Abstract
Time to complete two tests of manual dexterity, the 9-hole Peg Test and Grooved Pegboard Test, increases with advancing age. However, the adaptations responsible for the differences in pegboard times between middle-aged and older adults are largely unknown. Potential mechanisms include neuromuscular characteristics, cognitive function, and cutaneous sensation. To provide a tractable framework to address these gaps in knowledge, the purpose of the current study was to identify the latent variables underlying age-associated differences in time to complete the 9-hole and grooved pegboard tests. The approach involved an independent component analysis that identified associations between the two pegboard times for the two groups of participants with two to six secondary outcomes. The common association across three of the four conditions (two groups and two pegboard tests) was features derived from force-matching tasks requiring submaximal isometric contraction. In addition, there were significant associations for older adults between age, measures of cognitive function, and pegboard times. Nonetheless, the significant associations were unique for each age group and pegboard test. The results provide a framework for subsequent mechanistic studies to identify the adaptations underlying age-associated declines in manual dexterity.
Keywords: Pegboard tests, Middle-aged, Older adults, Manual dexterity, Latent variables, Independent component analysis
1. INTRODUCTION
Neurological health and function across the lifespan (3–85 years) can be characterized with the NIH Toolbox, which comprises tests to quantify an individual’s status in four domains: cognition, emotion, motor, and sensory (Gershon et al., 2013). The motor domain includes tests to assess performance in dexterity, balance, endurance, locomotion, and strength (Reuben et al., 2013). Manual dexterity, a measure of the ability to manipulate an object through skillful coordination of the hands and fingers, is often quantified by time to complete tests such as the Rolyan 9-hole Peg Test and the Lafayette Grooved Pegboard Test (Wang et al., 2011). Such tests combine elements of mental acuity, tactile sensibility, muscle strength, force control, and coordination, among others (Carey et al. 1997; Ashendorf et al. 2009; Marmon et al. 2011a, 2011b; Lawrence et al. 2015; Almuklass et al. 2016). The grooved pegboard test requires greater tactile acuity and imposes a greater cognitive demand than the 9-hole test (Ashendorf et al., 2009; Bowden and McNulty, 2013; Thompson-Butel et al., 2014; Wang et al., 2011). Manual dexterity is required for numerous activities of daily living, such as grooming, writing, and cooking, and is usually compromised with advancing age (Carey et al., 2008, 2002; Ostwald et al., 1989; Williams et al., 1982).
Neuromuscular characteristics have previously been shown to be strong predictors of pegboard performance (Marmon et al. 2011a, 2011b; Bowden & McNulty, 2013; Almuklass et al. 2016). In a study of 75 adults (18–89 yrs), for example, Marmon et al. (2011) found that a significant proportion of the variance (R2 = 0.36) in the time to complete the grooved pegboard test could be explained by two predictor variables: force steadiness during index finger abduction (partial r = 0.57) and handgrip strength (partial r = −0.34). When these data were examined for each age group (young, middle-aged, and older adults), however, a statistically significant regression model (R2 = 0.59) was only identified for older adults (75 ± 6 yrs, n = 25) and the predictor variables were age (partial r = 0.73), force steadiness during index finger abduction (partial r = 0.43), and pinch grip strength (partial r = 0.28). These findings indicate that much, but not all, of the variance in the time for older adults to complete the grooved pegboard test could be explained by measures related to force control and muscle strength, but these outcome variables were unable to explain the variance in the times observed for middle-aged adults (51 ± 6 yrs).
As an extension of this work, Almuklass et al. (2016) developed an expanded set of force steadiness tasks that was able to explain the variance in the time it took young adults (24.2 ± 4.1 yrs; n = 30) to complete the grooved pegboard test. The predictor variables (R2 = 0.70) were force steadiness during wrist extension (partial r = −0.475) and time to match the pinch grip target force as quickly as possible during concurrent wrist extension (partial r = 0.780). These results indicated that young adults with longer pegboard times were more accurate when matching a submaximal isometric contraction target force, but took longer to reach the target force.
The current study used a similar approach to identify age- and test-specific descriptors of pegboard times for middle-aged and older adults, but in addition examined the contribution of measures that assessed tactile sensation (Cole et al. 1998; Bowden & McNulty, 2013; Dunn et al. 2013) and cognitive function (Ashendorf et al. 2009; Guillery et al. 2013). The purpose of the study was to identify the latent variable descriptors underlying age-associated differences in time to complete the 9-hole and grooved pegboard tests. The hypothesis was that the descriptor variables would differ for middle-aged and older adults on both pegboard tests. The outcomes were expected to identify the neuromuscular properties, tactile sensibility, and cognitive characteristics that contribute to age-specific differences in performance on the two tests of manual dexterity.
2. MATERIALS AND METHODS
Twenty-five middle-aged (51.3 ± 6.8 yrs; 14 women) and 28 older adults (73.8 ± 6.9 yrs; 12 women) met inclusion criteria and provided informed consent to participate in the study. All participants were right-handed (97 ± 7; range: 75–100) according to the Edinburgh Handedness Inventory Short Form (Veale, 2014), free from neurological disease, had no reported orthopedic problems that could influence upper limb function, and were not taking any medications known to influence neuromuscular or cognitive function. The Institutional Review Board at the University of Colorado Boulder approved the protocol (Protocol # 14-0631).
All subjects participated in two experimental sessions that lasted ≤2 hours. Functional capabilities were assessed in one session using NIH Toolbox measures of manual dexterity (Wang et al., 2011), muscle strength (Reuben et al. 2013), cognition (Weintraub et al., 2013), and tactile discrimination (Carey et al., 1997). In the other session, force steadiness was measured during single-(Marmon et al., 2011b) and double-action (Almuklass et al., 2016) tasks that involved submaximal isometric contractions. The primary outcomes were the times to complete the two tests of manual dexterity with the right hand: 9-hole and grooved pegboard tests. The secondary outcomes were used to identify the latent variables associated with the times for the two groups of participants to complete the two pegboard tests.
2.1 Functional Assessments
The 9-hole pegboard test requires participants to place 9 cylindrical pegs into 9 holes arranged in a 3-by-3 grid as quickly as possible, and then to remove all pegs one at a time. Each subject performed the 9-hole test four times, twice with each hand: a familiarization trial followed by a timed trial. Normative data for time to complete the 9-hole pegboard test (mean ± SD) are 17.5 ± 2.8 s for middle-aged adults (46–65 yrs), 17.8 ± 2.7 s for older adults (66–75 yrs), and 21.0 ± 2.8 s for oldest adults (76–85 yrs) (Wang et al., 2011). The 25-hole grooved pegboard test requires participants to place keyhole-shaped pegs into 25 holes on a board as quickly as possible. The holes resemble keyholes that are arranged in a 5-by-5 matrix, with varied keyhole orientation across the board. The pegs are inserted one row at a time, with the peg insertion order determined by the tested hand. Right-hand peg placement is performed left to right from top to bottom, whereas left-hand peg placement is performed right to left but also from top to bottom. Subjects practiced the grooved pegboard test by completing the top row once and then the time to complete the test for each hand was recorded. Normative data for the time to complete the grooved pegboard test (mean ± SD) are 69.0 ± 18.0 s for middle-aged adults (46–65 yrs), 68.6 ± 17.9 s for older adults (66–75 yrs), and 86.7 ± 16.4 s for oldest adults (76–85 yrs) (Wang et al., 2011). Test order was counterbalanced across participants with dominant hand (right) trials performed first and the right-hand times were used for latent variable analysis.
Maximal hand grip strength was measured with a hand dynamometer (Hydraulic Hand Dynamometer, Baseline Evaluation Instruments, Irving, TX) (Reuben et al. 2013). Three maximal voluntary contractions (MVC) were performed for each hand, starting with the right hand and alternating trials between right and left hands. Subjects were instructed to increase force over 3 s to reach a maximal value. Strong verbal encouragement was provided during each MVC trial with at least 2 min of rest between trials. The maximal value recorded for each hand was used as MVC force.
The Tactile Discrimination Test characterized the ability to discriminate surface texture (Carey et al., 2002, 1997). The test comprises a forced-choice protocol that requires participants to identify the non-matching texture from a set of three pads with their right index finger; two pads have an identical texture and one differs. Participants were presented a prescribed series of pads in varying order of difficulty and a cumulative score was calculated for the right hand only (Carey et al. 1997).
Five tests from the Cognition Battery of the NIH Toolbox were used to assess executive function, attention, episodic memory, processing speed, and working memory (Weintraub et al., 2013):
Executive function was assessed with the Flanker Inhibitory Control and Attention Test and the Dimensional Change Card Sort Test. The Flanker test required participants to focus on a central directional arrow with 4 flanking arrows as distractions. The subject was asked to choose the correct direction of the central arrow. Scoring is based on a combination of accuracy and reaction time to measure participant attention and inhibitory control.
Attention denotes the capacity to deal with environmental information and was also tested with the Flanker test. The Dimensional test required participants to match images by either color or shape with a target image.
Episodic memory was measured with the Picture Sequence Memory Test by requiring participants to recall objects and activities in a storyboard order for a series of 18 pictures.
Processing speed was measured with the Pattern Comparison Processing Speed Test, which required participants to discern whether two side-by-side images are identical or not.
Working memory was measured with the List Sorting Working Memory Test, which required participants to recall and sequence different visual and orally presented stimuli in order of size from smallest to largest. The recalled sequence progressed from a single dimension (either animals or foods) to a double dimension (foods, then animals).
All cognitive tests were performed in accordance with NIH Toolbox specifications and instructions.
2.2 Force Steadiness
Force steadiness was measured as participants performed submaximal isometric contractions that comprised either single- and double-action force-matching tasks with the right hand and arm (Almuklass et al., 2016). The target forces were 5% and 10% MVC and each contraction was sustained for 30 s. The single-action tasks involved index finger abduction, thumb-index finger precision pinch, and wrist extension. The double-action tasks were achieved by combining index finger abduction or pinch (x axis) with wrist extension force (y axis). Force steadiness was quantified as the coefficient of variation for force in each direction and the net resultant force.
The subjects were seated with the right forearm resting on a metal stand, secured with polyester straps, and the hand pushing against force transducers. One transducer measured force during wrist extension (0.0057 V/N, JR3 Model 45E15A-U760-A, Woodland, CA) and another measured the pinch and index finger abduction forces (0.021 V/N, Futek LMD300 Pinch Load Cell, Irvine, CA). Participants completed MVCs for wrist extension, index finger abduction, and pinch in a counterbalanced order. Each task involved a gradual increase in force from rest to maximum in 3 s and then holding that maximal force for 2 s with vigorous encouragement. Subjects performed at least two MVCs for each action; if the difference in peak force between the two MVCs was >5%, subsequent MVC trials were performed until two were performed within 5%. No more than five MVC trials were performed for any task. At least 2 min of rest was provided between each MVC trial. The greatest peak MVC force was used to calculate the target forces for the force-matching tasks.
Each force-matching task was performed with visual feedback displayed on a computer monitor placed ~1.5 m in front of the seated participant (Almuklass et al., 2016). Screen dimensions were normalized from 0% to 20% MVC in both the x (pinch or index finger abduction force) and y (wrist extension force) directions for the tested actions. The feedback comprised a horizontal target line for wrist extension and a vertical target line for index finger abduction and index-thumb pinch. The resultant force was indicated by a single crosshair image. The visual angle during the acquisition of the target force was 9.3° for the 5% MVC target and 18.5° for the 10% MVC target, and was reduced to 0.69° and 0.86°, respectively, during the steady contraction.
Single-action tasks were performed at each target force (5 or 10% MVC force) with the isometric contractions (pinch, index finger abduction, or wrist extension) counterbalanced across participants. After one practice trial (~10 s), participants performed two trials in which the task was to exert a steady force for 30 s. At least 45 s of rest was provided between each trial. Participants were instructed to reach the target as quickly as possible upon hearing a ‘Go’ signal from one investigator. Double-action tasks were then performed in the same counterbalanced order and with the same instruction as the single-actions tasks.
Pinch and index finger abduction forces were amplified 200 times (V72-25A Resistive Bridge Strain Gage Coupler, Coulbourn Instruments, Whitehall, PA). Wrist extension forces were measured with the JR Universal Force-Moment Sensory System (JR3, Inc. Woodland, CA; UFS-800 control unit; 45E15A-UFS760 transducer) and processed with the Isolated Signal Interface/Processor (Coulbourn Instruments, Whitehall, PA; model V79-02). The force signals were low-pass filtered at 50 Hz (V75-48 High Performance Bandpass Filter, Coulbourn Instruments, Whitehall, PA) and sampled at 2000 Hz with an analog-to-digital converter (Power 1401, Cambridge Electronic Design, Cambridge, UK). All force data were obtained with Spike2 data acquisition software (Version 5.20, Cambridge Electronic Design, Cambridge, UK) and stored on a computer for offline analysis.
2.3 Data Analysis
Force trajectory during the single- and double-action tasks was quantified with custom MATLAB software (Mathworks, Natick, MA). The force trajectory was characterized by measuring initial force error, rise time, stabilization time, time to match, and coefficient of variation for force (Figure 1). Initial force error was identified by filtering the force trace using a 4 Hz low pass 4th order Butterworth filter and calculating the difference between the initial force plateau from the target force. Initial force plateau was defined as the first force sample where the difference between two consecutive force samples was zero. Rise time was calculated as the time from force onset to the initial force peak.
Figure 1.
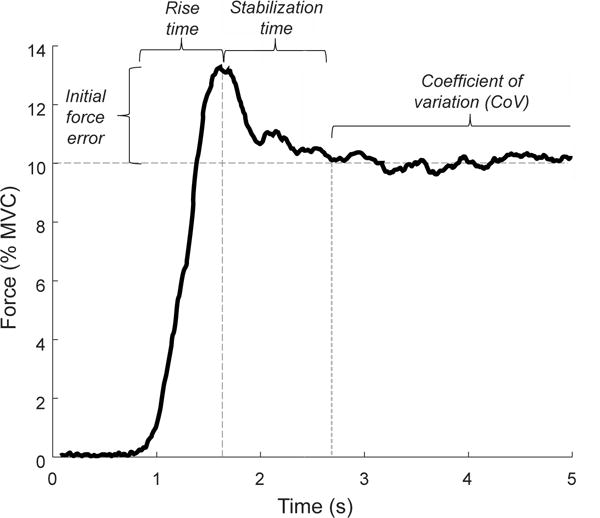
Force trajectory during a rapid isometric contraction to match a 10% MVC target force. Initial force error is the difference between the initial force peak and the target force. Rise time is the time from force onset to the initial force peak. Stabilization time is the time from the initial force peak to the beginning of the steadiness window. Coefficient of variation is the steadiest 10 s in the 30-s window.
All subsequent force trajectory characteristics were identified using a 20 Hz low-pass 4th order Butterworth filter. Stabilization time was defined as the time from the initial force plateau to the point where the force trace met the following criteria: a) the standard deviation of a moving 1-s sample was within ± 3 standard deviations of the last 5 s of the 30-s steadiness trial; b) the initial index of the 1-s sample was within a 0.03% window of the target force value. Time to match was then quantified as the sum of rise and stabilization time. The force-matching trial continued for 30 s from the index of time to match, from which the steadiest 10 s of the force-matching trial was used to calculate the coefficient of variation. The steadiest performance was quantified as the lowest standard deviation value of a moving 10-s window across the 30-s force-matching trial.
2.4 Statistical Analysis
The Shapiro-Wilk test was performed to test normality with Q-Q plots (theoretical vs. sample quantiles) and histograms used to verify distribution. The times for both pegboard tests were normally distributed for older adults and for the 9-hole test for middle-aged adults. There were two outliers (76.4 s and 84.5 s) in the grooved pegboard times for middle-aged adults, but these values fell within the range of times reported by Wang et al. (2011) and were retained in the analysis. Age-group comparisons were performed initially with a parametric t-test and verified with the non-parametric Mann-Whitney U test. Due to the absence of normal distributions, within-participant comparisons for force steadiness and force trajectory were performed with the Wilcoxon-Signed-Rank test. When more than two outcomes were compared, a Bonferroni adjusted alpha was used. Parametric effect size was quantified with Cohen’s d while non-parametric effect size was quantified with the Hodges-Lehmann estimator.
Due to the non-normal distribution of the force steadiness, force trajectory, cognitive, and tactile measures across participants, Spearman’s Rank-Order test was used to examine the correlation between right-hand times to complete the pegboard tests and the secondary outcome variables. Statistically significant correlations for each test and age group were entered into a principal component analysis (PCA) to identify the number of factors to be used in an independent component analyses (ICA). Eigenvalues greater than 1 indicated a significant factor, which corresponded to the number of factors used for the ICA model of each pegboard test and age group. Variables were entered into the model in order of decreasing correlation values, but were limited to one outcome for each task. The fastICA algorithm was then used to identify latent variables that characterized manual dexterity performance by age and pegboard test. All statistical procedures were performed using R (version 3.3.1) with the α set at 0.05.
3. RESULTS
The measures obtained from 53 participants comprised the times to complete the two tests of manual dexterity (9-hole and grooved pegboard tests), NIH Toolbox functional measures, force steadiness during isometric contractions, and force trajectory characteristics during rapid force-matching tasks. There were no significant differences in either height or body mass for the two groups of participants, and only 1 of the 5 measures of muscle strength exhibited statistically significant differences (P < 0.05) between groups (Table 1). Similarly, the lower scores for tactile discrimination achieved by the older adults were not statistically different from those for the middle-aged adults (P = 0.101, Cohen’s d = 5.5, Hodges-Lehmann estimate = 14.5). In contrast, the scores for three cognitive function tests were significantly less (P < 0.05) for the older adults than for the middle-aged adults (Table 1).
Table 1.
Anthropomorphic and functional measures
| Middle-aged | Older | |
|---|---|---|
| Age (yrs) | 51.3 ± 6.8 | 73.8 ± 6.9* |
| Height (m) | 1.74 ± 0.09 | 1.72 ± 0.10 |
| Body mass (kg) | 72 ± 12 | 74 ± 14 |
| 9-hole test (s) | 18.3 ± 2.8 | 21.1 ± 3.1* |
| Grooved pegboard test (s) | 60.0 ± 8.5 | 80.8 ± 18.1* |
| Grip strength, right (kg) | 42 ± 11 | 37 ± 11 |
| Grip strength, left (kg) | 41.5 ± 10.3 | 34.6 ± 9.2* |
| Wrist extension MVC (N) | 104 ± 42 | 95 ± 35 |
| Pinch MVC (N) | 36.7 ± 12.2 | 32.2 ± 8.9 |
| Index finger abduction MVC (N) | 25.4 ± 7.8 | 23.1 ± 10.1 |
| Tactile discrimination test (au) | 81 ± 19 | 72 ± 20 |
| Education (au) – mode (median) | 21 (21) | 21 (22) |
| Dimensional (au) | 29.6 ±0.6 | 28.9 ± 1.8 |
| Flanker inhibitory (au) | 20 ± 0 | 20 ± 0 |
| List sorting (au) | 18.4 ± 2.6 | 15.9 ± 3.0* |
| Pattern comparison (au) | 58 ± 12 | 43 ± 11* |
| Picture sequence (au) | 18.4 ± 8.6 | 11.5 ± 5.3* |
Values are mean ± SD, excluding Education which is represented as mode (median). MVC = maximal voluntary contraction
P < 0.05 relative to middle-aged adults.
3.1 Pegboard tests
Time to complete both the 9-hole test and the grooved pegboard test differed across age groups (Table 1). Relative to the middle-aged adults, the times to complete the 9-hole test were 13.3% longer for older adults (P < 0.05, Cohen’s d = 8.3, Hodges-Lehmann estimate = 2.8) and 25.7% longer for the grooved pegboard test (P < 0.05, Cohen’s d = 5.6, Hodges-Lehmann estimate = 18.6). The correlations between the times for the two pegboard tests were statistically significant for both age groups: Spearman’s rank correlation was 0.46 for middle-aged adults (P < 0.05) and the Pearson product correlation was 0.58 for older adults (P < 0.01). Pegboard times were generally faster for the right hand (dominant) in each age group, but the only statistically significant differences were for the grooved pegboard times of middle-aged adults (P < 0.001, 10.7% change, Hodges-Lehmann estimate = 5.3) and the 9-hole times for older adults (P < 0.05, 6.0% change, Cohen’s d = 0.4).
3.2 NIH Toolbox
Education levels did not differ between the two groups (P = 0.127, Cohen’s d = 4.4, Hodges-Lehmann estimate = 1.3e–5). In contrast, older adults had lower scores on the List sorting working memory test (P < 0.01, −15.7% change, Cohen’s d = 7.5, Hodges-Lehmann estimate = 2.0), Pattern comparison processing speed test (P < 0.001, −34.6% change, Cohen’s d = 5.2, Hodges-Lehmann estimate = 14.0), and the Picture sequence memory test (P < 0.01, −60% change, Cohen’s d = 2.56, Hodges-Lehmann estimate = 6.0). However, the differences in executive function were not statistically significant between groups as indicated by the scores on the Dimensional change card sort test (P = 0.111) and the Flanker inhibitory control and attention test (P = 1.0). Furthermore, the only statistically significant difference in muscle strength was a 20% lower value for left hand maximal grip strength for older adults (P < 0.05, Cohen’s d = 5.1, Hodges-Lehmann estimate = 6.0).
3.3 Force steadiness
Table 2 indicates the statistically significant differences in force steadiness (coefficient of variation for force) during the force-matching task between age groups, single- and double-action tasks, and target force. The older adults were less steady (greater values) than the middle-aged adults in 12 of the 14 comparisons with only wrist extension during double-action 10% pinch (P = 0.025; Bonferroni adjusted significance < 0.0167) and wrist extension during index finger abduction at 10% MVC not differing (P = 0.071) between the two groups. Force steadiness was worse (greater values) for 11 of the 16 double-action tasks relative to the single-action tasks (Table 2). Six of these statistically significant differences were observed for the middle-aged adults (three at each target force) and five for the older adults. The most consistent difference in force steadiness between the single- and double-action tasks was observed for the pinch grip; all values were greater when the pinch grip was performed with concurrent wrist extension (Table 2). Additionally, force steadiness was superior (lower values) at the greater target force (10% MVC; Table 2).
Table 2.
Coefficients of variation for force during single and double actions at the two target forces for middle-aged and older adults
| Middle-aged | Older | |||
|---|---|---|---|---|
| 5% | 10% | 5% | 10% | |
| Wrist extension | ||||
| Single action | 2.9 ± 1.3 | 1.8 ± 0.8 * | 3.3 ± 1.3 ‡ | 2.1 ± 0.8 *‡ |
| With pinch | 3.6 ± 2.3 † | 2.1 ± 1.1 *† | 4.3 ± 1.7 †‡ | 2.6 ± 1.0 *†‡ |
| With index abduction | 3.0 ± 1.6 | 2.6 ± 3.5 * | 3.6 ± 1.5 †‡ | 2.2 ± 0.8 *‡ |
| Pinch grip | ||||
| Single action | 3.5 ± 1.1 | 2.2 ± 0.6 * | 4.7 ± 2.3 ‡ | 2.8 ± 1.3 *‡ |
| With wrist extension | 4.5 ± 2.1 † | 3.1 ± 1.7 *† | 5.9 ± 2.8 †‡ | 3.7 ± 2.0 *† |
| Index finger abduction | ||||
| Single action | 4.9 ± 1.5 | 3.2 ± 1.1 * | 7.3 ± 3.1 ‡ | 4.8 ± 2.2 *‡ |
| With wrist extension | 6.1 ± 2.3 † | 4.6 ± 3.0 *† | 7.8 ± 3.2 ‡ | 5.4 ± 2.5 * |
Values (in %) are means ± SD.
P < 0.05 relative to 5% target force.
P < 0.05 relative to single action.
P < 0.05 relative to middle-aged adults.
3.4 ICA Models
The ICA model for the 9-hole pegboard times of the middle-aged adults comprised four factors (Figure 2): 9-hole pegboard time, force error during double-action wrist extension at 10% target force, time to match a 5% target force during index finger abduction, and education level. These descriptive variables were moderately correlated with the 9-hole pegboard times for the middle-aged adults; the Spearman’s rho correlation values were −0.50 (error double wrist 10%), 0.41 (match time index 5%), and −0.35 (education). The ICA analysis indicated that the first independent component (IC) explained 38% of the covariance and shows that the 9-hole times were negatively correlated (−0.84) with this first IC, whereas the second largest contribution to the first IC was force error during the double-action wrist extension to the 10% target force (0.45). This finding indicates that those middle-aged participants who overshot the target force had a faster time for the 9-hole test. The second IC explained an additional 30% of the covariance and identified force error during the double-action wrist extension at 10% target force as the strongest correlate (0.83). The third IC explained an additional 19% of the covariance with time to match the 5% target force during index finger abduction being the strongest correlate (0.85) and education being the second highest (0.43).
Figure 2. ICA model for middle-aged 9-hole pegboard test.
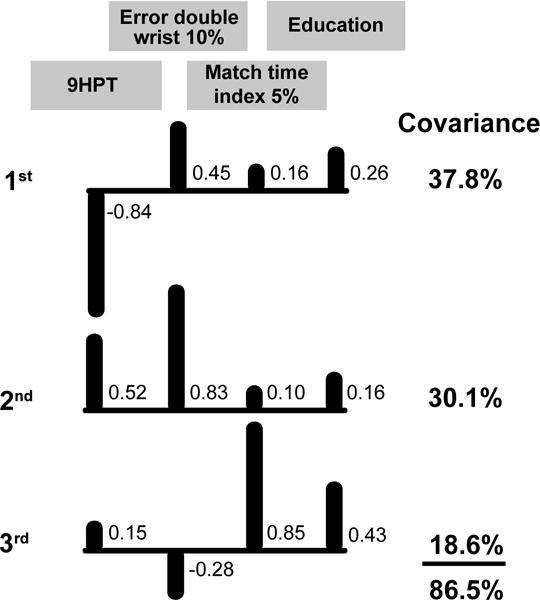
Correlations between the four outcome measures and the covariance they explained for the three significant independent components. Upward bars indicate positive correlations and downward bars denote negative correlations. 9HPT = time to complete the 9-hole pegboard test, Error double wrist 10% = force error of double-action wrist extension at 10% MVC, Match time index 5% = time to match a 5% index finger abduction force target, and Education = ordinal measure of education level from the NIH Toolbox.
The ICA model for the 9-hole pegboard times of older adults included six factors (Figure 3): 9-hole time, age (rho = 0.57), sex (rho = −0.50), rise time to the 5% target force for the pinch (rho = 0.43), picture sequence memory test score (rho = −0.42), and time to match the 10% target force during the double-action pinch/wrist extension task (rho = 0.41). The first IC explained 39% of the covariance with 9-hole pegboard times being the strongest correlate (0.73) and age the second strongest (−0.57). The second IC explained an additional 24% of the covariance with age being the strongest correlate (−0.68) and 9-hole times being the second strongest (−0.49). The third IC explained an additional 13% of the covariance with time to match the 10% target force during double-action pinch/wrist extension being the strongest correlate (0.75) and age the second strongest (0.39).
Figure 3. ICA model for older adult 9-hole pegboard test.
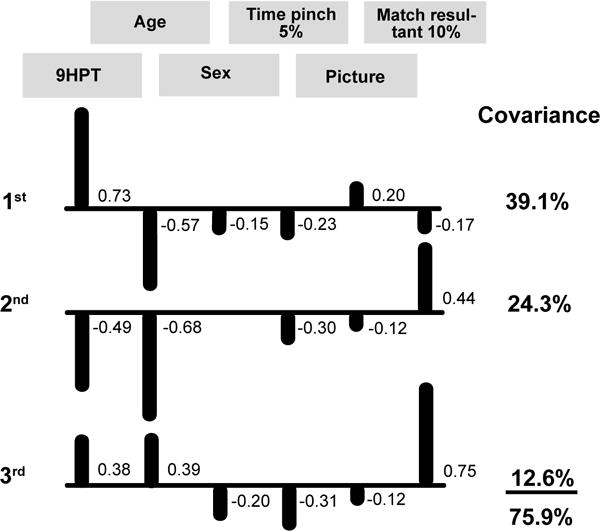
Correlations between the six outcome measures and the covariance they explained for the three significant independent components (ICs). Correlations <0.10 are not shown. Upward bars indicate positive correlations and downward bars denote negative correlations. 9HPT = time to complete the 9-hole pegboard test, Age = age in years at the time of assessment, Sex = binary measure of sex where 0 = women and 1 = men, Time pinch 5% = rise time to the 5% MVC pinch target, Picture = NIH Toolbox Picture sequence memory test score, and Match resultant 10% = time to match double-action pinch/wrist extension resultant 10% MVC target force.
The ICA model for the grooved pegboard test of middle-aged adults comprised 6 factors (Figure 4): grooved pegboard time, force steadiness for index finger abduction during the double-action task at 5% target force (rho = 0.58), index finger abduction strength (rho = −0.45), force steadiness for index finger abduction during the double-action task at 10% target force (rho = 0.41), force steadiness for index finger abduction at the 5% target force (rho = 0.39), and rise time to a 5% target force for the double-action wrist extension task (rho = −0.38). The first IC explained 29% of the covariance with grooved pegboard test times being the strongest correlate (0.61), force steadiness for index finger abduction during the double-action task at 10% target force (0.53) the second strongest, and force steadiness for index finger abduction during the double-action task at 5% target force (0.41) the third strongest. The second IC further described an additional 23% of the covariance with force steadiness of index finger abduction at 5% MVC (−0.57) being the strongest correlate, grooved pegboard times (−0.51) the second strongest, and force steadiness for index finger abduction during the double-action task at 5% target force (0.46) the third strongest. The third IC explained an additional 21% of the covariance with force steadiness of index finger abduction at 5% target force again being the strongest correlate (0.78), grooved pegboard times the third strongest (−0.57), and force steadiness for index finger abduction during the double-action task at 5% target force (0.15) the third strongest.
Figure 4. ICA model for middle-aged grooved pegboard test.
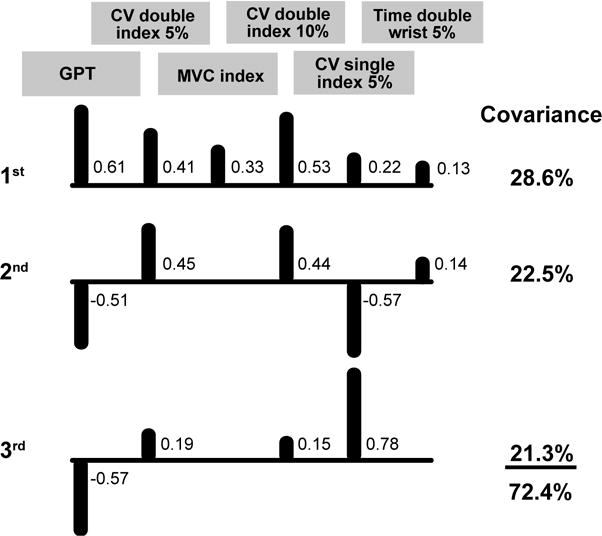
Correlations between the six outcome measures and the covariance they explained for the three significant independent components (ICs). Correlations <0.10 are not shown. Upward bars indicate positive correlations and downward bars denote negative correlations. GPT = time to complete the grooved pegboard test, CV double index 5% = force steadiness (coefficient of variation for force) during double-action index finger abduction at 5% MVC, MVC index = index finger abduction strength, CV double index 10% = force steadiness of double-action index finger abduction at 10% MVC, CV single index 5% = force steadiness of index finger abduction at 5% MVC, and Time double wrist 5% = rise time to a 5% double-action wrist extension target.
The ICA model for the grooved pegboard test of older adults comprised three factors (Figure 5): grooved pegboard time, age (rho = 0.76), and list sorting working memory scores (rho = −0.64). The first IC described 47% of the covariance with grooved pegboard times being the strongest correlate (0.70) and list sorting (working memory) score (0.66) being the second strongest. The second IC described an additional 45% of the covariance with the strongest correlate being the list sorting score (0.72) and the second strongest being the grooved pegboard test times (−0.69). The third IC explained an additional 7% of the covariance with age being the strongest correlate (0.96), the list sorting score being the second strongest (0.22), and grooved pegboard times being the third strongest (0.18).
Figure 5. ICA model for older adult grooved pegboard test.
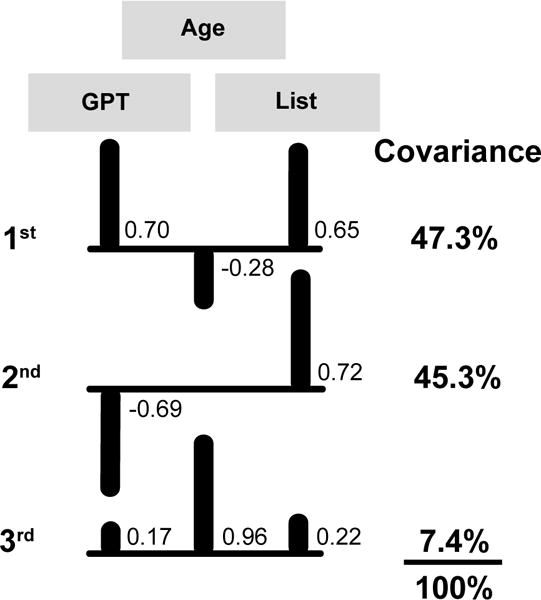
Correlations between the three outcome measures and the covariance they explained for the three significant independent components (ICs). Correlations <0.10 are not shown. Upward bars indicate positive correlations and downward bars denote negative correlations. GPT = time to complete the grooved pegboard test, Age = age in years at the time of assessment, and List = NIH Toolbox List sorting working memory test score.
4. DISCUSSION
The main findings of the current study were that the time to complete the two pegboard tests of manual dexterity was longer for older adults than for middle-aged adults and the outcome variables most strongly associated with the pegboard times differed for the two tests and the two age groups.
A previous study from our laboratory (Marmon et al. 2011) found that 59% of the variance in the time it took older adults to complete the grooved pegboard test could be explained by age, force steadiness during a submaximal isometric contraction with the index finger abductor muscles, and pinch grip strength. However, it was not possible to develop a regression model that could explain the variance in the grooved pegboard times for middle-aged adults. To provide a framework that can be used to address this gap in knowledge about age-associated differences in manual dexterity, the current study examined a broader survey of outcome variables and extended the approach to include the 9-hole pegboard test. The framework is based on a data-reduction technique that describes concisely the characteristics for each age cohort on the two pegboard tests. The approach comprised an independent component analysis, which performs blind-source separation to provide a lower dimensional approximation of the original dataset in terms of latent variables. To be conservative, only the first three latent variables are reported as they explain most of the covariance (72–100%) in the outcome measures (Lawrence et al., 2015).
4.1 9-Hole Pegboard Test
The cumulative covariance for the first three independent components of the 9-hole pegboard test as performed by the middle-aged adults accounted for 86.5% of the covariance between the outcome measures. The largest contributions to these latent variables were two timing variables: force error and time to match a target force. Force error indicates the ability to match a prescribed target force using feedforward motor planning. The force error observed during the double-action wrist extension task to a 10% target force was negatively correlated with 9-hole pegboard times, which indicated that those participants with a positive force error (exceeded the target force) had faster pegboard times. Consistent with this finding, Vieluf et al. (2013) reported that late middle-aged adults (55–65 years) have greater difficulty than young adults in rapidly producing a prescribed force with a precision pinch, despite the two groups having similar pinch strength.
The other descriptive covariate with the 9-hole pegboard time for middle-aged adults was the time to match a 5% target force with the index finger abductors, which is a measure of visuomotor function during a force-matching task (Almuklass et al., 2016). There was a positive correlation between the 9-hole pegboard times and time to match the target, indicating that those middle-aged adults who had faster times to match the submaximal target force had superior manual dexterity.
The cumulative covariance for the first three independent components of the 9-hole pegboard test as performed by older adults accounted for 75.9% of the covariance between the outcome measures. The largest contributors to the latent variables included age and feedback mechanisms. Age was positively correlated with 9-hole pegboard times, demonstrating a more general age-related decline in manual dexterity for older adults. The second largest descriptor was the time for the resultant force to match the 10% MVC target force during concurrent pinch and wrist extension. As with middle-aged adults, the positive correlation between time to match a target force and 9-hole pegboard times for older adults indicates that those participants who matched the target force more quickly had superior manual dexterity.
4.2 Grooved Pegboard Test
The cumulative covariance for the first three independent components of the grooved pegboard test as performed by middle-aged adults accounted for 72.4% of the covariance between the outcome measures. The largest contributors to the latent variables included measures of force steadiness, which provides an index of the effective neural drive to muscle (Negro et al., 2009). Force steadiness (coefficient of variation for force) during two force-matching tasks were correlated with grooved pegboard time: index finger abduction during concurrent wrist extension to 10% target force and index finger abduction to 5% target force. The two measures of force steadiness were positively correlated with middle-aged grooved pegboard times, indicating that those participants with greater force variability, hence greater variability in the common modulation of motor unit discharge rates (Farina and Negro, 2015), had slower times on the test of manual dexterity.
The cumulative covariance for the three independent components of the grooved pegboard test as performed by older adults accounted for 100% of the covariance between descriptive outcome measures. These descriptors included one NIH Toolbox measure of cognition and age. Scores on a test of working memory were negatively correlated with grooved pegboard times, indicating that the higher test scores were associated with faster pegboard times. The working memory test (List Sorting Working Memory Test) required participants to recall and sequence different visual and orally presented stimuli in order of size from smallest to largest (Gershon et al., 2013; Weintraub et al., 2013). When performing the grooved pegboard test, short-term memory is required to remember the peg orientation for insertion based on visual and cutaneous information about the peg held in the hand. Furthermore, age of the older adults was positively correlated with grooved pegboard times, similar to the results for the 9-hole pegboard test.
4.3 Age differences
Advancing age is a common descriptor and predictor of declines in manual dexterity (Reuben et al., 2013; Wang et al., 2015, 2011). Previous research suggests age-specific neural adjustment strategies (Christou et al., 2007), increased endpoint variability (Christou and Enoka, 2011), decreased force steadiness (Marmon et al., 2011a), and age-specific strength/dexterity interactions of late middle-aged and older adults (Martin et al., 2015) account for decreased manual dexterity with advancing age. In the current study, time to complete the two pegboard tests was longer for older adults than middle-aged adults and was associated with lower scores on three cognitive tests (Table 1), weaker left-hand grip strength (Table 1), and greater force variability during steady contractions (Table 2) for the older adults.
There were some similarities and differences in the outcome measures that contributed to the latent variables for the two pegboard tests performed by each group (Figures 2–5). The similarities for the 9-hole pegboard test were that each group included two measures derived from the force-matching tasks. The differences were that the middle-aged group included a measure of education, whereas the older group included age, sex, and the score on a cognitive test. Interestingly, middle-aged and older adults only differed in force-matching ability on two tasks: time to match a 5% target force during wrist extension and force error during a pinch to the 10% target. However, neither of these outcomes emerged as descriptors of manual dexterity.
Additionally, higher education levels have been found to be associated with better performance on tests of manual dexterity (Buchman et al., 2007; Ruff and Parker, 1993). The results of the current study extend this finding to include manual dexterity assessed by the 9-hole test for middle-aged adults. Although education was not found to be a descriptor for older adults, episodic memory—a memory construct that involves storage of unique events or experiences in a time-specific manner (Weintraub et al., 2013)—was negatively correlated with 9-hole pegboard times for older adults. Episodic memory is often the first measure of cognitive function to show an age-related decline and is susceptible to brain trauma and neurodegenerative disease (Albert, 1996; Weintraub et al., 2013).
In contrast to the 9-hole test, the outcome variables that contributed to the three latent variables for the grooved pegboard test differed for the two age groups. The correlated measures for the middle-aged adults comprised three characteristics derived from the force-steadiness tasks, one measure of muscle strength, and one force trajectory measure, which contrasted with the correlated measures for the older adults of age and the score on a cognitive test. Although the older adults generally had greater force variability during steady contractions than middle-aged adults (Table 2), measures of force steadiness did not contribute to the latent variables for the older adults. Instead, older adults had significantly lower scores on the List-sorting working memory test (Table 1; P < 0.05), despite similar education levels for the two groups of participants.
The results of the current study suggest that middle-aged adults should be encouraged to engage in activities that challenge manual dexterity and the underlying motor control mechanisms. Previous studies and interventions have mainly focused on differences between young and older adults (Christou, 2011; Christou et al., 2007; Kornatz et al., 2005; Poston et al., 2008), but future interventions need to include middle-age adults before neurological adaptations begin to constrain performance. One consistent, but somewhat surprising feature of the current work, is the strength of the association between steadiness tasks involving index finger abduction and performance on tests of manual dexterity. Tasks requiring index finger abduction are predictive of pegboard performance (Almuklass et al., 2016; Marmon et al., 2011b). This action is likely more novel than pinching or wrist extension, which suggests that the adaptations underlying the decline in manual dexterity do not progress uniformly among the muscles involved in such tasks.
5. CONCLUSION
Older adults were slower than middle-aged adults on both the 9-hole and grooved pegboard tests. The main descriptors associated with these age differences in pegboard times differed for the two tests. Measures derived from force-matching tasks were associated with 9-hole pegboard times for both groups of participants, but with grooved pegboard times for only the middle-aged adults. Grooved pegboard time for middle-aged adults was the only one of the four conditions (two tests and two age groups) that was associated with force steadiness. Both pegboard tests appear to challenge cognitive function in older adults, but the 9-hole test is also associated with an element of fine motor control. These differences provide a framework for subsequent work on the adaptations responsible for increases in pegboard times—a consensus index of manual dexterity—in middle-aged and older adults.
Highlights.
Latent variables underlying age-related differences in pegboard times were identified.
Significant associations were unique for each age group and the two pegboard tests.
Results provide a framework to identify responsible adaptations in pegboard times.
Acknowledgments
This work was supported by the T32 Grant [AG000279] awarded to Robert S. Schwartz.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. doi: https://doi.org/10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuklass A, Price R, Gould J, Enoka R. Force steadiness as a predictor of time to complete a pegboard test of dexterity in young men and women. J Appl Physiol. 2016 doi: 10.1152/japplphysiol.01051.2015. in press. [DOI] [PubMed] [Google Scholar]
- Ashendorf L, Vanderslice-Barr JL, McCaffrey RJ. Motor tests and cognition in healthy older adults. Appl Neuropsychol. 2009;16:171–176. doi: 10.1080/09084280903098562. [DOI] [PubMed] [Google Scholar]
- Bowden JL, McNulty PA. The magnitude and rate of reduction in strength, dexterity and sensation in the human hand vary with ageing. Exp Gerontol. 2013;48:756–765. doi: 10.1016/j.exger.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–9. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Egan GF, Donnan GA. Reproducible activation in BA2, 1 and 3b associated with texture discrimination in healthy volunteers over time. Neuroimage. 2008;39:40–51. doi: 10.1016/j.neuroimage.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Carey LM, Matyas TA, Oke LE. Evaluation of impaired fingertip texture discrimination and wrist position sense in patients affected by stroke: comparison of clinical and new quantitative measures. J Hand Ther. 2002;15:71–82. doi: 10.1016/S0894-1130(02)50012-0. [DOI] [PubMed] [Google Scholar]
- Carey LM, Oke LE, Matyas TA. Impaired Touch Discrimination After Stroke: A Quantiative Test. Neurorehabil. Neural Repair. 1997;11:219–232. doi: 10.1177/154596839701100404. [DOI] [Google Scholar]
- Christou EA. Aging and variability of voluntary contractions. Exerc Sport Sci Rev. 2011;39:77–84. doi: 10.1097/JES.0b013e31820b85ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EA, Enoka RM. Aging and movement errors when lifting and lowering light loads. Age (Omaha) 2011;33:393–407. doi: 10.1007/s11357-010-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EA, Poston B, Enoka JA, Enoka RM. Different neural adjustments improve endpoint accuracy with practice in young and old adults. J Neurophysiol. 2007;97:3340–3350. doi: 10.1152/jn.01138.2006. [DOI] [PubMed] [Google Scholar]
- Dunn W, Griffith JW, Morrison MT, Tanquary J, Sabata D, Victorson D, Carey LM, Gershon RC. Somatosensation assessment using the NIH Toolbox. Neurology. 2013;80:S37–S40. doi: 10.1212/WNL.0b013e3182876e0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Negro F. Common Synaptic Input to Motor Neurons, Motor Unit Synchronization, and Force Control 23–33. 2015 doi: 10.1249/JES.0000000000000032. [DOI] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98:2072–2080. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- Lawrence EL, Dayanidhi S, Fassola I, Requejo P, Leclercq C, Winstein CJ, Valero-Cuevas FJ. Outcome measures for hand function naturally reveal three latent domains in older adults: strength, coordinated upper extremity function, and sensorimotor processing. Front Aging Neurosci. 2015;7:1–8. doi: 10.3389/fnagi.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmon AR, Gould JR, Enoka RM. Practicing a functional task improves steadiness with hand muscles in older adults. Med Sci Sports Exerc. 2011a;43:1531–1537. doi: 10.1249/MSS.0b013e3182100439. [DOI] [PubMed] [Google Scholar]
- Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011b;43:560–567. doi: 10.1249/MSS.0b013e3181f3f3ab. [DOI] [PubMed] [Google Scholar]
- Veale J. Edinburgh handedness inventory - short form: a revised version based on confirmatory factor analysis. Laterality. 2014;19:164–177. doi: 10.1080/1357650X.2013.783045. [DOI] [PubMed] [Google Scholar]
- Martin JA, Ramsay J, Hughes C, Peters DM, Edwards MG. Age and grip strength predict hand dexterity in adults. PLoS One. 2015 doi: 10.1371/journal.pone.0117598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol. 2009;587:5925–5938. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostwald SK, Snowdon DA, Rysavy SDM, Keenan NL, Kane RL. Manual dexterity as a correlate of dependency in the elderly. J Am Geriatr Soc. 1989;37:963–969. doi: 10.1111/j.1532-5415.1989.tb07282.x. [DOI] [PubMed] [Google Scholar]
- Poston B, Enoka JA, Enoka RM. Practice and endpoint accuracy with the left and right hands of old adults: the right-hemisphere aging model. Muscle Nerve. 2008;37:376–86. doi: 10.1002/mus.20954. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Magasi S, McCreath HE, Bohannon RW, Wang YC, Bubela DJ, Rymer WZ, Beaumont J, Rine RM, Lai JS, Gershon RC. Motor assessment using the NIH Toolbox. Neurology. 2013;80:S37–40. doi: 10.1212/WNL.0b013e3182876e0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R, Parker S. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the finger tapping and grooved pegboard tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- Thompson-Butel AG, Lin GG, Shiner CT, McNulty PA. Two Common Tests of Dexterity Can Stratify Upper Limb Motor Function After Stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314523678. [DOI] [PubMed] [Google Scholar]
- Wang YC, Bohannon RW, Kapellusch J, Garg A, Gershon RC. Dexterity as measured with the 9-Hole Peg Test (9-HPT) across the age span. J Hand Ther. 2015;28:53–60. doi: 10.1016/j.jht.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Wang YC, Magasi SR, Bohannon RW, Reuben DB, McCreath HE, Bubela DJ, Gershon RC, Rymer WZ. Assessing dexterity function: A comparison of two alternatives for the NIH toolbox. J Hand Ther. 2011;24:313–321. doi: 10.1016/j.jht.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkins J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S37–40. doi: 10.1212/WNL.0b013e3182876e0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Hadler NM, Earp JAL. Manual ability as a marker of dependency in geriatric women. J Chronic Dis. 1982;35:115–122. doi: 10.1016/0021-9681(82)90112-6. [DOI] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL, Harper JG. Tactile impairments cannot explain the effect of age on a grasp and lift task. Exp Brain Res. 1998;131:263–269. doi: 10.1007/s002210050459. [DOI] [PubMed] [Google Scholar]
- Vieluf S, Godde B, Reuter EM, Voelcker-Rehage C. Age-related differences in finger force control are characterized by reduced force production. Exp Brain Res. 2013;1:107–117. doi: 10.1007/s00221-012-3292-4. [DOI] [PubMed] [Google Scholar]
- Guillery E, Mouraux A, Thonnard JL. Cognitive-motor interference while grasping, lifting and holding objects. PLoS ONE. 2013;11:14–20. doi: 10.1371/journal.pone.0080125. [DOI] [PMC free article] [PubMed] [Google Scholar]


