TO THE EDITOR
It has been appreciated that a substantial proportion of patients that are thought to have Autoimmune Lymphoproliferative Syndrome (ALPS) do not formally meet ALPS criteria1. Recognizing the arbitrary nature of classification and diagnostic criteria for ALPS, some of these patients have been diagnosed with “probable” ALPS or with “ALPS-like” disease, depending on which diagnostic criteria are fulfilled. This latter group of disorders includes patients with defects in caspase 8, KRAS, and NRAS, as well as a group of patients with unknown molecular defects1.
The use of next-generation sequencing methodology, including exome sequencing, has uncovered new genetic defects in patients previously considered to have ALPS-like disease. Among those are patients with autosomal dominant mutations in the gene encoding Cytotoxic T-lymphocyte Associated protein 4 (CTLA-4) (CTLA4, OMIM# 616100)2.
CTLA-4 is an inhibitory receptor expressed by activated T cells and FOXP3+ regulatory T lymphocytes (Tregs), which inhibits proliferation of activated T cells upon binding to CTLA-4 ligands3. CTLA-4 haploinsufficiency results in an immune dysregulation syndrome characterized by lymphoproliferation, characteristic lymphocytic infiltration in non-lymphoid organs, autoimmune cytopenias, hypogammaglobulinemia, and recurrent infections2,4.
Murine studies demonstrated a regulatory role of FOXP3+CXCR5+BCL6+ follicular regulatory T cells (TFR) in the germinal center response5. Further studies revealed that deletion of CTLA-4 causes increased numbers of follicular helper T cells (TFH) and TFR cells6. Upon antigenic stimulation, loss of CTLA-4 in TFH cells result in increased antigen-specific B-cell responses, whereas loss of CTLA-4 in TFR cells resulted in defective suppression of antigen-specific and auto antigen-specific antibody responses6.
A recent study in human monogenic primary immune disorders (PID) investigated the signaling pathways and cellular interactions required for the development and function of TFH cells, and demonstrated the impact of PID-associated mutations on the quantity and quality of TFH cells7. While this study highlighted the importance of assessing TFH cells in PIDs, CTLA-4 haploinsufficiency was not one of the PIDs studied.
Here, we describe a patient with autoimmune lymphoproliferative disorder who was found to have CTLA-4 haploinsufficiency. We report increased CXCR5+PD1+TFH and decreased CXCR5+PD1+FOXP3+CD4+TFR cells in peripheral blood, impaired Treg suppression, and characteristic histopathology.
The patient is a 15-year-old female with a history of recurrent stomatitis, chronic waxing-waning lymphadenopathy, and multi-lineage autoimmune cytopenias, who was referred for suspected ALPS. Informed consent was obtained from the patient and family. Bone marrow studies revealed normal trilineage hematopoiesis and a normal female karyotype. Viral serology remained negative. She had received IVIG twice for thrombocytopenia, including 6 months prior to referral, but no immunosuppressive therapy. Although she did not meet ALPS criteria and had no defect in in vitro apoptosis, she was found to have borderline elevated peripheral blood DNTs and elevated serum IL-18 levels (supplementary file Table-E1 summarizes the immune profile). Next-generation sequencing of FAS and related genes, including FADD, FASL, CASP8, CASP10, NRAS, KRAS, ITK, and MAGT1, was negative. Based on these results, CTLA-4 haploinsufficiency was considered. CTLA4 sequencing revealed a previously published pathogenic variant; c.223C>T (p.R75W)2. Sequencing revealed the same mutation in her asymptomatic mother.
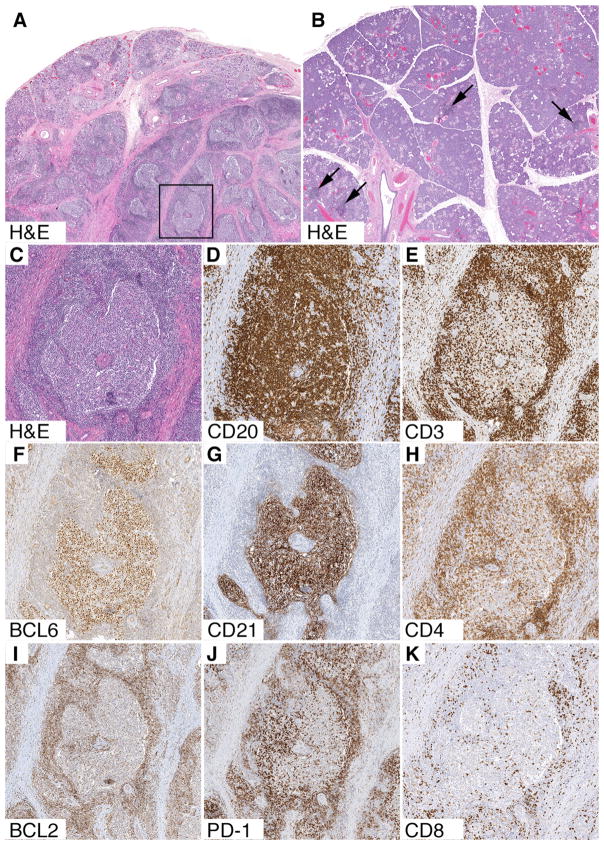
At the age of 13, the patient underwent an excisional biopsy of a – presumed - submandibular lymph node at another institution. The histopathology revealed a submandibular salivary gland with destruction of nearly all of the acini, preservation of residual salivary gland ducts, thick bands of collagen fibrosis, and abundant ectopic (inducible) mucosa-associated lymphoid tissue (MALT) (Fig 1). The ectopic MALT contained expanded secondary lymphoid follicles with fully developed architecture, which contained germinal centers with a mixture of follicular center cells, follicular dendritic cells, and PD1-positive follicular helper/regulatory T cells (TFH and TFR cannot be distinguished by routine immunohistochemistry). The borders between mantle zones and germinal centers were distinct, and intermingling of mantle cells and follicular center cells, characteristic of progressive transformation of germinal centers (PTGC), was not present. There was no obvious increase in double negative (CD4-CD8-) T cells in paracortical zones, and no evidence of MALT lymphoma or follicular lymphoma.
FIG 1. Histopathology of submandibular gland.
A, Low power view (H&E, 11x) shows thick pink bands of collagen fibrosis, destruction of acini, and ectopic (inducible) MALT with secondary lymphoid follicles containing expanded germinal centers. A single secondary lymphoid follicle is highlighted by the box. B, Low power view (H&E, 11x) of a normal gland from a control patient shows an absence of fibrosis and preserved acini. Arrows point to a few subtle tiny lymphoid aggregates without germinal centers, which are not considered to be ectopic MALT. C, High power view (H&E, 44x) of the secondary lymphoid follicle, highlighted by the box. The center contains an expanded, pale-staining germinal center, which is surrounded by a dark blue outer mantle zone. D-K, Immunohistochemical stains (44x) confirm that the secondary lymphoid follicle has fully developed architecture. Abundant CD20+ B cells are present in both the germinal center and mantle zone, whereas relatively few CD3+ T cells are present in the germinal center. CD4+ T cells outnumber CD8+ T cells and there is no obvious increase in double negative T cells. The germinal center contains a mixture of BCL6+ follicular center cells, CD21+ follicular dendritic cells, and PD1+ follicular helper/regulatory T cells. BCL2 stains the B cells in the mantle zone and all of the T cells. It does not stain the BCL6+ follicular dendritic cells.
In vitro studies of peripheral blood TFH, TFR and Treg lymphocytes were performed as published previously8, and revealed a normal % of Tregs with decreased CD25 and CTLA4 expression in both patient and mother (Supplementary Fig. E1A). Further studies demonstrated increased TFH cells with concomitantly decreased TFR cells in peripheral blood (Supplementary Fig. E1B), and defective Treg suppression in the patient, compared to healthy controls. Despite the same CTLA4 mutation, these studies were normal in the mother (Supplementary Fig. E1C). Additionally, patient’s circulating TFH cells expressed more IL-21, but not IL-2, than healthy controls, suggesting a more activated status of TFH cells in the patient (Supplementary Fig. E1B).
This case highlights the need to screen for CTLA-4 haploinsufficiency in patients with autoimmune lymphoproliferative disorders, resembling ALPS, who not meet ALPS criteria. It also emphasizes two important features: distinct histopathology and a distinct Treg, TFH and TFR profile in CTLA-4 haploinsufficiency.
With regard to the former, our finding of ectopic (inducible) MALT in a non-lymphoid organ, with extensive collage fibrosis, as well as an absence of PTGCs and an absence of an overtly expanded double negative (CD4-CD8-) T-cell population, raise the possibility that patients with CTLA-4 haploinsufficiency have a histopathology that is distinctive from patients with typical ALPS9. Consistent with this conclusion, a lung biopsy from another patient with the same R75W mutation in CTLA4 revealed similar findings, including “lymphoid fibrotic lesions”, ectopic MALT with secondary lymphoid follicles with germinal centers, and a predominance of CD4-positive T cells in between the follicles2.
With regard to the latter, our results showing a profile of increased TFH cells with low frequency of TFR cells, linked to impaired Treg suppression, might serve as a biomarker profile for disease (prediction) in individuals with CTLA4 mutations; relevant given the published variable penetrance of disease in families, and perhaps pointing towards the existence of yet unknown modifying genes2,4. Lastly, our results invite further analysis of TFH, TFR and Treg populations in the broader context of classification of ALPS and related diseases.
In conclusion, our detailed clinical, immunological and histopathological characterization adds to a growing body of data indicating an important role for CTLA-4 in the regulation of the germinal center response. This is likely relevant for immunologic features in these patients, such as autoimmunity and an increased risk of lymphoma, and might facilitate a better demarcation of CTLA-4 haploinsufficiency from other autoimmune lymphoproliferative disorders, and – ultimately – a more accurate and pathogenesis-driven classification of these rare disorders.
Methods
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue was cut to 4 micron thickness and adhered to charged slides. All subsequent steps, including deparaffinizing, antigen retrieval, application of antibodies, antibody detection (DAB chromogen), and counterstaining were done on an automated system according to manufacturer recommendations (Ventana Benchmark Ultra, Ventana Medical Systems, Tucson, AZ). All antibodies were prediluted by the manufacturer to recommended titers. The following antibodies were used. BCL2: 124; mouse monoclonal (Ventana Medical Systems, Tucson, AZ); BCL6: GI191E/A8; mouse monoclonal (Cell Marque, Rocklin, CA); CD3: 2GV6; rabbit monoclonal (Ventana Medical Systems, Tucson, AZ); CD4: SP35; rabbit monoclonal (Ventana Medical Systems, Tucson, AZ); CD8: SP57; rabbit monoclonal (Ventana Medical Systems, Tucson, AZ); CD20: L26; mouse monoclonal (Ventana Medical System, Tucson, AZ); CD21: 2G9; mouse monoclonal (Cell Marque, Rocklin, CA); PD-1; NAT105; mouse monoclonal (Cell Marque, Rocklin, CA).
Acquisition of histopathology images
Glass slides were converted to high-resolution digital slides using an Aperio AT2 slide scanner (Aperio Digital Pathology, Leica Biosystems Inc., Buffalo Grove, IL). Images from selected areas on the digital slides were captured as files in TIFF format using Aperio Image Scope software. Adobe Photoshop software (Adobe Systems Inc., San Jose, CA) was used to crop images to uniform size and make minor white balance adjustments in order to optimize image quality. Magnifications were computed by taking the width of each image as it will appear in the printed journal, dividing it by the physical width of the corresponding region on the glass slide, and rounding to the nearest whole number (e.g., for Figure 1A, the image width in the printed journal will be 85.725 mm and the physical width of the region on the glass slide is 7.5 mm; 85.725/7.5 = 11.4x; this was rounded to 11x).
Supplementary Material
Acknowledgments
The in vitro studies reported here has been supported by the National Institutes of Health, (R01AI085090, T. Chatila, MD MSc).
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116(14):e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–6. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–7. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–39. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma CS, Wong N, Rao G, Avery DT, Torpy J, Hambridge T, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993–1006. e1. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135(1):217–27. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153(5):1541–50. doi: 10.1016/S0002-9440(10)65742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.