2%, and 76.5%(HR, 1.06; 97.5%CI, 0.78–1.45; P = .66, sunitinib vs placebo; and HR, 0.80; 97.5%CI, 0.58–1.11; P = .12 for sorafenib vs placebo). There was no difference by dose quartile.
CONCLUSIONS AND RELEVANCE
Neither prognostic category of the tumor nor dose intensity of therapy altered the lack of difference in DFS or OS in this population of patients with high-risk ccRCC.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00326898
We recently reported no improvement in disease-free survival (DFS) in our primary analysis of the first and largest adjuvant vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) trial in primary resected renal cell carcinoma (RCC), E2805/ASSURE.1 Some current adjuvant trials of VEGF-TKIs include only patients with higher-stage RCC (pT3/4 or node-positive) and clear cell histology, because this population has a median time to progression of less than 1 year.2,3
Given recently published results of a 750-patient randomized trial, S-TRAC, (sunitinib 50 mg daily [4/2 schedule] vs placebo in clear cell predominant pT3-4 or node-positive disease) that show improved DFS,4 the appropriate adjuvant strategy for high-risk patients is unclear. Herein we provide an updated analysis of a high-risk subset in ASSURE (pT3/4 or node-positive and clear cell histology). The objectives of this analysis were first to evaluate DFS and OS in this population, and second, to evaluate the impact of dose intensity on outcome.
Methods
Patients
This analysis included patients with clear cell (histologically >25%) only disease, high-risk patients with pT3, pT4, or node-positive disease.5
Treatment
Patients were randomized in double-blind fashion to receive 54 weeks of either sunitinib, 50 mg, oral daily for 28 of 42 days per cycle, or sorafenib, 400 mg, oral twice daily continuously, or placebo. In 2009, to address toxic effects, starting doses were amended to 37.5 mg (sunitinib/placebo), or 400 mg (sorafenib/placebo), for the first 1 to 2 cycles of therapy, and escalated to full doses if adverse effects were grade 2 or lower. Dose reductions down to 25 mg daily for sunitinib/placebo or 400 mg every other day for sorafenib/placebo for toxic effects were permitted. Compliance was assessed using a pill diary and pill count.
Study Oversight
The E2805 trial was led by the Eastern Cooperative Oncology Group (ECOG-ACRIN) with Southwest Oncology Group, Alliance, and National Cancer Institute of Canada Clinical Trials Group participation. It was overseen by an independent data monitoring committee, and approved by the institutional review board at each center with informed written consent obtained from the participants.
The clinical database as of July 16, 2016, was analyzed by the study statistician (J.M.).
Statistical Analysis
Disease-free survival was defined as time from randomization to recurrence, second primary cancer, or death without recurrence or second primary. Patients free of these events were censored at the date of last disease evaluation. Patients with residual disease after surgery were considered to have recurred on day 1. Overall survival was defined as time from randomization to death. Patients alive at analysis were censored at the date of last contact.
Key Points.
Questions
Do high-risk clear cell renal cancer (ccRCC) (≥pT3 or node-positive) patients receiving adjuvant sunitinib or sorafenib have improved disease-free survival, and does the dose intensity of either drug affect outcome?
Findings
In a secondary analysis of a high-risk subset of patients in the ASSURE randomized trial, 5-year disease-free survival rates were 47.7%, 49.9%, and 50.0% respectively for sunitinib, sorafenib, and placebo. Dose intensity did not affect outcome.
Meaning
There was no benefit to adjuvant sunitinib or sorafenib in this high-risk ccRCC population, and there was no difference in outcome by dose quartile.
Groups were compared using the Wald test from Cox proportional hazards models stratified on histology, performance status, type of nephrectomy, and risk category.6 Because there were 2 arm-wise comparisons, 97.5% CIs are shown. These are primarily post hoc subset analyses. Given the retrospective nature of these analyses, the type I error is not controlled, and P values should be interpreted with caution. Quartiles of dose were formed by summing the total doses taken per cycle reported for each patient across all cycles, dividing by the number of cycles, and finding the quartiles of the resulting distribution. Dose intensity was affected by both holds and reductions.
The efficacy population includes all patients meeting subset inclusion criteria, as randomized. Analyses of dosing exclude patients who withdrew before treatment. The full protocol, with the original statistical analysis plan, is provided in the Supplement. Analyses were conducted using R statistical software(version 3.0.2, R Foundation), using the survival package.
Results
Patients
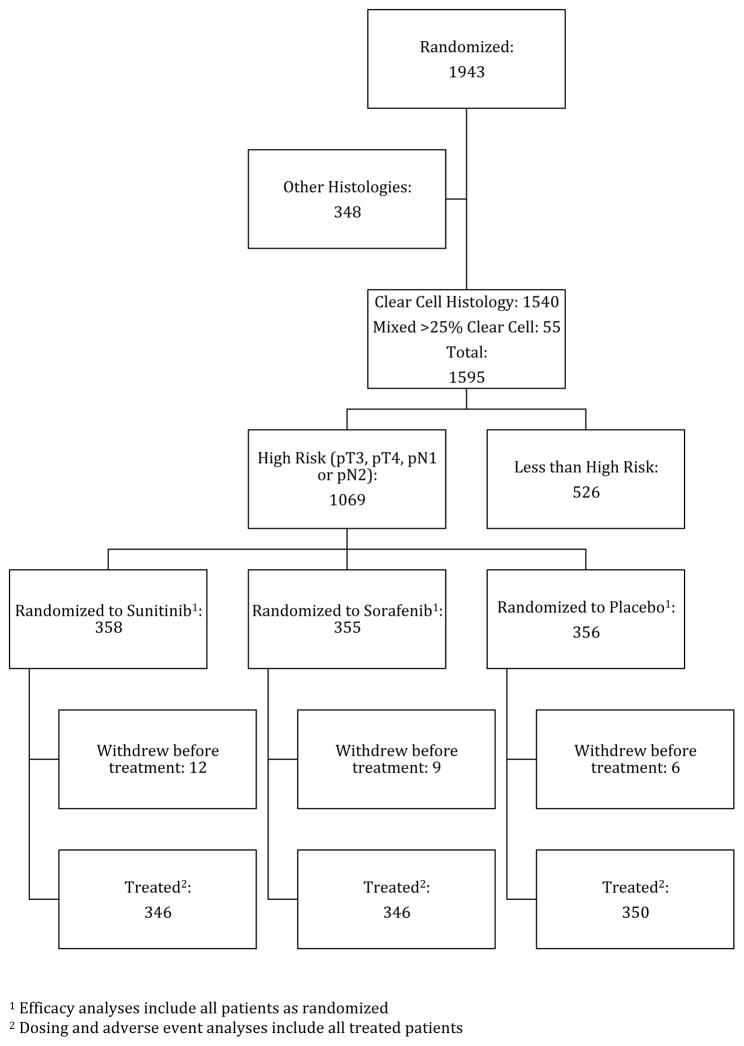
Of 1943 total patients accrued (from April 2006 to September 2010), 1069 were defined as pT3 and higher or node-positive with clear cell histology and are included in this analysis (eTable 1 in the Supplement). Figure 1 is a CONSORT diagram showing the disposition of cases. Patients were staged by American Joint Committee on Cancer 2002 classifications.7
Figure 1. CONSORT Diagram of Participant Selection.
aEfficacy analyses include all patients randomized.
bDosing and adverse event analyses include all treated patients.
Efficacy
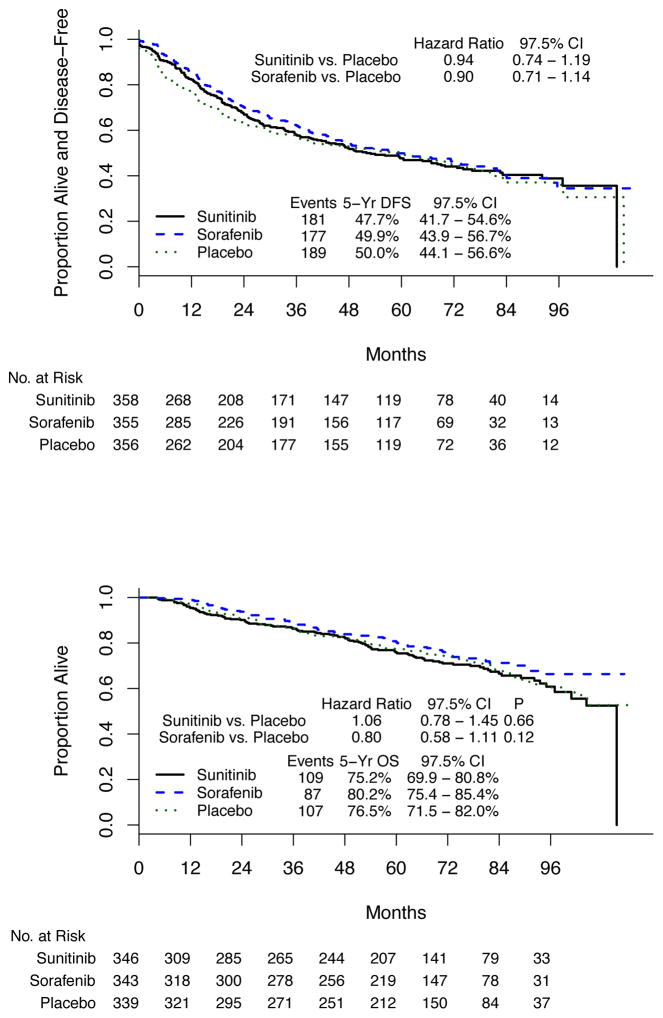
The 5-year DFS rate in high-risk patients with clear cell histology for sunitinib was 47.7%, for sorafenib was 49.9%, and for placebo was 50.0%. Differences in DFS were not statistically significant (sunitinib vs placebo:hazard ratio [HR], 0.94; 97.5% CI, 0.74–1.19; stratified log rank P = .54; sorafenib vs placebo: HR, 0.90; 97.5% CI, 0.71–1.14, stratified log rank P = .30) (Figure 2, A).
Figure 2. Disease-Free and Overall Survival by Treatment Arm in the High-Risk Clear Cell Cohort.
A, Disease-free survival. B, Overall survival.
Outcome Analysis by Dose Intensity Quartile
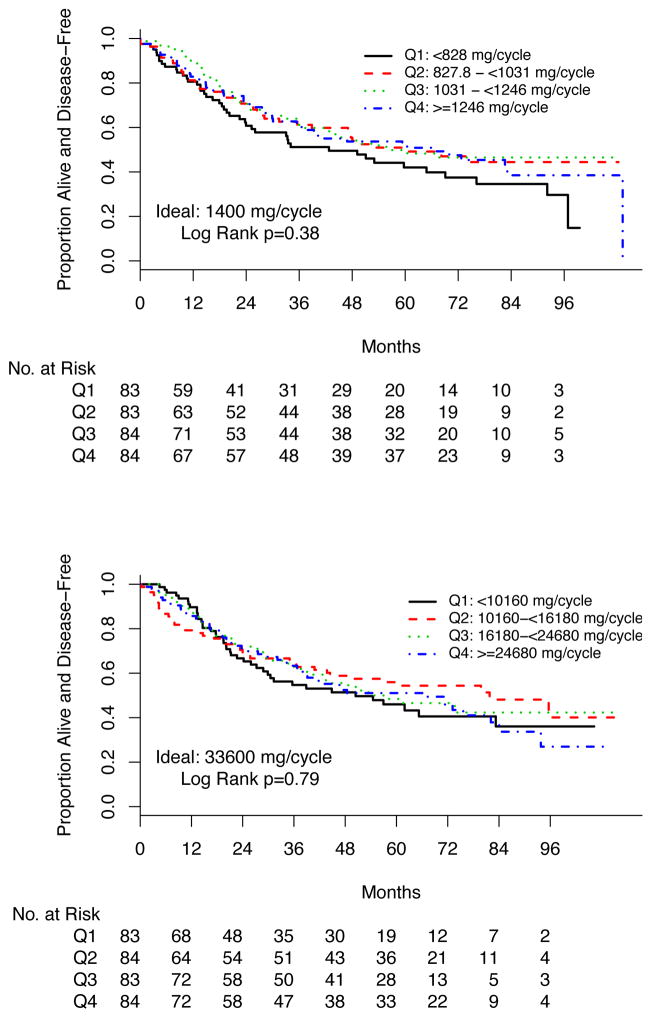
We analyzed DFS outcomes for high-risk ccRCC patients by agent (sunitinib or sorafenib) in quartiles of total dose per 6-week cycle. The expected full doses per 6-week cycle were sunitinib, 1400 mg, and sorafenib, 33600 mg. DFS for each agent by quartile is shown in Figure 3. There was no difference in DFS associated with quartile of average dose per cycle (log rank P = .38 and .79 for sunitinib and sorafenib, respectively). Of 98 patients, 45 (45.9%) taking sunitinib, 35 of 102 (34.3%) taking sorafenib, and 84 of 97 (86.6%) taking placebo in this analysis successfully escalated from reduced starting dose to full dose by cycle 3, similar to each arm in the primary analysis.
Figure 3.
Disease-Free Survival by Quartile of Average Dose Received per 6-Week Cycle
Overall Survival
At the time of this analysis, high-risk ccRCC patients on the 3 arms had 5-year OS of 75.2%(97.5%CI, 69.9%–80.8%), 80.2% (97.5%CI, 75.4%–85.4%), and 76.5%(97.5%CI, 71.5%–82.0%) for sunitib, sorafenib, and placebo, respectively. There were no differences associated with treatment (sunitinib vs placebo [HR, 1.06; 97.5%CI, 0.78–1.45; P = .66], sorafenib vs placebo [HR, 0.80; 97.5%CI, 0.58–1.11; P = .12]). This is shown in Figure 2, B. Overall survival by dose per cycle quartile among patients randomized to sunitinib or sorafenib is shown in eFigure 2, A and B, in the Supplement.
Adverse Events
Grade 3 or higher adverse events were reported by 66%, 72%, and 28% of patients in this cohort randomized to sunitinib, sorafenib, and placebo, respectively (eTable 2 in the Supplement), underscoring a high rate of adverse effects.
Discussion
High-risk patients might be the ideal population in which to detect activity of adjuvant sunitinib or sorafenib. Consistent with the primary analysis,1 our analysis of E2805 patients with ccRCC and high-risk (pT3 and higher or node-positive) disease showed no statistically significant benefit from these agents vs placebo. In addition, we observed no difference in outcome by dose quartile. This high-risk population had a better 5-year recurrence-free rate (around 50%) than expected (41.9% for high-risk disease and 36.0% for node-positive disease),5 possibly a result of better surgical technique, more accurate staging, or unknown biologic factors. Five-year DFS was similar to that observed in another contemporary trial.8 Ongoing correlative analyses are designed to identify subsets that may benefit from adjuvant therapy.
We asked if patients who received higher doses of sunitinib or sorafenib might have fared differently than those who received lower doses. Our DFS results did not show a meaningful difference by dose quartile. Duration of therapy or starting dose, likewise, had no effect on our primary outcome of DFS.
Limitations
This analysis has limitations: the ccRCC cohort was prespecified but analysis of dosing cohorts and higher stage were posthoc; the trial was not powered to detect differences in these subsets. However, the sample size was large enough that a clinically meaningful difference would be observable in the graphical portrayal of DFS. The subsets that involve dosing are not free of bias, because they were formed using information that arose after randomization.
Conclusions
These analyses shed light on the impact of adjuvant therapy and dose in this high-risk population. Using key questions to examine failed primary outcomes of randomized clinical trials,9 our subgroup findings did not elicit positive signals in these patients. Furthermore, the lack of efficacy is not attributable to low dose-intensity. Based on this analysis, a rationale for adjuvant therapy in this high-risk population is not elucidated.
Supplementary Material
Acknowledgments
Funding/Support: This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180867, CA180821, CA31946, CA077202, CA180863, CCSRI021039, CA32102, CA105409, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The trial was supported by the Cancer Trials Evaluation Program, National Cancer Institute, who provided drugs and placebos through agreements with Bayer and Pfizer.
Role of the Funder/Sponsor: ECOG/ACRIN provided personnel for the support of the conduct of the study as well as for the collection of data. Review and approval of the manuscript was provided by ECOG/ACRIN and CTEP. The decision to submit the manuscript for publication was made by the authors.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Haas and Ms Manola had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Haas, Manola, Flaherty, Atkins, Dipaola, Choueiri.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Haas, Manola, Flaherty, Atkins, Dipaola, Choueiri.
Critical revision of the manuscript for important intellectual content: Haas, Manola, Dutcher, Flaherty, Uzzo, Atkins, Choueiri.
Statistical analysis: Manola, Uzzo.
Administrative, technical, or material support: Flaherty, Dipaola, Choueiri.
Supervision: Dutcher, Uzzo, Dipaola, Choueiri.
References
- 1.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal SK, Haas NB. Adjuvant therapy for renal cell carcinoma: past, present, and future. Oncologist. 2014;19(8):851–859. doi: 10.1634/theoncologist.2014-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SP, Crispen PL, Thompson RH, et al. Assessment of the pathologic inclusion criteria from contemporary adjuvant clinical trials for predicting disease progression after nephrectomy for renal cell carcinoma. Cancer. 2012;118(18):4412–4420. doi: 10.1002/cncr.26695. [DOI] [PubMed] [Google Scholar]
- 4.Ravaud A, Motzer RJ, Pandha HS, et al. S-TRAC Investigators. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–2254. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 5.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174(2):466–472. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 6.Cox DR. Regression models and life tables. J Roy Statist Soc B. 1972;34:181–220. [Google Scholar]
- 7.Greene FL, Page D, Morrow M, et al., editors. AJCC Cancer Staging Manuel. 6. New York, NY: Springer; 2002. [Google Scholar]
- 8.Chamie K, Donin NM, Klöpfer P, et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the Ariser randomized clinical trial [published online October 27, 2016] JAMA Oncol. doi: 10.1001/jamaoncol.2016.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pocock SJ, Stone GW. The primary outcome fails–what next? N Engl J Med. 2016;375(9):861–870. doi: 10.1056/NEJMra1510064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.