Abstract
Background
Adherence to dietary prescriptions is critical for successful weight loss and weight loss maintenance. However, research on specific instances of inadherence (lapses) is limited, and findings regarding the frequency, nature and causes of lapses are mixed. Additionally, no studies have examined lapses over the course of a weight loss program.
Purpose
In the context of a reduced calorie diet prescribed as part of a behavioral treatment, we aimed to characterize lapse occurrence, examine lapse frequency across treatment, examine predictors of lapses, and assess the relationship between lapses and weight loss.
Methods
Adults (n = 189) enrolled in a 12-month behavioral weight loss program completed ecological momentary assessment (EMA) at baseline, mid-treatment, and end-of-treatment. At each EMA survey, participants indicated whether a lapse had occurred, and responded to questions assessing situational, environmental, and affective states.
Results
Lapse frequency showed a curvilinear relationship over time, such that frequency first decreased and then increased. More frequent lapses at baseline were associated with less early and overall weight loss. Lapses occurred most often at home, in the evenings, on the weekends, and entailed eating a forbidden food. Greater overall levels of assessed affective and environmental triggers predicted lapses; greater momentary hunger and deprivation, and the presence of palatable food also prospectively predicted lapses.
Conclusions
In addition to characterizing lapse frequency, the current study identified prospective predictors of lapses across treatment. These findings support the importance of lapses to weight control, and provide insight for potential targets of intervention to prevent lapse occurrence.
Keywords: dietary lapses, adherence, ecological momentary assessment, EMA, behavioral weight loss, overweight
Over two-thirds of Americans are overweight or obese (1) and 48% are currently attempting to follow a lower-calorie diet in order to control weight (2). However, results from self-guided and formal weight loss programs are disappointing. Even the 5–10% weight loss produced by our most effective non-surgical treatments (i.e., intensive behavioral weight loss programs) is far less than is expected given prescribed caloric intakes (3, 4). Further, these treatments produce less weight loss than is experienced by individuals in controlled environments (e.g., hospital inpatient programs) whose caloric intake is tightly managed (5–7). Thus, weight control failure is partially attributable to an inability to remain consistently adherent to an intended diet. Specific instances of inadherence, i.e., dietary lapses, potentially play an important role in weight control by accumulating to threaten weight loss or weight loss maintenance. Moreover, lapses are known to provoke feelings of hopelessness (8, 9), which may lead to abandonment of weight control goals altogether (i.e., relapse).
Despite the fact that lapses presumably play a central role in weight control outcomes, we know relatively little about them, especially in the context of highly structured weight control prescriptions. According to goal conflict theory, an empirically-based model of self-regulation, the eating behavior of chronic dieters is governed by two opposing goals: the hedonic drive to consume palatable (and likely high-calorie) foods and the desire to lose weight (10). This model posits that failures to adhere to dietary recommendations (i.e., lapses) are likely a result of exposure to internal (e.g., hunger, fatigue) and external (e.g., presence of delicious food, socializing) cues that activate biological drives to maintain hedonic states and therefore override dieting goals (10, 11). Empirical support for the goal conflict theory can be extrapolated from self-reports and laboratory-based studies about lapses and associated cues. For example, weight control participants have reported that the factors most responsible for their dietary lapses are negative affect, food-related cues, cravings, hunger, and being in social situations (12, 13). However, findings obtained from self-report and laboratory-based studies are limited by bias in recall and ecological validity, respectively (14). Alternatively, studies have successfully studied lapses and their antecedents using ecological momentary assessment (EMA), which utilizes event and/or signal-based recording (i.e., recording at a lapse occurrence and when a time-based prompt occurs) to repeatedly assess current (or very recent) behaviors and psychological processes in their real-world context (8, 9, 15–17). The unique assessment capabilities of EMA allow for more accurate examination of lapse frequency and nature, as well as for identification of triggers for lapsing (18, 19).
Frequency and Nature of Lapses
Numerous studies have utilized EMA to examine general eating behaviors, overeating episodes, and eating disordered behaviors (e.g., binge eating) in a variety of populations (see Engel et al., 2016). However, only three studies have used EMA to report lapse characteristics among overweight and obese adults attempting to lose weight. Interestingly, reported mean frequency in these studies has ranged from 2.7 to 11.8 lapses/week (8, 9, 15), most likely because of differences in weight control program (formal weight loss treatment versus self-guided dieting), assessment point (during versus end of program), and participant characteristics (middle-aged women versus undergraduate dieters). Perhaps for the same reason, findings regarding the location of lapses have also varied. For example, lapses of young adults but not of post-menopausal women were more likely to occur at home (8, 9). Only one study examined timing of lapses, reporting that lapses appeared equally likely to occur on weekends and weekdays, but were more likely to occur in the evening (17). The data demonstrating equal risk for lapse on weekends vs weekdays are surprising, given that epidemiological data from the National Health and Nutrition Examination Survey have indicated that on Saturdays, compared to weekdays, calorie intake is significantly higher (an increase of 181 kcal, on average) and dietary quality is significantly worse (20). Restaurant and fast food eating is more prevalent on weekends, which is one factor that could possibly lead to greater risk of lapse (20).
Gold-standard behavioral weight loss interventions currently contain strategies for preventing dietary lapses, such as stimulus control (to reduce visible temptation), planning for high-risk situations (e.g., social events), and managing emotions that have the potential to provoke overeating. However, without further understanding the nature of dietary lapses, especially during behavioral weight loss treatment, the efficacy of these intervention components may be limited. Of note, no work has yet characterized, over multiple time points, dietary lapses that occur in the context of a behavioral weight loss program (e.g., clarify how often individuals attempting weight loss lapse, when and where these lapses most often occur, and whether lapse frequency or characteristics differ over time). Typically, participants lose weight during the initial phases of an intervention when acquisition of targeted skills/strategies is greatest and intensity (and thus accountability) is high, but then experience weight regain as the program lessens in intensity or ceases altogether. One explanation for this pattern is that reinforcement for weight control behaviors, and thus motivation, is likely to be greatest during active weight loss (e.g., especially at the outset of treatment) and lowest during weight loss maintenance (21). During maintenance phase of treatment, when accountability and intensity of the treatment program is at its lowest and reinforcement from weight loss is minimal (because the rate of weight loss typically is very low or zero), participants may begin to have trouble maintaining motivation to continue with challenging weight control behaviors (e.g., self-monitoring food intake, modifying one’s food environment, planning meals, physical activity). As such, lapse frequency might decrease during initial phases of a program but subsequently increase. Information about changes in lapse frequency, as well as in other characteristics of lapses, has the potential to inform weight control interventions, whether conventional or momentary, such that they improve the ability to prevent lapses.
Association of Lapses with Weight Loss
To date, no direct examinations of the relationship between lapse frequency and concurrent weight loss have been conducted. One study examined lapse frequency at the end of a treatment program and determined frequency was not significantly related to weight loss during the program (22). The lack of relationship could be due to the timing of assessment or perhaps participants compensating for lapse episodes by restricting subsequent eating and/or increasing physical activity. Another important question concerns the ability of lapse frequency observed during the initial measurement period (which is normally confined to a short, discrete period due to concerns about participant burden) to predict overall weight loss outcomes. The fact that early weight loss predicts future, long-term weight loss (23, 24) suggests that dietary adherence remains relatively consistent throughout a weight control program. As such, initial lapse frequency (as a quantification of adherence) might predict long-term weight loss outcomes. Notably, to our knowledge, no studies have examined the predictive value of lapse frequency for future weight loss. Should early lapse frequency predict either concurrent or later weight loss, strategies for increasing adherence early in behavioral weight loss program could be warranted.
Triggers of Dietary Lapses
Consistent with the goal conflict theory of self-regulation, studies of eating behavior have indicated that the hedonic drives that instigate lapses are triggered by the presence of food cues in the environment (10, 11, 25). Further, internal states serve as cues for consuming foods that are typically high in fat and sugar (26). These cues drive behavior on an implicit level and so valuable information about triggers for lapse can be extrapolated from studies utilizing momentary assessment. Only a handful of EMA studies have examined lapse occurrence among overweight and obese adults who are dieting. These studies have identified specific internal and external cues that predict lapse and are therefore presumed to activate hedonic drives rather than dieting goals. In particular, the presence of any internal trigger such as hunger, an overall negative or positive mood, stress, several specific affective states (e.g., boredom, sadness, nervousness), and feelings of deprivation (8, 9) is prospectively predictive of lapse occurrence. Research using retrospective recall has also detected an association between lapses in the context of a behavioral weight loss program and negative affect (27). External factors that have predicted lapses include being or socializing with others, being in a tempting environment, eating with others, conflict, and watching television (8, 9, 17). Notably, several triggers (e.g., specific mood states) have emerged as predictors in some studies but not others, again likely because of differences in sample characteristics. Further, nothing is known about how triggers of lapses evolve over the course of a behavioral weight loss program, which would provide information about how to target lapses throughout the course of a weight loss intervention.
Current Study
As described above, the small body of EMA findings related to dietary lapse frequency and related factors among overweight and obese dieting individuals is limited. Additionally, results have been relatively mixed, likely due to whether participants were enrolled in a behavioral weight loss program, when lapses were assessed, and potential differences in participants’ understanding of lapses due to differing dietary goals. Moreover, no prior studies that we are aware of have assessed dietary lapses among the same group over multiple time points.
As such, the current study sought to extend the existing research on dietary lapses by utilizing EMA methods to assess lapse behavior throughout a 12-month behavioral weight loss program. Specifically, the study aimed to (1) characterize lapses from a prescribed weight loss diet in terms of frequency, type (i.e., eating more than intended, eating a food that one hadn’t intended to eat, and eating at a time one hadn’t intended), location, and time; (2) test the hypothesis that lapse frequency will decrease from the beginning-of-treatment to mid-treatment (due to weight control skill acquisition) and then increase to end-of-treatment (due to behavioral fatigue); (3) test the hypothesis that lapse frequency at the start of treatment will be associated with both concurrent and future weight change; (4) test the hypothesis that lapses will occur more frequently on the weekends than weekdays, and (5) test the hypothesis that internal (stress, boredom, irritation, sadness, loneliness, deprivation, hunger, and fatigue) and external (availability of delicious food) cues will prospectively predict future dietary lapses (at the next survey, within the same day).
Method
Participants
The sample was comprised of 189 overweight participants (82.0% female; 70.9% Caucasian; Mage = 51.81 ±9.76 years; MBMI = 36.93 ±5.83 kg/m2) enrolled in a structured behavioral weight loss program as part of a larger study; see Forman and colleagues (28) for details. All participants were weight loss-seeking, and motivations generally included health, mobility and appearance factors.
Intervention
The intervention was comprised of 25 group sessions of decreasing frequency over 12 months, and included all features of standard behavioral weight loss including weight goals, regular weighing, calorie and physical activity goals/self-monitoring, and guidance in identifying and following a balanced deficit diet that would comport with calorie goals. Each 75-minute session consisted of a brief check-in from each group member, introduction or review of a weight control skill, and goal-setting. Treatment sessions were delivered by doctoral-level clinicians and a trainee who served as a co-leader. If a participant was absent from a particular group session, he/she completed a brief (20-minute) individual makeup session with the group co-leader to review the session material. Participants were asked to carefully track all food/beverage consumption and calculate caloric intake so as to ensure that they met their calorie goals. One participant from the parent study did not participate in the EMA protocol and was excluded from analyses.
Procedure
Participants were loaned an Android player (essentially a smartphone without phone capabilities; Samsung Galaxy Player 4.0) pre-loaded with a custom-designed EMA smartphone application (DrexelEMA). The EMA protocol occurred for 14 days within the first three weeks of treatment (baseline), 7 days at 6-months (mid-treatment), and 7 days at 12 months (end-of-treatment). Prior to each period of EMA data collection, participants were given oral and written instructions on how to use the EMA app and how to identify a dietary lapse (see Ecological Momentary Assessment, below).
Measures
Weight
Weight was measured prior to each of the 25 sessions as well as at pre-treatment, mid-treatment and end-of-treatment with the participant in street clothes (without shoes) using a standardized Seca scale accurate to 0.1 kg. Height was measured with a stadiometer to establish BMI.
Ecological Momentary Assessment (EMA)
As is common in EMA studies (29, 30), participants received six, semi-random prompts daily (i.e., ±30 minutes of 9:30 am, 12:00 pm, 2:30 pm, 5:00 pm, 7:15 pm, 9:30 pm) in order to ensure adequate sampling throughout waking hours. Participants were also instructed to initiate an EMA survey whenever they experienced a dietary lapse. At each EMA report, the time of the survey response was automatically recorded by the device (though specific clusters of timestamps required semi-manual adjustment due to technical problems related to the Android operating system’s treatment of time zones). At each EMA report, participants also provided information about cues and whether or not a lapse had taken place (see below). If participants did not respond immediately to the prompt, they were signaled every 5 minutes until they responded to the prompt or the prompt expired (i.e., after 45 minutes). Participants lost $1 from a potential maximum of $42 in compensation for every prompt not responded to.
At each EMA survey prompt, participants were asked to indicate whether they had experienced a dietary lapse since last completing an EMA survey. A lapse was defined as “eating or drinking likely to cause weight gain, and/or put weight loss/maintenance at risk.” In the event of an affirmative response, participants were asked to respond to several items assessing the timing (i.e., date and time), the type (I ate a larger portion of a meal or snack than I intended, I ate when I hadn’t intended to eat, or I ate a type of food that I intended to avoid), and the location (At home, At school, At work, At a restaurant, café or other eating location, or Other) of the lapse. If two lapses occurred within 20 minutes, the lapses were treated as one lapse episode, and only the first of the reported lapses was used in analyses.
Based on an adapted version of the PANAS (31), negative affect (sad, lonely, bored, angry/irritated, stress) and non-affective (hunger, perceived deprivation, tiredness) states were measured on a 5-point Likert scale (1 = Not at all; 2 = Slightly; 3 = Moderately; 4 = Very; 5 = Extremely). We formatted non-affective items in a manner similar to the PANAS in order to keep EMA questions in a similar format to facilitate efficient responding by the participant (e.g., “Right now, how strongly do you feel hungry?”). Presence of tempting foods was evaluated by asking users to report “Since the last time you completed this survey, was delicious food or drink available that would put your calorie goal/weight control at risk?” (Yes/No). Social situations were not directly assessed due to a desire to limit the number of EMA questions in order to maximize compliance with the six-surveys-a-day protocol.
Statistical Approach
Lapse type, time, and location were characterized using percentages by assessment point. Lapse triggers were characterized by examining means and standard deviations for each trigger across assessment points and participants. Variability in triggers was examined by calculating mean square successive difference (MSSD), which is a measure of variability that takes into account the temporal order of observations. MSSD was calculated for each trigger on the daily level for each participant. We then characterized variability by examining the mean MSSD values and the standard deviation of mean MSSD values across assessment points and participants. A generalized estimating equation (GEE) based upon a negative binomial distribution with a log link function and an AR(1) matrix structure was used to compare daily lapse frequency across assessment points. Day of assessment (for each assessment point) was included to control for potential changes in lapse frequency across the assessment periods. Assessment point was included in the model as a continuous predictor. Both linear and quadratic terms were included in the model to assess the nature of the change in lapse frequency over time. The assessment variable was centered prior to squaring.
The relationships between lapse frequency and weight loss were assessed using linear and multiple linear regressions. Maximum likelihood multiple imputation was used to account for missing weights at both the end of the baseline EMA assessment period and at the end of treatment. The relationship between baseline lapse frequency and overall weight change in the program was assessed both with and without including weight change during the baseline EMA period as a covariate. This was done to examine the predictive value of baseline lapse occurrence independently and over and above the effect of early weight loss, respectively.
A GEE model based upon a negative binomial distribution with a log link function and an AR(1) matrix structure was also used to compare lapse frequency at the daily level on weekdays versus weekends. Assessment point was included in the model as a categorical predictor, and potential interactions between lapse frequency and assessment point were examined, with mid-treatment serving as the reference category. Separate GEE models based on a negative binomial distribution with a logit link function and an AR(1) matrix structure were used to examine whether each trigger predicted lapse occurrence at the next survey. All models controlled for between-subject effects (i.e., participants’ mean level of each potential trigger), as well as whether a lapse was reported at the current survey. Assessment point was also included as a predictor in each model and potential interactions between assessment point and both between- and within-subject predictors were examined. All between-subjects variables were grand mean centered. Within-subjects effects for continuous predictor variables were centered within person prior to computing interaction terms.
Results
Compliance
A total of 189 participants completed EMA at baseline, 163 completed EMA at mid-treatment, and 147 completed EMA at end-of-treatment. Among these participants, the mean response rate to prompted surveys was 82.4% (SD = 13.3%; range = 26.0–100.0%) at baseline, 82.2% (SD = 15.5%; range = 17.0–100.0%) at mid-treatment, and 84.1% (SD = 15.5%; range = 33.0–100.0%) at end-of-treatment. Based upon observed compliance distributions, data from participants with less than 40% compliance at a given assessment point were excluded from analyses for that assessment point only. Thus, the final analysis sample size was 186 at baseline, 162 at mid-treatment, and 143 at end-of-treatment (equivalent to 98.4%, 99.4%, and 97.3% of available data at each time point, respectively).
Data descriptives
A total of 24,772 (13,402 baseline, 6,017 mid, 5,353 end-of-treatment) EMA recordings were provided by 186 participants representing 4,568 participant days. Descriptive information for survey type, response days, and compliance by assessment point and overall are provided in Table 1. As noted above, sample size was not equal across assessments (baseline n = 186, mid-treatment n = 162, end-of-treatment n = 143). Logistic regression revealed that baseline lapse frequency did not significantly predict missing data at mid- (χ2 = .58, p = .45, df = 1) or end-of-treatment (χ2 = .02, p = .89, df = 1), suggesting that participants who lapsed more frequently at baseline were not at significantly greater risk of attrition from the EMA protocol. Analyses were conducted with participants who had eligible data (i.e., compliance ≥ 40%) at any assessment point, and with completers only (i.e., individuals who completed EMA at all three assessment points with ≥ 40%, n = 138). A similar pattern of results was observed, so we present results using all eligible data.
Table 1.
EMA survey type and compliance data by assessment point
| Assessment Point | |||
|---|---|---|---|
| Baseline | Mid-Treatment | End-of-Treatment | |
|
|
|||
| Sample size | 186 | 162 | 143 |
| Total number of recordings | 13,402 | 6,017 | 5,353 |
| Prompted | 11,541 | 5,065 | 4,736 |
| User-Initiated | 1,861 | 952 | 617 |
| Recording days | |||
| Mean (SD) | 13.3 (2.1) | 6.9 (1.5) | 6.9 (0.8) |
| Range | 3 to 18 | 1 to 13 | 2 to 10 |
| Compliance with prompted surveys | |||
| Mean (SD) | 83.2% (11.5%) | 82.7% (14.7%) | 85.5% (13.2%) |
| Range | 45% to 100% | 42% to 100% | 42% to 100% |
Lapse frequency
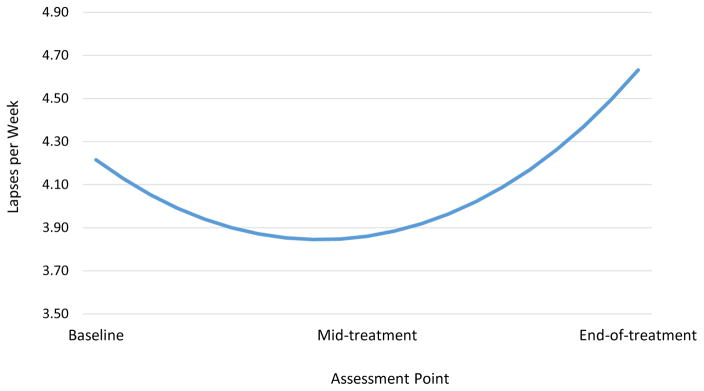
Participants reported a total of 2,786 lapses (1,452 at baseline, 667 at mid-treatment, and 667 at end-of-treatment). A GEE revealed a significant quadratic effect of time on lapse frequency, b = .14, SE = .06, Wald χ2 (1, N = 4,578) = 5.62, p = .02. The linear term was not significant, b = −.04, SE = .05, Wald χ2 (1, N = 4,578) = .61, p = .43. The estimated mean number of lapses per day was 0.60 at baseline, 0.55 at mid-treatment, and 0.66 at end-of-treatment. When translated to the weekly level, mean number of lapses were 4.22 at baseline, 3.86 at mid-treatment, and 4.63 at end-of-treatment (see Figure 1). The number of participants who reported no lapses at each assessment point was as follows: 14 at baseline (7.52% of participants completing EMA), 29 at mid-treatment (17.90% of participants completing EMA); and 25 at end-of-treatment (17.48% of participants completing EMA). Thus, the actual number of participants contributing data for EMA analyses at each time point were 172 at baseline, 133 at mid-treatment, and 118 at end-of-treatment. The range in number of lapses per participant (adjusted to 7 day total) was 0 to 16 at baseline, 0 to 21 at mid-treatment, and 0 to 21 at end-of-treatment.
Figure 1.
Estimated mean number of lapses per week per participant by assessment point.
Note: Values are based on predicted values from a GEE examining the effect of time on lapse frequency, when controlling for all other predictors in the model. Values are presented at the weekly level for interpretative purposes.
Lapse Type, Timing, and Location
Table 2 displays the percentage of each type of lapse, lapse timing, and lapse location reported at each assessment point. Across all assessments, eating an unintended type of food was the most commonly reported type of lapse. Lapses occurred most often at home. Evening was the most common time of lapse; early afternoon, late afternoon, and late evening were other common times of lapses. Of note, lapse type, timing, and location also were examined after first calculating person-level averages for each variable (so that individuals with more lapses did not skew the distribution); a nearly identical pattern was observed.
Table 2.
Lapse characteristics by assessment point.
| Assessment Point | |||
|---|---|---|---|
| Baseline (%) | Mid-treatment (%) | End-of-treatment (%) | |
|
|
|||
| Lapse Type | |||
| Unintended food | 44.6% | 42.2% | 45.8% |
| Unplanned time | 30.3% | 27.6% | 25.0% |
| Larger portion | 25.2% | 30.2% | 29.2% |
| Lapse Location | |||
| Home | 48.6% | 46.0% | 46.7% |
| Work/School | 16.6% | 14.6% | 19.5% |
| Eating Out | 19.8% | 19.2% | 16.9% |
| Other | 16.6% | 20.1% | 16.8% |
| Time of Lapse | |||
| Early morning (6:00–8:59 AM) | 2.0% | 2.5% | 3.7% |
| Late morning (9:00–11:59 AM) | 10.5% | 11.2% | 11.8% |
| Early afternoon (12:00–2:59 PM) | 18.9% | 20.7% | 20.5% |
| Late afternoon (3:00–5:59 PM) | 18.3% | 16.5% | 19.3% |
| Evening (6:00–8:59 PM) | 28.9% | 31.8% | 28.8% |
| Late evening (9:00–11:59 PM) | 20.1% | 14.8% | 14.1% |
| Overnight (12:00 AM–5:59 AM) | 1.3% | 2.4% | 1.6% |
Note: Percentages reflect all available data from completed survey items. Percentages may not add up to 100% due to rounding.
Relationship of Lapses to Weight Change
Weight change during the EMA assessment period (M = −3.09%, SD = 1.50%) and between baseline and end-of-treatment (M = −12.04%, SD = 7.86%) were both positively predicted by number of lapses during the baseline EMA period (b = .08, SE = .03, p = .01, and b = .46, SE = .17, p = .009, respectively). A multiple linear regression model containing number of reported lapses per week at baseline and weight change during the baseline EMA period indicated that lapse frequency also predicted percent weight change during treatment (b = 0.34, SE = .17, p = .05) when controlling for weight change during the baseline EMA period (b = 1.49, SE = .37, p < .001).
Lapses on Weekend versus Weekdays
Results from a GEE model indicated that participants were more likely to lapse on Saturdays or Sundays (EMM = .13, SEM = .008) than on weekdays (EMM = .11, SEM = .006), Wald χ2 (1, N = 24,772) = 7.05, p = .008 across all assessments.
Relationship between cues and subsequent lapse
Table 3 presents descriptive information for potential lapse triggers. Mean levels and variability of most triggers were fairly low. Table 4 presents model information for triggers for each GEE model. Report of a lapse at the current survey significantly predicted lapse occurrence at the subsequent survey in all models (ps < .001). Consistent with the lapse frequency analyses, assessment point also predicted lapse occurrence in most models. No significant interactions between assessment point and potential lapse triggers were observed, with the exception of the presence of delicious foods (see below). For ease of interpretation, full statistical information for current lapse (yes/no) and assessment point predictors, as well as for the interaction terms between assessment point and each trigger (both between- and within-), are not included in Table 4.
Table 3.
Descriptive data for lapse triggers across all EMA surveys
| Trigger | Mean Response | MSSD | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Sadness | 1.29 | 0.43 | 0.30 | 0.38 |
| Loneliness | 1.22 | 0.39 | 0.24 | 0.38 |
| Boredom | 1.17 | 0.30 | 0.28 | 0.41 |
| Anger/Irritation | 1.35 | 0.39 | 0.51 | 0.56 |
| Stress | 1.74 | 0.56 | 0.69 | 0.49 |
| Hunger | 1.75 | 0.42 | 1.49 | 0.93 |
| Deprivation | 1.61 | 0.53 | 0.70 | 0.64 |
| Fatigue | 1.84 | 0.57 | 0.73 | 0.55 |
| Presence of Delicious Food | 0.41 | 0.27 | -- | -- |
Note: The range for all variables except presence of delicious food was 1 to 5. Availability of delicious food was dichotomous; thus, MSSD values are not provided.
Table 4.
Between- and within-person effects for lapse triggers.
| B | SE | 95% CI | Wald χ2 | p | |
|---|---|---|---|---|---|
| Between-person Effects | |||||
| Sadness | .30 | .14 | [.04, .57] | 4.95 | .03 |
| Loneliness | .39 | .13 | [.12, .65] | 8.37 | .004 |
| Boredom | .52 | .16 | [.21, .84] | 10.76 | .001 |
| Anger/Irritation | .61 | .17 | [.27, .95] | 12.20 | <.001 |
| Stress | .57 | .14 | [.29, .85] | 23.66 | <.001 |
| Hunger | .58 | .16 | [.26, .90] | 12.36 | <.001 |
| Deprivation | .41 | .13 | [.16, .65] | 10.53 | .001 |
| Fatigue | .49 | .14 | [.22, .76] | 12.43 | <.001 |
| Presence of Delicious Food | .68 | .27 | [.14, 1.22] | 6.20 | .01 |
| Within-person Effects | |||||
| Sadness | −.20 | .36 | [−.90, .50] | .31 | .58 |
| Loneliness | .15 | .12 | [−.09, .39] | 1.59 | .21 |
| Boredom | .17 | .10 | [−.03, .38] | 2.66 | .10 |
| Anger/Irritation | .02 | .08 | [−.15, .18] | .06 | .81 |
| Stress | −.04 | .08 | [−.20, .11] | 4.15 | .59 |
| Hunger | .30 | .06 | [.19, .41] | 28.58 | <.001 |
| Deprivation | .27 | .07 | [.14, .40] | 16.04 | <.001 |
| Fatigue | .10 | .06 | [−.02, .21] | 2.73 | .10 |
| Presence of Delicious Food | .42 | .12 | [.19, .66] | 12.19 | <.001 |
Note: Each potential trigger was examined in a separate GEE that contained both between- and within-person effects for that trigger.
Between-subject effects
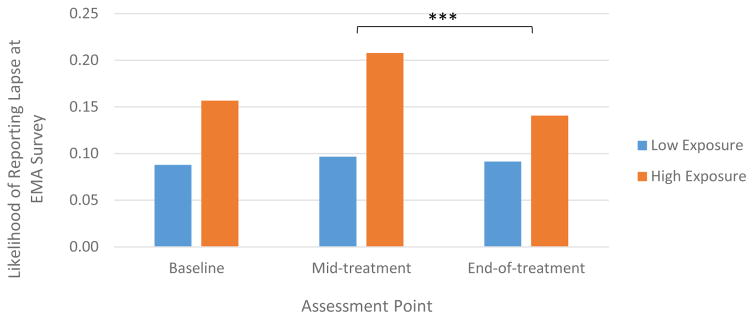
Significant between-subject effects were observed for all models, indicating that participants who reported greater-than-average sadness, loneliness, boredom, anger/irritation, stress, hunger, deprivation, fatigue, and exposure to delicious foods across EMA surveys relative to other participants in this sample were more likely to lapse at any given survey (see Table 4). In the case of exposure to delicious food, the predictive effect of the general presence of delicious food (i.e., the average level of exposure to delicious food) on lapse occurrence was moderated by assessment point, such that the strength of this effect was attenuated at end-of-treatment compared to mid-treatment (b = .81, SE = .27, 95% CI = .27 to 8.83, Wald χ2 = 6.51, p = .003; Figure 2).
Figure 2.
Average level of exposure to delicious food differentially predicts lapse occurrence from mid-treatment to end-of-treatment.
Note: Low exposure and high exposure represents values −/+ 1SD of mean exposure level to delicious foods. Values reflect estimated marginal means when controlling for all other variables in the GEE model.
Within-subject effects
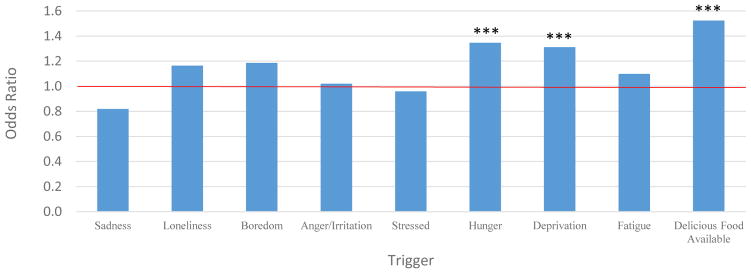
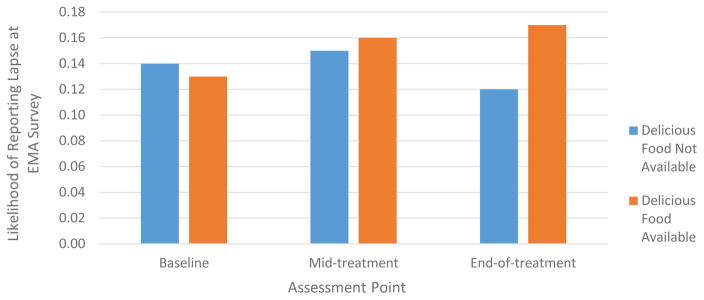
When controlling for between-subject effects, hunger, deprivation and availability of delicious food prospectively predicted lapse occurrence (Table 4). Specifically, the likelihood of a lapse increased by approximately 1.3x for each incremental increase in hunger and deprivation, and by 1.5x on average across assessment points if delicious food was available. The odds of lapsing at the next survey based on current level of each predictor is displayed in Figure 3. Of note, the extent to which the presence of delicious food raised the probability of lapsing increased by assessment point (baseline versus mid-treatment: b = −.51, SE = .15, 95% CI = −.81 to −.21, Wald χ2 = 11.01, p = .001; mid-treatment versus end-of-treatment: b = −.38, SE = .15, 95% CI = −.67 to −.09, Wald χ2 = 6.51, p = .01; Figure 4).
Figure 3.
Odds of lapse by trigger type
Note. *** p < .001. Red line indicates level at which the odds of lapsing would be constant regardless of trigger level.
Figure 4.
Momentary availability of delicious food differentially predicts lapse occurrence across assessment points
Note: Values reflect estimated marginal means when controlling for all other variables in the GEE model.
Discussion
This study represents the first-ever investigation to use EMA to prospectively track triggers of dietary lapses and dietary lapse frequency over multiple time points during a full weight control intervention. It is also the first study to examine the relationships between lapse frequency and concurrent and future weight loss, which is important for understanding the relevance of lapses to weight control efforts. These data are unique in that participants were enrolled in a gold standard behavioral weight loss program with structured dietary guidance, and in that we repeated EMAs at baseline, mid- and end-of-treatment.
Characterizing frequency, type, timing and location of lapses
Our findings revealed that participants in the weight loss program lapsed, on average, between 4 and 5 times per week. This frequency is somewhat higher than some previous reports (2.7 to 6.0 lapses/week; 8, 15), while lower than others (11.8 lapses/week; 9). One factor that may have contributed to the lapse rate in this study being greater than those observed in some previous studies is the difference in how dietary lapses were defined in the present study. Whereas several prior studies have defined lapses as “an incident where you felt you broke your diet (e.g., overeat, eat a forbidden food, etc.)” (8, 9, 17), lapses were defined as instances of “eating or drinking likely to cause weight gain, and/or put weight loss/maintenance at risk” in the present study, and participants were forced to categorize their lapse type (i.e., eating a larger portion than intended, eating at an unintended time, eating a food one was trying to avoid). Being required to identify a lapse type may have provided participants with a more elaborated and concrete understanding of what would constitute as a lapse in daily life, thus leading to better identification of and more frequent reports of lapses. Other possible explanations for the greater lapse rate are that the dietary guidelines in the current study were more clear-cut, making lapses more likely, obvious, and easier to report, and that the assessment of lapses in this study was more comprehensive in general and placed a greater emphasis on lapses (i.e., when training participants) than previous studies have, leading participants to be more aware of potential lapses. The one study that revealed a higher lapse rate (9) was conducted with undergraduate students who were self-dieting; differences in sample characteristics, approach to weight loss, and participants’ environment may help to explain why the lapse rate was lower in this study.
Lapse frequency was reasonably stable throughout the three assessment periods, but did show a hypothesized curvilinear relationship over time, such that lapse frequency appeared to decrease from baseline to mid-treatment and then increase from mid- to end-of-treatment. While no previous study has examined changes in lapse frequency over time, the observed pattern is highly consistent with the many findings of weight loss intervention participants demonstrating weight loss during active treatment and then weight regain (or at least slowed weight loss) as treatment tapers or stops (28, 32–34). Motivation is likely highest at the beginning of the program, in part because of the reinforcement of easier and more demonstrable weight loss (35). In addition, participants are benefiting at the outset of the program from skills training in shopping, food selection, cooking and normalizing eating to maximize satiety while minimizing calorie intake (36, 37). The increase in lapse frequency from mid- to end-of-treatment may be due to motivational degradation to practice key behaviors (35, 38). For instance, declines in motivation over time (likely resulting from plateaus or regaining weight) have been found to be associated with poor adherence to self-monitoring food intake (a key weight control behavior) (35). Such findings indicate that motivation is crucial for maintaining adherence to behavioral skills that ultimately impact outcomes, a finding echoed by other research (39). Further research should directly investigate the relationship between motivational states and lapse frequencies throughout behavioral weight loss programs.
It should be emphasized that reported lapses are also a reflection of the participants’ understanding of the dietary prescription and how stringently they were attempting to follow it. For example, it is conceivable that lapse reports at mid-treatment and end-of-treatment are more meaningful than those at baseline, because participants’ understanding of the dietary prescription they were attempting to follow deepened with exposure to treatment. In partial support of this hypothesis, lapse frequency at mid- and end-of-treatment were more strongly correlated (r = 0.73, p < .001) than were lapse frequency at baseline and mid-treatment (r = 0.52, p < .001) and at baseline and end-of-treatment (r = 0.56, ps < .001), suggesting participants’ standards for what qualified as a lapse may have been more similar later in treatment.
Results also revealed that frequency of lapses varied by type and location. For example, eating a food one had not intended to eat was by far the most frequent type of lapse perhaps because of the power of cues from desired foods. The home was the most common location of lapses, which is not surprising given the amount of time people spend at home and that it is a location where desirable food is likely to be readily available. On a related note, lapses occurred most often in the evenings, and were more likely to occur on the weekends than on weekdays. This result is consistent with other studies showing that weight control behaviors show different patterns on weekends versus weekdays, and makes sense given that lapsing is associated with being at home, having easy access to food and having unscheduled time (40). In addition, more lapses occurring later in the day is consistent with the limited resource model of self-control (41) which posits that individuals have a limited capacity for self-control, and that exerting self-control more often throughout the day can deplete one’s self-regulatory resources.
These results extend previous findings among individuals presenting for treatment that lapses occur most frequently at home and in the evening (8, 9, 17). As such, the current study’s findings add to the argument that modification of the home environment (to remove cues and easy access to high-calorie food), especially in the evening, represents a crucial intervention target (42). The present study showed that, while exposure to palatable foods decreased over time (indicating that interventions successfully reduce exposure to tempting foods), the momentary relationship between tempting food in the environment and dietary lapses strengthened. Perhaps some “tempting” foods (e.g., leftovers) could not necessarily be removed from the environment, suggesting that other interventions (e.g., portion control) may be necessary. Alternatively, stimulus control interventions may need to be further strengthened, for example, by involving partners or other family members and through home visits (43). Future research could investigate the utility of using EMA to identify individual-level triggers in order to personalize treatment targets (e.g., focusing on boredom and the workplace for one individual, but social occasions and evenings for another). In addition, current behavioral weight loss intervention components that aim to prevent dietary lapses (e.g., stimulus control, planning, reducing emotional eating) were not originally informed by research using ecologically-valid measurement methods, which suggests that this research could inform ways to enhance these intervention components.
Clinical significance of lapses and association with concurrent weight gain
The clinical significance of the reported lapses is difficult to judge given that we did not attempt to record how sizeable they were in calories. However, conservatively estimating that the average lapse was a modest 150 kcal produces an estimate of 600–750 kcal of out-of-plan consumption per week, on average. According to metabolic calculations, adding this amount of caloric intake to the diet of an individual in a weight control program would significantly impact weight trajectory (44). A more direct measure of the impact of lapses is that lapse frequency was associated with co-occurring weight change. In fact, the effect size indicated a 1.27 lb. difference in weight loss between a participant of mean weight for this sample at baseline (i.e., 222.0 lbs.) whose lapse frequency was one standard deviation below the mean (.80 lapses) and one standard deviation above the mean (7.37 lapses). This magnitude of difference over just a two-week period does appear to be clinically meaningful, especially given it could multiply across time and the fact that desired weight loss in behavioral treatments is 1–2 lbs. per week (7). Moreover, baseline lapse frequency predicted weight change at end-of-treatment, even controlling for co-occurring early weight loss, suggesting that early inadherence, above and beyond early weight loss success, is predictive of eventual success in the program. Put simply, early adherence appears to predict adherence in the program generally, which translates into overall weight loss by the end of the program.
Predictors of lapse
Potential triggers of lapsing were found to be associated with lapses in two different ways. First, participant’s average level of sadness, loneliness, boredom, anger/irritation, stress, hunger, deprivation, fatigue, and exposure to delicious foods was positively associated with likelihood of lapsing. In other words, people who were generally at higher levels of aversive internal states and who were more often around delicious foods during the EMA period lapsed more often. Interventions that provide skills to generally ease unpleasant emotional and physical states might then aid in weight control efforts, as would interventions designed to remove tempting food from the personal food environment. Second, an increase in an individual’s level of hunger and deprivation (relative to, and controlling for, his or her average level) and availability of delicious food at a given time point (controlling for average level) prospectively predicted lapse occurrence at the subsequent time point. In fact, the likelihood of a lapse increased 30–50% for each increase in level of a trigger variable. For example, someone who is two points above her average on the deprivation scale is 2.6 times as likely to lapse as when she is at her average level of deprivation. These particular results support the notion that momentary changes in the level of internal and external cues have a significant impact on eating decisions. Of note, the influence of these cues on a person’s eating decisions potentially takes place implicitly without his or her conscious knowledge (45–47)
The identification of specific cues that prospectively predict lapses from a formal weight control program has clinical implications. For example, intervention programs could emphasize the insidious nature of cues like hunger, feelings of deprivation, and tempting food, and introduce skills designed to be aware of and manage these states. Moreover, technology-driven, tailored, in-the-moment approaches, such as smartphone-based just-in-time adaptive interventions, could be developed that track cues known to prospectively predict lapses and to deliver specific coping skills designed to prevent a lapse from occurring (48). Of note, systems that help people recognize and interrupt patterns leading to dietary lapse might be equally useful for those in less formal weight loss programs as many of these individuals would face the same challenge of adhering to an eating plan and would be subject to the same forces pushing against adherence (49).
Of interest, momentary increases in physically aversive states, but not emotional states, significantly increased the likelihood of subsequent lapses. Perhaps negative reinforcement effects are stronger in the former case and thus play a larger role in driving off-plan eating. In any case, the pattern of findings are at odds with self-report studies showing that emotions drive overeating and that emotional eating is associated with overweight (50–52). One possibility is that self-reported emotional eating is a product of retrospectively accounting for behavior at odds with intentions. It must also be acknowledged that the triggers with the lowest mean levels across participants and the least variability were loneliness, boredom, and sadness. Therefore, it is possible that a more sensitive measure (perhaps with anchors to compare momentary states to normative emotional experiences) is required to detect more subtle distinctions in emotional states for the purpose of predicting lapse behavior.
The availability of delicious food was more predictive of lapses at certain assessment points compared to others. In particular, from mid- to post-treatment, lapse likelihood differed by average level of self-reported exposure to delicious foods such that the increased risk of lapsing from general level of exposure to delicious foods was attenuated. Interestingly, however, after controlling for individuals’ average level of exposure, the momentary risk of lapsing after exposure to delicious food increased over time. A post-hoc analysis revealed that the likelihood of reporting that delicious foods were available decreased slightly from baseline to end-of-treatment (42.4% to 39.1% of surveys; p = .05). Thus, perhaps participants became better at removing food cues from their personal environments (resulting in a weaker relationship between general food exposure level and lapse occurrence), meaning that when food was availability it was especially influential.
Limitations
The findings of the current study must be considered within the context of several limitations. First, our investigation is limited by the number of variables that could be included within the EMA protocol. Given the burden of completing EMA, we did not include certain variables that may be important in predicting dietary lapse, such as social or interpersonal interactions, TV watching, self-efficacy or motivation, exercising, lack of healthy food availability, and alcohol intake. In particular, social cues or interactions were not directly assessed despite their established influence on lapse frequency (9, 17, 41, 53). Second, the items used to assess physical states were constructed for the present study and were not subjected to a formal factor analysis. Although these items were modeled off of the PANAS, which has been previously used in EMA research, it is possible that the physical state questions functioned somewhat differently than the items assessing affective states. In addition, although EMA is more ecologically valid than retrospective self-reports, it is still subject to limitations of participant insight, awareness, and recall (15). Another limitation is the fact that reporting lapses is confounded with the participant’s understanding of what constitutes a lapse, stringency of the participant’s intended diet, and with the participant’s conscientiousness in reporting lapses. Further, the analyses implemented in the current study only examined whether a cue predicted lapse at the next survey, when in fact, a cue could increase likelihood of lapsing later than the next EMA time point. Moreover, we did not attempt to examine the interacting effect of cues (e.g., if boredom only increases the risk of lapsing when paired with readily available desirable food). Finally, the attrition from the EMA procedures was quite high. Although baseline lapse frequency did not predict missing data at mid- or end-of-treatment and a similar pattern of results was observed when running EMA analyses only with participants who provided EMA data at all three time points, it is possible that the pattern of results would differ if all individuals had completed all assessments.
Future Directions and Conclusions
Future research should attempt to widen the scope of data recorded. For example, recording the size of a lapse (e.g., caloric value, types of food eaten) would help understand the contribution of certain types of lapses to slowed weight loss or weight regain, and it would be beneficial to assess a wider range of potential triggers of lapses, including social situations. Assessing temptations (dietary and otherwise) in addition to lapse occurrences would add to our understanding of how individuals successfully resist temptations, and would allow for examination of how repeatedly exerting self-control throughout the day may contribute to later lapses as hypothesized by the limited resource model of self-control (41). In addition, use of other devices (e.g., watch) that could passively record other variables (e.g., GPS location, accelerometer, heart rate variability) would provide rich, non-self-report information about the predictors and correlates of dietary lapse. A more structured diet (e.g., meal replacements) could allow for a more exact lapse definition, and perhaps more accurate recording (although measurement of lapses in such a study would also be less generalizable to gold-standard interventions where flexibility with content of meals is central). Additionally, it would be beneficial to examine whether greater program adherence was associated with greater reductions in lapse frequency across the assessments, as well as other mediators of change in lapse frequency. Such analyses will help to identify what key components of interventions drive positive changes in behavior and facilitate the development of more effective interventions. Lastly, future research should examine whether just-in-time interventions increase awareness of cues, and thus prevent dietary lapses from occurring.
In sum, the current study provides the first information about EMA-measured dietary lapses and lapse cues throughout a behavioral weight loss intervention. Results point to the relevance of early dietary lapses to ultimate weight loss, and the ability of momentary physical states to prospectively predict dietary lapses. These results provide ideas for intervention development to enhance outcomes from behavioral weight loss treatment.
Acknowledgments
This project was wholly funded by an R01 grant (R01DK095069) to the first author from the National Institute of Diabetes and Digestive and Kidney Diseases. We also want to thank the participants of this study as well at its research coordinator, Andrew Frohn.
Footnotes
Conflict of Interest: Dr. Forman reports grants from the National Institute for Diabetes and Digestive and Kidney Diseases (award # R01 DK095069), during the conduct of the study. Dr. Crosby reports personal fees from Health Outcome Solutions, outside the submitted work. None of the remaining authors report a conflict of interest.
References
- 1.Yang L, Colditz GA. Prevalence of Overweight and Obesity in the United States, 2007–2012. JAMA Internal Medicine. 2015;175:1412–1413. doi: 10.1001/jamainternmed.2015.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaemsiri S, Slining MM, Agarwal SK. Perceived weight status, overweight diagnosis, and weight control among US adults: the NHANES 2003–2008 Study. International Journal of Obesity. 2011;35:1063–1070. doi: 10.1038/ijo.2010.229. [DOI] [PubMed] [Google Scholar]
- 3.Weinsier RL, Nagy TR, Hunter GR, et al. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. The American journal of clinical nutrition. 2000;72:1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 4.Lowe MR. Self-Regulation of Energy Intake in the Prevention and Treatment of Obesity: Is It Feasible? Obesity research. 2003;11:44S–59S. doi: 10.1038/oby.2003.223. [DOI] [PubMed] [Google Scholar]
- 5.Brownell KD, Jeffery RW. Improving long-term weight loss: pushing the limits of treatment. Behavior Therapy. 1987;18:353–374. [Google Scholar]
- 6.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. The American journal of clinical nutrition. 1997;66:239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 7.Wilson GT. Behavioral treatment of obesity: Thirty years and counting. Advances in Behaviour Research and Therapy. 1994;16:31–75. [Google Scholar]
- 8.Carels RA, Douglass OM, Cacciapaglia HM, O’Brien WH. An ecological momentary assessment of relapse crises in dieting. Journal of consulting and clinical psychology. 2004;72:341. doi: 10.1037/0022-006X.72.2.341. [DOI] [PubMed] [Google Scholar]
- 9.Carels RA, Hoffman J, Collins A, et al. Ecological momentary assessment of temptation and lapse in dieting. Eating Behaviors. 2002;2:307–321. doi: 10.1016/s1471-0153(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 10.Stroebe W, Mensink W, Aarts H, Schut H, Kruglanski AW. Why dieters fail: Testing the goal conflict model of eating. Journal of Experimental Social Psychology. 2008;44:26–36. [Google Scholar]
- 11.Forman EM, Butryn ML. A new look at the science of weight control: how acceptance and commitment strategies can address the challenge of self-regulation. Appetite. 2015;84:171–180. doi: 10.1016/j.appet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. Journal of consulting and clinical psychology. 1989;57:488. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- 13.Wing RR. Changing diet and exercise behaviors in individuals at risk for weight gain. Obesity research. 1995;3:277s–282s. doi: 10.1002/j.1550-8528.1995.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz N, Sudman S, Schuman H, et al. Context Effects in Social and Psychological Research. 1992. [Google Scholar]
- 15.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual review of clinical psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 16.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavorial medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- 17.McKee HC, Ntoumanis N, Taylor IM. An ecological momentary assessment of lapse occurrences in dieters. Annals of Behavioral Medicine. 2014;48:300–310. doi: 10.1007/s12160-014-9594-y. [DOI] [PubMed] [Google Scholar]
- 18.Tomiyama AJ, Mann T, Comer L. Triggers of eating in everyday life. Appetite. 2009;52:72–82. doi: 10.1016/j.appet.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiffman S, Stone AA. Ecological momentary assessment: A new tool for behavioral medicine research. Technology and methods in behavioral medicine. 1998:117–131. [Google Scholar]
- 20.An R. Weekend-weekday differences in diet among US adults, 2003–2012. Annals of epidemiology. 2016;26:57–65. doi: 10.1016/j.annepidem.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, Butryn ML, Sarwer DB, et al. Comparison of Psychosocial Status in Treatment-Seeking Women with Class III vs. Class I–II Obesity. Obesity. 2006;14:90S–98S. doi: 10.1038/oby.2006.288. [DOI] [PubMed] [Google Scholar]
- 22.Carels RA, Cacciapaglia HM, Douglass OM, Rydin S, O’Brien WH. The early identification of poor treatment outcome in a women’s weight loss program. Eating Behaviors. 2003;4:265–282. doi: 10.1016/S1471-0153(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 23.Stotland S, Larocque M. Early treatment response as a predictor of ongoing weight loss in obesity treatment. British journal of health psychology. 2005;10:601–614. doi: 10.1348/135910705X43750. [DOI] [PubMed] [Google Scholar]
- 24.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? International journal of behavioral medicine. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe M, Annunziato R, Riddell L, et al. Reduced energy density eating and weight loss maintenance: 18-month follow-lip results from a randomized controlled trial. Obesity research. 2003 [Google Scholar]
- 26.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Drapkin RG, Wing RR, Shiffman S. Responses to hypothetical high risk situations: do they predict weight loss in a behavioral treatment program or the context of dietary lapses? Health Psychology. 1995;14:427. doi: 10.1037//0278-6133.14.5.427. [DOI] [PubMed] [Google Scholar]
- 28.Forman EM, Butryn ML, Manasse SM, et al. Acceptance-Based versus Standard Behavioral Treatment for Obesity: Results from the Mind Your Health Randomized Controlled Trial. Obesity. 2016;24:2050–2056. doi: 10.1002/oby.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth JM, Wonderlich SA, Heron KE, et al. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of consulting and clinical psychology. 2007;75:629. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JG, Doshi S, Crosby RD, Lowe MR. Ecological Momentary Assessment of Obesogenic Eating Behavior: Combining Person-Specific and Environmental Predictors. Obesity. 2011;19:1574–1579. doi: 10.1038/oby.2010.335. [DOI] [PubMed] [Google Scholar]
- 31.Watson D, Clark LA, Tellegen A. Development of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 32.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obesity research. 2004;12:151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 33.Byrne SM, Cooper Z, Fairburn CG. Psychological predictors of weight regain in obesity. Behaviour research and therapy. 2004;42:1341–1356. doi: 10.1016/j.brat.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Forman EM, Butryn ML, Hoffman KL, Herbert JD. An open trial of an acceptance-based behavioral intervention for weight loss. Cognitive and Behavioral Practice. 2009;16:223–235. [Google Scholar]
- 35.Webber KH, Tate DF, Ward DS, Bowling JM. Motivation and its relationship to adherence to self-monitoring and weight loss in a 16-week Internet behavioral weight loss intervention. Journal of nutrition education and behavior. 2010;42:161–167. doi: 10.1016/j.jneb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. Handbook of obesity treatment. 2002;1:357–379. [Google Scholar]
- 37.Wing RR. Behavioral weight control. Handbook of obesity treatment. 2002;2:301–317. [Google Scholar]
- 38.West DS, Gorin AA, Subak LL, et al. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. International Journal of Obesity. 2011;35:259–269. doi: 10.1038/ijo.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira PJ, Silva MN, Mata J, Palmeira AL, Markland D. Motivation, self-determination, and long-term weight control. International Journal of Behavioral Nutrition and Physical Activity. 2012;9:22. doi: 10.1186/1479-5868-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Racette SB, Weiss EP, Schechtman KB, et al. Influence of weekend lifestyle patterns on body weight. Obesity. 2008;16:1826–1830. doi: 10.1038/oby.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann W, Vohs KD, Baumeister RF. What people desire, feel conflicted about, and try to resist in everyday life. Psychological Science. 2012;23:582–588. doi: 10.1177/0956797612437426. [DOI] [PubMed] [Google Scholar]
- 42.Carels RA, Young KM, Koball A, et al. Transforming your life: An environmental modification approach to weight loss. Journal of health psychology. 2011;16:430–438. doi: 10.1177/1359105310380986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorin AA, Raynor HA, Fava J, et al. Randomized controlled trial of a comprehensive home environment-focused weight-loss program for adults. Health Psychology. 2013;32:128. doi: 10.1037/a0026959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redman L, Heilbronn L, Martin C, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PloS one. 2008;4:e4377–e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friese M, Hofmann W, Wänke M. When impulses take over: Moderated predictive validity of explicit and implicit attitude measures in predicting food choice and consumption behaviour. British Journal of Social Psychology. 2008;47:397–419. doi: 10.1348/014466607X241540. [DOI] [PubMed] [Google Scholar]
- 46.Houben K, Roefs A, Jansen A. Guilty pleasures. Implicit preferences for high calorie food in restrained eating. Appetite. 2010;55:18–24. doi: 10.1016/j.appet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Richetin J, Perugini M, Adjali I, Hurling R. The moderator role of intuitive versus deliberative decision making for the predictive validity of implicit and explicit measures. European Journal of Personality. 2007;21:529–546. [Google Scholar]
- 48.Nahum-Shani I, Hekler EB, Spruijt-Metz D. Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychology. 2015;34:1209. doi: 10.1037/hea0000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. British journal of health psychology. 2010;15:1–39. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eating Behaviors. 2003;3:341–347. doi: 10.1016/s1471-0153(02)00100-9. [DOI] [PubMed] [Google Scholar]
- 51.Faith M, Allison D, Geliebter A, Dalton S. Emotional eating and obesity: theoretical considerations and practical recommendations. Overweight and weight management: the health professional’s guide to understanding and practice. 1997:439–465. [Google Scholar]
- 52.Lowe MR, Fisher EB., Jr Emotional reactivity, emotional eating, and obesity: A naturalistic study. Journal of behavioral medicine. 1983;6:135–149. doi: 10.1007/BF00845377. [DOI] [PubMed] [Google Scholar]
- 53.Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers*. Annu Rev Nutr. 2004;24:455–479. doi: 10.1146/annurev.nutr.24.012003.132140. [DOI] [PubMed] [Google Scholar]