Abstract
Nanoparticle drug formulations have been extensively investigated, developed, and in some cases, approved by the Food and Drug Administration (FDA). Synergistic combinations of drugs having distinct tumor-inhibiting mechanisms and non-overlapping toxicity can circumvent the issue of treatment resistance and may be essential for effective anti-cancer therapy. At the same time, co-delivery of a combined regimen by a single nanocarrier presents a challenge due to differences in solubility, molecular weight, functional groups and encapsulation conditions between the two drugs. This review discusses cellular and microenvironment mechanisms behind treatment resistance and nanotechnology-based solutions for effective anti-cancer therapy. Co-loading or cascade delivery of multiple drugs using of polymeric nanoparticles, polymer-drug conjugates and lipid nanoparticles will be discussed along with lipid-coated drug nanoparticles developed by our lab and perspectives on combination therapy.
Keywords: combination, nanoparticle, tumor microenvironment, multi-drug resistance
Graphical Abstract
Introduction
In the past decade, nanotechnology has been extensively pursued and is now being applied in the field of pharmaceutics as a method to improve drug biodistribution and limit toxicity [1–4]. Additionally, nanotechnology offers promising potential to screen, formulate, and administer drugs whose use was previously limited due insolubility [5, 6]. Yet, the majority of nanomedicine applied for cancer therapy have failed to meet the Food and Drug Administration (FDA) approval, due to the development of drug resistance, tumor relapse and failure to elicit the enhanced anticancer efficacy [4, 7].
To overcome the issue of poor efficacy, synergistic combinations of different therapeutics are often applied in cancer therapy [2, 8–11]. Existing treatments generally consist of a combination of cytotoxic drugs such as cisplatin, paclitaxel (PTX), and doxorubicin (DOX) that nonspecifically kill all rapidly dividing cells [12–15]. Oftentimes, drugs that induce efficacy through unique mechanisms prove difficult to formulate by presenting different solubility, molecular weights, and other chemical or physical properties. While existing chemotherapy is designed to target malignant cells, an increasing body of evidence suggests the role of the tumor microenvironment (TME) in the progression of cancer and metastasis [16–18]. Specifically, the microenvironment limits the delivery and penetration of particles into the tumor. Abnormally elevated interstitial fluid pressure (IFP) within tumors hinders this process [19]. In the light of this issue, drugs designed to modulate the stroma such as anti-angiogenic drugs [20, 21], TGF-β inhibitors [22, 23] and hedgehog inhibitors, provide a new strategy to overcome this type of drug resistance and improve the anti-cancer therapy.
Several approaches of drug co-loading are reviewed herein. Firstly, amphiphilic polymers can be synthesized to encapsulate multiple hydrophobic drugs through a nanoprecipitation technique [24]. To co-deliver nucleic acid with a hydrophobic drug, cationic, amphiphilic polymers are often used [25–27]. To control the molar ratio of combined drugs with diverse properties in a single formulation, a polymer-drug conjugate presents the best option [12]. Slow hydrolysis, however, can be one major drawback considering for drug conjugates. Secondly, liposomes can be used to deliver a combination of hydrophobic and hydrophilic drugs [28]. Our laboratory has previously developed lipid coated calcium phosphate cores and lipid coated cisplatin cores [6, 29]. A variety of therapeutics, such as siRNA, cisplatin, DNA, peptides and other chemo-drugs can be encapsulated and dispersed into nanocore with the outer leaflet of lipid on these NPs [30–32]. The same cores may also be precisely encapsulated into poly(lactic-co-glycolic acid) (PLGA) NPs [9, 33]. In addition to co-delivery of drugs or genes in a same nano-carrier, delivery of drugs in separate particles targeting different stromal populations, or sequential/cascade delivery of a stroma-modulating agent with a nano-chemotherapeutics has also been reviewed and summarized. By using one regimen to target the TME to inhibit angiogenesis, disrupt the extracellular matrix (ECM), normalize tumor-associated fibroblasts (TAFs), the penetration and distribution of simultaneously or sequentially delivered therapeutics can be significantly enhanced, leading to increased efficacy and greater therapeutic outcome. While the advantages are plentiful, the challenges and future directions of combination therapy are also discussed.
2. Rationales and significance of traditional combination therapy
Clinical cases and animal studies suggest that chemotherapy is most effective when multiple drugs are given in combination to achieve drug synergy [12, 24, 34]. The basic rationale for combinatory anti-tumor therapy is to simultaneously or sequentially deliver therapeutics that each utilizes unique molecular mechanisms [24]. This allows for decreased therapeutic doses while reducing the chance of multidrug resistance (MDR) and minimizing overlapping toxicity. The aforementioned advantages will be discussed in detail below:
2.1 Overcoming intrinsic or acquired multidrug resistance
2.1.1 Overcoming hindrance of efflux transporters
MDR is one of the major obstacles in the treatment of cancer. MDR refers to a state of resilience against functionally and structurally unrelated chemotherapeutic drugs, which can be intrinsic or acquired through exposure to chemotherapeutic agents [2, 35]. Many mechanisms are known to contribute to MDR, such as the presence of a multidrug efflux pump or efflux transporters. Drug efflux transporters are more prevalent in drug-resistant tumor cells, which actively pump chemotherapeutic drugs out of the cell [36]. Among them, P-glycoprotein (P-gp), encoded by the MDR1/ABCB1 gene in humans, is one of the most well characterized efflux ATP binding cassette (ABC) transporters that show broad substrate specificity [37, 38]. Its overexpression in cancer has been considered as one of the major hindrances in anticancer therapy. Pharmaceuticals that block or bypass the P-gp efflux are considered as P-gp inhibitors or P-gp modulators [39]. To this end, co-/concurrent treatment of chemotherapy with small-molecule P-gp inhibitors or siRNA against P-gp mRNA have become clinical strategies to overcome MDR [40]. Recently, Hubensack et al. demonstrated increased levels of PTX accumulation in the brain of the nude mice when treated with elacridar or tariquidar (both are third-generation P-gp inhibitors) owing to the downregulation of P-gp expressed at the blood–brain barrier [41]. Other P-gp modulators, such as the first generation of P-gp inhibitor, verapamil [42], or the natural product extract, curcumin [43], can also inhibit P-gp pumps and sensitize cells to primary chemotherapeutic P-gp substrate, such as irinotecan [43], PTX [44], adriamycin [45, 46], and vinblastine[47]. In one such study, nano-delivery of curcumin was utilized to overcome P-gp mediated efflux of PTX in MDR ovarian cancer (SKOV3TR) [35, 48]. In another preclinical study, Meng et al. used a high throughput-screening assay to identify P-gp siRNA as an optimal siRNA to overcome doxorubicin (DOX) resistance in the MCF-7/MDR cell line. Co-delivery of P-gp siRNA with DOX in a single multifunctional mesoporous silica nanoparticle (MSN) was used to overcome DOX resistance in an MDR human breast cancer xenograft [26, 49]. The concept of combining P-gp inhibitors with chemotherapy has also been applied clinically. In a Phase I clinical trial, zosuquidar was co-delivered with daunorubicin and cytarabine to patients bearing acute myeloid leukemia. The combination therapy demonstrated enhanced anticancer activity [50].
Several other ABC transporters that are associated with MDR include multi-drug resistance protein 1 (MRP-1, ABCC1), breast cancer resistance protein (BCRP, ABCG2), the mitoxantrone resistance protein (MXR/BCRP, ABCG2) and ABCB4 (MDR3) [1, 35]. These efflux transport proteins alone, or in conjunction with several cell membrane pump proteins can also inhibit drug internalization and decrease intracellular drug concentrations. In addition to P-gp inhibitors, modulators of these efflux transporters have been administrated in combination with chemotherapy to reverse MDR. BCRP has been shown to acquire resistance to a series of anticancer agents such as 7-ethyl-10-hydroxycamptothecin (SN-38), topotecan, and mitoxantrone [1]. Yanase et al have indicated that gefitinib, a selective epidermal growth factor receptor tyrosine kinase inhibitor, sensitized human colon cancer HT-29 cells, which endogenously express BCRP, to SN-38 [51].
2.1.2 Modulating DNA repair systems
The acquired resistance of DNA alkylating agents (e.g. cisplatin) differs from other chemotherapy agents in that several cellular pathways are activated in response to their interaction with DNA. These pathways include those that modulate DNA repair mechanisms. DNA repair mechanisms consist of a complex network of proteins (e.g. ATM, ATR, Chk1/2, BRCA1 or p53) that able to identify DNA damage induced by the alkylating agent [35, 52], and also include enzymes that repair the damage using nucleotide excision repair (NER) and mismatch repair (MMR) [53, 54]. DNA repair mechanisms also include translesion DNA synthesis (TLS) by specialized DNA polymerases through repairing DNA damage [55]. The therapeutic agent is therefore unable to induce apoptosis in affected tumor cells. To this end, co-/concurrent delivery of agents that inhibit DNA repair with DNA alkylating agents (e.g. cisplatin) would be another promising combination strategy.
Indeed, overexpression of kinase-inactive ATR has been shown to increase sensitivity of cancer cells to cisplatin and ionizing radiation (IR) in tissue culture [56, 57]. Consistent with these data, ATR kinase inhibitors such as AZD6738 sensitized lung cancer to cisplatin and IR [58]. Another example regards the first-line treatment of bladder cancer: gemcitabine and cisplatin [53]. Gemcitabine can potentiate the cytotoxicity of cisplatin and inhibit the repair of cisplatin-DNA damage by deregulation of ERCC1 and XPA, two major proteins in the NER system [53]. Therefore, the removal of platinum-DNA adducts can be inhibited [59]. A combination of gemcitabine monophosphate and cisplatin simultaneously in a single PLGA NP has demonstrated significant synergistic efficacy in treating aggressive bladder cancer xenografts [33]. Recent studies also showed that the suppression of gene products (e.g., REV1, REV3L) involved with the error-prone translesion DNA synthesis also sensitizes resistant tumors to chemotherapy and prevents drug resistance in relapsed tumors. For instance, the Rev1/Rev3L/Rev7-dependent error-prone TLS pathway has plays an important role in cisplatin-induced mutations. Suppression of error-prone TLS activity in mammalian cells by knocking down Rev1 or Rev3L has been shown to inhibit cisplatin-induced mutagenesis; thus relapsed tumors remain sensitive to subsequent treatment [55, 60].
2.1.3 Modulating other significant genes
Changes in survival/apoptotic pathways are another cause of multi-drug resistance [26]. Chemotherapy has been shown to induce overexpression of gene amplification or mutation of oncogenes (e.g. p53, Kras, c-Myc) [32, 61–64], regulators of drug metabolism (e.g. pregnane X receptor, PXR) [65, 66], and anti-apoptotic proteins (e.g. Bcl-2) [61, 67, 68], as well as inducing apoptosis by suppressing transcription factor nuclear factor kappa B (NF-κB) [69]. To this end, combination therapy involving a chemotherapeutic agent while inhibiting the aforementioned pathways can effectively treat tumor cells with acquired resistance [32, 62, 63, 67, 70–74].
2.2 Cancer stem cells and EMT
Cancer stem cells (CSCs) are distinguished from other cancer cells by hallmarks such as self–renewal, relative quiescence, low metabolic activity, resistance to drugs through protective apoptotic signaling pathways, active DNA-repair capacity and overexpression of several ABC drug efflux transporters [75]. There are two major scenarios that explain the origin of CSCs. The first happens by direct, malignant transformation of normal stem cells that accumulated in the oncogenic insults over time, an example being CD34+, CD38− leukemia initiating cells [76]. The second scenario involves dedifferentiation of terminally differentiated cells to a primitive stem cell-like state, similar to epithelial to mesenchymal transition (EMT). EMT not only increases the probability of metastasis, but also contributes to drug resistance. Tumor cells with mesenchymal features are more likely to achieve CSC features and multidrug resistant properties [77].
Many studies suggest that tumors are enriched with CSCs at the completion of primary therapy, and the remaining CSCs contribute to drug resistance and ultimately result in disease recurrence. Studies of EMT-related CSC suggest another strategy to overcome drug resistance. Sources of CSC origin could be decreased through inhibition of pathways involved in EMT transition, for example, rapamycin combined with other traditional chemo-drugs can inhibit PI3K/PTEN/mTOR signaling and block activation of the TGF-β pathway and prevent CSC maintenance [77]. Aside from regulating the source of CSCs, another strategy may be to specifically target and kill existing CSCs based on antibody-directed recognition of unique molecular targets specific to CSCs. In this regard, several drug efflux transporters have been identified in CSCs, including P-gp, multidrug resistance-associated proteins (MRP) and BCRP [43]. Along this approach, fumitremorgin C (FTC) has been used as an inhibitor of the ABCG2 transporter which has shown efficacy when combined with imatinib, which targets the leukemia stem cells [78]. Another strategy is to selectively target CSCs through high-throughput compound screening, such as using the antibiotic salinomycin and an HDAC inhibitor [77].
2.3 Synergistic effect of combinatory therapy
One of the prime benefits of combination therapies is the potential of providing synergistic effects. The term synergistic effect means that, the overall therapeutic benefits of the combinatory drugs were found to be greater than the sum of the effects of the drugs individually. These advantages have driven the efforts of drug discovery toward the search for combination therapies [24]. The optimal drug combination with maximal antitumor efficacy can be calculated using combinational drug effect/combination index (CI) isobologram analysis, an effective way to indicate that drugs are working synergistically [24, 79]. The mechanism of synergistic effects following combinational drug treatment could be targeting multiple sites of several different pathways, leading to cumulative regulation of targeting activity and simultaneous enhancement of the positive effects or reduction of negative effects at comparatively lower doses [24]. Hence, in addition to combination of MDR modulators with chemotherapeutic reagents, synergistic effects can be observed between two chemotherapeutic agents targeting different pathways; for example a DNA damaging/intercalating agent in combined with an anti-mitotic microtubule stabilizing agent. An example illustrated here is the combination of DOX, the intercalating agent, and PTX, which is considered the first-line treatment of multiple malignancies in clinical trials [12]. In addition to DOX, cisplatin is often co/concurrently-delivered with PTX to achieve synergistic antitumor effect. Cisplatin is always combined with PTX in clinical and preclinical treatment of gynecological tumors, such as ovarian and cervical cancers [80]. Gemcitabine and PTX, also a combination of a DNA-damaging agent with a microtubule stabilizer, have shown synergistic effects in treating breast cancer xenografts when delivered in a single MSNP particle [81]. In addition, several investigations have reported that the combination treatment of PTX and combretastatin A4, two microtubule-associated inhibitors, displayed synergistic effects on tumor cells and tumor vasculature [80]. The antitumor effect of PTX could also be enhanced by tetrandrine, which increased the level of intracellular reactive oxygen species [82]. Recently, Chiang et al. elucidated the synergistic interaction of RAD001 (an mTOR inhibitor) with gemcitabine or PTX for anticancer treatment in a heterogeneous group of non-Hodgkin lymphoma (NHL) cell lines [83, 84].
Curcumin is another important component often used in combination with other chemotherapy drugs. Accordingly, the synergistic effects between curcumin and various anticancer agents have been previously explored. Curcumin has potential anticancer effects via multiple signaling pathways [85]. Increasing evidence demonstrates that curcumin reverses chemo-resistance and sensitizes cancer cells to chemotherapy in cancer cells [20]. The nanoformulation of curcumin is versatile. Free curcumin, polymeric, liposomal and other types of formulations of curcumin can be co/concurrently-delivered with chemotherapy, such as camptothecin (CPT) [86], etoposide [45, 87], platinum [88], PTX [48, 89] and DOX [90, 91] to induce synergy. One study utilized flaxseed oil nanoemulsion to effectively encapsulate PTX and curcumin to enhance the cytotoxicity against both wild-type and resistant ovarian tumor cell line SKOV3[48]. Oral curcumin was able to enhance the oral bioavailability of etoposide due to the inhibition of the P-gp efflux pump [45]. A recent study also elucidated the synergy of platinum prodrug MPEG-b-P(LA-co-DHC/Pt) conjugates and curcumin [88].
2.4 Non-overlapping toxicity
Dose-dependent toxicity to other non-cancerous cells is among the largest hurdles encountered in cancer therapy. However, unlike single-agent therapy, synergistic combinations of two or more agents can overcome toxicity and other adverse effects associated with high doses of individual drugs by countering biological compensation and allowing reduced dosage of each compound [24]. Curcumin, a natural product with high biosafety, has again been chosen as a promising candidate. Sreekanth et al. co-administered curcumin or curcumin liposome with the cytotoxic anticancer drug PTX to reduce the dose-limiting toxicity exerted by PTX upon systemic delivery. They demonstrated that combination therapy not only reduced the dose of PTX but also increased the anticancer cytotoxic activity compared with dosing PTX alone [89]. In addition to curcumin, other combinatory chemotherapies have also shown advantages of decreased toxicity. In another study, Dasanu et al. showed that the combination of two different drugs (i.e. carboplatin and gemcitabine) in a small group of patients with metastatic ovarian cancer resulted in significant antitumor activity with little hematological toxicity [92].
2.5 Current combination chemotherapy in clinical trials
As mentioned above, multiple mechanisms demonstrate the importance of combination chemotherapy, basically including non-overlapping toxicity, decreased resistance and increased tumor cell-killing efficacy. Therefore, combination chemotherapy has been applied widely in clinical trials for decades. Traditional anti-cancer drug combinations, as shown in some of examples described in earlier sections, could be classified into three groups: the anthracycline-based combinations, the methotrexate-based combinations, and paclitaxel (PTX)-based combinations [11, 12]. Anthracycline-based chemotherapeutics, including DOX, daunorubicin, epirubicin, idarubicin and valrubicin, are often combined with 5-fluorouracyl (5-FU), gemcitabine and cyclophosphamide. 5-FU and gemcitabine functions as nucleoside analog that replaces the building blocks of nucleic acid during DNA replication, leading to the formation of “faulty” nucleoside, resulting in apoptosis, while cyclophosphamide acts as DNA alkylating agent to prevents the DNA replication by forming intrastrand and interstrand DNA crosslinking [93]. Methotrexate, an antimetabolite and antifolate drug, also plays vital roles when combined with 5-FU, gemcitabine and cyclophosphamide due to the capability of inhibiting the metabolism of folic acid [12]. PTX-based combinations are another commonly used traditional chemotherapy. PTX has been reported to be combined with cisplatin, DOX, combretastatin A4, gemcitabine and so on [12].
3. Nanoformulation-mediated co-delivery of small-molecule chemotherapy
3.1 Rationale and advantages of nanoformulation-mediated co-delivery
An important factor that separates in vitro success of combination therapy from significant clinical benefit is the versatile pharmacokinetics of the combined regimens [12]. To achieve more effective combination therapy in the clinics, developing technologies for precise and controlled delivery of multiple drugs is of vital importance. Nevertheless, the effective administration and delivery of multiple drugs at an optimal dose ratio is complicated due to the dissimilar pharmacokinetics and biodistribution of drugs within the body. This is because that drugs often have different physical properties, and consequently disparate rates of metabolism. Nanoformulations can help avoid such limitations by carrying multiple therapeutic agents with different physico-chemical properties and pharmacological behaviors simultaneously in a single nanoparticle (NP). In particular, several current nanoformulations allow for the co-encapsulation of hydrophobic and hydrophilic drugs with adjustable ratiometric drug loading [33, 94]. NPs are able to maintain the optimized synergistic drug ratio in a single carrier from administration to the point of intracellular uptake within the target cancer cell. This ratio is difficult to maintain if separate carriers are used wherein each carrier encapsulates only a single drug. In addition to the design of ratiometric loading and delivery, NPs can also be designed for cascade, sequentially [95] or spatiotemporally controlled release [81, 94] and stimuli-responsive properties [80, 96–98] based on different therapeutic purposes.
The loading of drugs into the nano-carrier can be achieved via physical encapsulation, chemical conjugation, or a combination of both methods [12]. Partner drugs can also be covalently conjugated to the matrix or the carrier and subsequently attached on the surface of drug-loading nanoparticle to form the combinatory NPs. Additionally, electrostatic interactions can also be applied to assemble combination NPs utilizing ionic interactions to facilitate encapsulation of both drugs [35, 99]. Currently, this novel “two in one” approach is under preclinical and clinical investigation (Figure 1, Table 1) [99]. Liposomal formulations have been clinically utilized to simultaneously encapsulate multiple small-molecule drugs but the efficacy was limited by poor solubility, systemic toxicity and unsuitable pharmacokinetics. One example is the Phase III clinical trial regards CPX-351. CPX-351 is a liposomal NP developed to treat acute myeloid leukemia. CPX-351 simultaneously encapsulates daunorubicin and cytarabine in an optimized 5:1 molar ratio [99, 100]. Other combinations, such as CPX-1 (irinotecan/floxuridine), are also investigated in the preclinical/clinical studies [3, 99, 101].
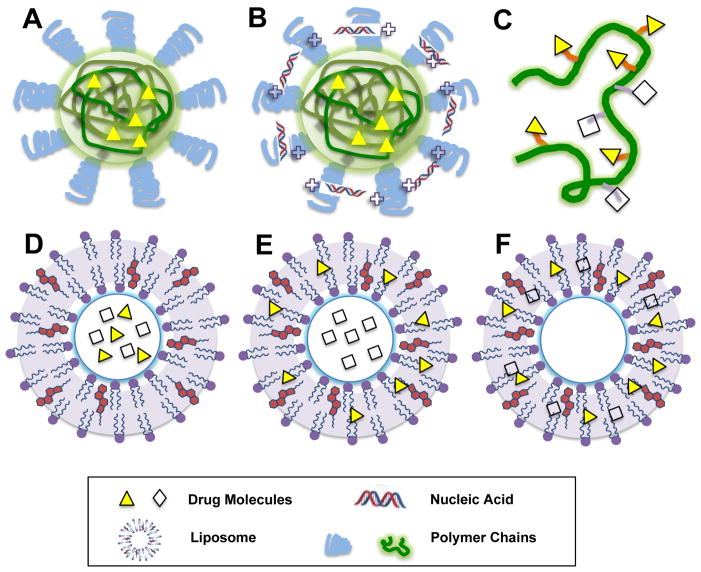
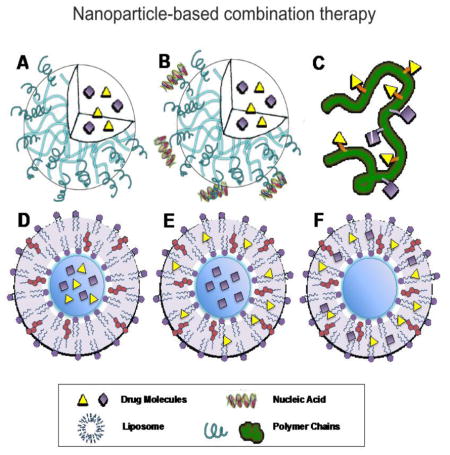
Figure 1. Nanoformulations for combination therapy.
A. Polymeric nanoparticles for two hydrophobic drug. B. Polymeric nanoparticles for one hydrophobic drug and one nucleic acid. C. polymer-drug conjugates. D. Liposomes for two hydrophilic drugs. E. Liposomes for one hydrophilic drug and one hydrophobic drug. F. Liposomes for two hydrophilic drugs.
Table 1.
Recent update of nanoparticle-based combination therapy with drugs and genes
| Formulation Category | Encapsulation Method | Drug Characteristics | Materials | Combination Drugs | Disease Model | Ref |
|---|---|---|---|---|---|---|
| Polymer-based nano-formulations | Physical encapsulation & physical encapsulation | Hydrophobic drugs | PLGA-PEG NPs | DOX and cisplatin | - | [127] |
| Hydrophobic drugs | Chitosan or HA functionalized PLGA NPs | Camptothecin and cisplatin | Colon cancer | [86, 128] | ||
| Hydrophobic drugs | nanoemulsion | PTX and curcumin | Ovarian cancer | [48] | ||
| Hydrophobic drug & hydrophilic drug | water-in-oil-in-water double emulsion | Catechin and curcumin | - | [129] | ||
| Hydrophobic drug & hydrophilic drug | PLGA-PEG NPs with nanocores | Cisplatin and Gemcitabine or rapamycin | Bladder cancer or melanoma | [9, 33] | ||
| Chemical conjugate & chemical conjugate | Hydrophobic drug & hydrophilic drug | PTX-Vitamin E conjugate & 5-FU-TPGS conjugate | PTX and 5-FU | PTX-resistant cancer | [111] | |
| Chemical conjugation & electrostatic adsorption | Hydrophobic drug & hydrophilic protein | TMC-based nanocomplexes | DOX-CA-TMC conjugate and interleukin-2 (rhIL-2) | Hepatic cancer | [130] | |
| Physical encapsulation, physical encapsulation, electrostatic adsorption, & surface coating | Hydrophobic & hydrophilic drugs, siRNA, and PTT agent | PDA coated P (MEO2MA-co-OEGMA-co-DMAEMA)-b-PLGA core-shell structured NPs | PTX, DOX, survivin siRNA, polydopamine (PDA) | Triple negative breast cancer | [131, 132] | |
| Physical encapsulation & electrostatic adsorption | Hydrophobic drug & siRNA | EGF modified mPEG-PLGA-PLL NPs | DOX, Bcl-2 siRNA | Lung cancer | [70] | |
| Hydrophobic drug & siRNA | PEI-glycyrrhetinic acid (PEI-GA) | DOX, Akt-1 shRNA | Hepatic cancer | [133] | ||
| Hydrophobic drug & siRNA | HA-ss-(OA-g-bPEI) | PTX, AURKA siRNA | - | [134] | ||
| Hydrophobic drug & siRNA | PEG-PLL-PLLeu | Docetaxel, Bcl-2 siRNA | Breast cancer | [73] | ||
| Drug as carrier, prodrug, electrostatic interaction | Hydrophobic drug & cationic drug | PolyMet | Cisplatin-glutamic acid conjugate and PolyMet | Lung cancer | [135] | |
| Chemical conjugation & physical encapsulation | Hydrophobic & hydrophilic drugs | elastin-like polypeptide (iTEP)-based NPs | PTX and Salinomycin (Sali) polypeptide conjugate | Triple negative breast cancer | [112] | |
| Hydrophobic drugs | PEG-based NPs | PEG-DOX conjugate and curcumin | Hepatic cancer | [90] | ||
| Inorganic nano-formulations | Chemical conjugation & physical encapsulation | Hydrophobic drugs (PDT agent) | MSNs | Cisplatin-MSN conjugate and chlorin e6 (Ce6) | Lung cancer | [136] |
| Chemical conjugation | Hydrophilic & hydrophobic drugs | Iron Oxide and SiO2 NPs | AICAR and DOX | - | [137, 138] | |
| Physical encapsulation & chemical conjugation | Hydrophobic drug | Iron Oxide Nanocarrier | Pt(IV) prodrug and dsRNA | - | [139] | |
| Physical encapsulation & electrostatic adsorption | Hydrophobic drug & siRNA | MSNs | DOX and Pgp siRNA | Breast cancer | [26, 49] | |
| Drug as a carrier | Electrostatic interaction | A cationic hydrophilic drug & siRNA | PolyMet | PolyMet and VEGF or Bcl-2 siRNA | Lung caner | [71] |
| Physical interaction | Hydrophobic drugs | HCPT and DOX | HCPT and DOX | - | [105] | |
| Lipid-like NPs | Chemical conjugation & electrostatic interaction | Cationic hydrophilic drug & gene | Lipid-metformin | Lipid-metformin and TRAIL plasmid | Lung cancer | [140] |
| Physical encapsulation | Hydrophobic drugs | Lipid NPs | etoposide and curcumin | Gastric cancer | [87] | |
| Electrostatic adsorption | RNAs | Lipidoid NPs | siRNA and miRNA | Lung Cancer | [63, 141] | |
| Electrostatic adsorption | RNAs | DOPC lipid | siRNA and miRNA | Ovarian cancer | [142] | |
| Physical encapsulation &electrostatic adsorption | Hydrophilic drug & siRNA | Calcium phosphate lipid NPs | Gemcitabine monophosphate and c-Myc siRNA | Lung cancer | [32] |
3.2 Nanoparticle formulations utilized to co-deliver small-molecule chemotherapy
3.2.1 Polymeric nanoparticles and micelles
Poly (lactic-co-glycolic acid) (PLGA) is an FDA-approved biodegradable copolymer that can encapsulate hydrophobic drugs via hydrophobic interactions to form NPs with high efficiency [42]. Wang et al have loaded PTX and etoposide into PLGA NPs for enhanced cytotoxic effects [102]. However, pure PLGA polymeric particles suffer from low stability within the aqueous phase and have limited loading capacity. Amphiphilic copolymers, for example, PLGA-PEG, are able to self-assemble into micellar NPs with a hydrophobic core and a hydrophilic corona, and with increased solubility and stability [9]. The amphiphilic copolymers have also demonstrated better loading capacity for the encapsulated drugs. Mishra et al. have encapsulated PTX and rapamycin (RAPA) into PEG-b-PLA for synergistic antitumor therapy [103]. To increase the cellular uptake efficiency, chitosan was also used as a material for surface functionalization of NPs. The cationic chitosan functionalized NPs were able to load the hydrophobic plant alkaloid, CPT and the hydrophobic polyphenol curcumin with unified release profiles and enhanced synergy [86]. Microenvironment-simulative NPs were also designed for the co-delivery of two hydrophobic drugs. To this end, PLGA NPs were further functionalized with pH sensitive moieties, such as a charge reversible pullan-based (CAPL) shell to facilitate the release of co-encapsulated PTX and combretastatin A4 [80]. Hydrogel based local delivery systems can also be designed to simultaneously delivery multiple moieties. Zhang et al have used mPEG-PCL doped gelatin hydrogels to co-deliver tetrandrine and PTX for synergistic antitumor efficacy [82]. Advances in polymer chemistry have facilitated the development of polymer-based combination therapy. Recently, Liao et al have used a convergent synthetic platform to precisely ratiometric load three chemo-drugs, including cisplatin, DOX and CPT. Based on the “brush-first” ring-opening metathesis polymerization (ROMP) method, nanoscopic brush-arm star polymers conjugated with DOX and CPT were developed, and further cross-linked with Pt(IV) diester derivative as a novel crosslinker [104].
3.2.2 Drug-drug interaction and polymer-drug conjugates
Carrier-free pure nanodrugs (PNDs) that are composed entirely of pharmaceutically active molecules are regarded as promising candidates for the next generation of drug combination formulations. The combinatory drugs are mainly formulated from supramolecular self-assembly of drug molecules [105]. For example, DOX has the characteristic structure of a surfactant with the unsaturated anthracycline rings acting as the hydrophobic part and the saturated end of the ring system as a hydrophilic part. Furthermore, DOX contains abundant hydroxyl groups adjacent to the amino sugar. HCPT molecules are also hydrophobic; therefore, Zhao et al have shown that DOX could potentially be used for solubilizing and nanosizing HCPT through hydrophobic-hydrophobic interaction. Indeed, the water-solubility of HCPT was improved 50-fold after co-assembly with DOX [105]. The intermolecular forces or hydrophobic interaction between combinatory drug moieties were not only observed in these carrier-free pure nanodrugs but also when loading simultaneously in one polymeric micelles or particles (formulations described in section 3.1) [9, 106]. These findings indicate that the interaction between two different drugs can allow enhanced loading of both therapeutics into a single NP.
Single or multiple drug-conjugated polymers exhibiting amphiphilic properties were also used to encapsulate small-molecule drugs for combination therapy based on the supramolecular interaction between drug-conjugated polymer carriers and the other drugs [90, 107–110]. For example, the platinum (IV) prodrug is conjugated to a PLA backbone via an ester bond to form the PLA-Pt(IV) conjugate [108, 109]. Docetaxel (Dtxl) was then encapsulated into PLA-Pt(IV) NPs due to the hydrophobic interaction between Pt(IV) prodrug and Dtxl. In addition, conjugation of hydrophilic drugs with hydrophobic polymeric or lipid chain enhances the hydrophobicity of the drugs, enabling the encapsulation of hydrophilic drugs into polymeric particles together with hydrophobic drugs, solving the issue of ratiometric loading of drugs with different solubilities. To this end, Ma and the coworkers developed the core-matched nanoemulsions (NEs) functionalized by vitamin E (VE) and tocopherol poly(ethylene glycol)succinate (TPGS) to co-deliver hydrophobic PTX and hydrophilic 5-FU. The matching between VE and TPGS provides the basis of co-loading. The core-matched NEs exhibited entrapment efficiency of >90% and advanced anti-tumor efficacy against both PTX-sensitive and PTX-resistant human epidermal carcinoma [111]. Drugs can also be conjugated with peptide to form the peptide prodrug. The example regards the selective inhibitor of cancer stem cells (CSCs), Salinomycin (Sali) conjugated to immune-tolerant elastin-like polypeptides (iTEPs) through a pH-sensitive linker. The iTEP-Sali-ABA conjugates were self-assembled into NPs, and simultaneously used to encapsulate PTX. These combinations not only inhibited tumor growth but also suppressed tumor metastasis [112]. Furthermore, multiple drugs can be separately or simultaneously covalently conjugated onto the same polymer to unify the physical property and facilitate co-delivery. Cisplatin (IV) prodrug and paclitaxel were conjugated to the same biodegradable and amphiphilic block copolymer MPEG-b-P(LA-coMCC) respectively for combination therapy [106]. Usually, the releasing of drugs from the polymers would be one issue to tackle. This could be solved by linking polymer and drugs via environment-or enzyme-sensitive linkers [113, 114].
Currently, more than fourteen polymer–drug conjugates have undergone clinical investigation and a polyglutamic acid (PGA)-paclitaxel conjugate (CT-2103, OPAXIO®, previously known as Xyotax®) is expected to be marketed in the future [115]. Clinical combination studies focus on combination of concurrent delivering polymer-drug conjugate separately with other small-molecule drugs. For example, a phase III study was carried out with advanced lung cancer testing the combination of PGA-paclitaxel with carboplatin [116]. To date, the co-delivery of drug-polymer conjugate with free drugs or drug-polymer in one single NP has not been explored clinically, despite the extensive exploration in preclinical studies.
3.2.3 Lipid nanoparticles
Liposomes are spherical vesicles consisting of amphiphilic phospholipid bilayers and an inner aqueous core, which are employed to load lipophilic and hydrophilic drugs. To co-deliver a hydrophobic drug and a hydrophilic small-molecule drug, Dai et al. loaded the antiangiogenic agent combretastatin A4 in the lipid bilayer and the anticancer drug DOX in the aqueous core of PEGylated liposomes [117]. In a separate study, vincristine (VCR) and topotecan (TPT) were co-loaded into a nanoliposome using the remote loading method (LipoViTo) [118]. Two hydrophilic drugs, cytarabine and Daunorubicin. HCl at a 5:1 molar ratio were also encapsulated into the aqueous space of bilayer liposomes [119].
Lipid and polymer can also be combined for the purpose of co-delivery. Recently, Li et al developed a layer-by-layer delivery system by using lipid-polymer hybrid to co-deliver two hydrophilic drugs Oxa and 5-FU and one hydrophobic drug CPT [120]. Within the hybrid NPs, 5-Fu was encapsulated into the hydrophilic core of the NPs, while CPT was loaded into the hydrophobic layer. In addition, Oxa was inserted in the interlayer of polymeric core and lipid bilayer [12].
3.2.4 Other types of delivery systems
Many other NPs capable of delivering multiple drugs have been developed, such as gold nanoparticles (GNPs), iron oxide nanoparticles (IONPs) and mesoporous silica nanoparticles (MSNs) [121–124]. GNPs and IONPs can be readily modified with a layer of functional polymer and further used to conjugate small-molecule drugs or condense nucleic acid. For instance, Xiao et al have designed a gold (Au) nanorod-based carrier capable of co-delivering siRNA and DOX [125]. DOX was conjugated onto the surface of the nanorod; whereas, siRNA was electrostatically condensed with a cationic polymer that covalent linked to the nanorod. The advantages of using GNPs and IONPs as a combination vector are mostly attributed to their tunable size and shape. In addition, GNPs and IONPs also allow facile tracking and imaging of the systemic distribution of the combinatory regimens through X-ray computed tomography (CT) imaging and magnetic resonance imaging (MRI), respectively. However, the clinical translation of these two delivery systems are limited by their intrinsic instability and potential toxicity. Furthermore, the therapeutic agents are only encapsulated to the surface of the cargos with relatively low payloads [121–124]. Compared to GNPs and IONPs, drugs are loaded into the pores of MSNs or absorbed on the surface of MSNs [26]. Thus, drugs with various physicochemical properties can be loaded simultaneously to MSNs with high loading efficiency. Liu et al have reported the co-delivery of a hydrophobic small molecule and a hydrophilic peptide using MSNs [126].
4. Combination of siRNA/chemodrugs in a single carrier
4.1 Rationale and advantages of nanoformulation-mediated co-delivery
The combination of chemotherapy with RNAi is also a promising synergistic strategy for cancer treatment, mainly aiming to overcome the aforementioned tumor-specific MDR. Given the ability to target and silence nearly any gene of interest, specific siRNAs can be constructed to target genes encoding proteins involved in the MDR pathway (i.e., efflux transporters, DNA repair and apoptotic pathways) or oncogenes (c-Myc, Kras). Advantages of incorporating siRNA as compared to small-molecule drugs include (1) siRNA with known sequences can selectively seek out and degrade mRNA that is complementary to the antisense strand, allowing for specific and efficient knockdown of the genes of interest. (2) Meanwhile, siRNAs demonstrate lower toxicity compared to small-molecule drugs, which often have multiple off-target binding sites. (3) Although the functions of siRNA are versatile, the structure of all siRNAs is highly uniform and stable, as the similarity in siRNA structures enables formulations of different siRNA using similar methods, significantly easing manufacturing techniques.
While “naked”, chemically modified siRNA has shown efficacy in certain physiological settings such as the brain and the lung, an additional delivery system is still required to maintain the RNA stability, improve cellular uptake and facilitate transfection [40, 62]. Synthetic nanomaterials have demonstrated potentials as effective non-viral siRNA delivery carriers. These carriers can be further designed to facilitate co-delivery of siRNA with hydrophobic small-molecule drugs. Given the physical characteristics of siRNA, such as high molecular weight and negative charges, most materials designed to co-encapsulate siRNA with small-molecule drugs focus on utilizing electrostatic interactions. They are basically amphiphilic materials involving hydrophilic, cationic moieties to bind siRNA and a hydrophilic pocket to load hydrophobic drugs. In addition, once entering the cytosol, siRNA should be incorporated into a protein complex called the RNA-induced silencing complex (RISC), to be cleaved and bind with target mRNA. Therefore, when designing an siRNA carrier, sufficient endosomal escape and cytosolic release, should be considered, and would be two prerequisites for efficient RNAi-mediated gene knockdown [143].
4.2 Nanoformulations utilized to co-deliver chemotherapy with siRNA
4.2.1 Polymeric nanoparticles
PLGA is an FDA approved carrier for small-molecule delivery. Yet, the lack of positive charges and slow drug release limit its application as a carrier for siRNA delivery. To facilitate co-delivery of siRNA with small-molecule drugs, an amphiphilic block copolymer composed of conventional monomethoxy (polyethylene glycol)-poly (D,L-lactide-co-glycolide)-poly (L-lysine) (mPEG-PLGA-b-PLL) was synthesized [144]. The functional side groups composed of PLL cationic polypeptide provide the ability to anchor siRNA through ionic interactions. mPEG-PLGA-b-PLL have been used to co-deliver adriamycin or DOX with Bcl-2 siRNA as a potential treatment strategy for lung cancer [70, 144]. Similarly, other cationic, amphiphilic copolymers have been synthesized to facilitate the loading of siRNA or DNA with other small-molecule drugs. Amphiphilic poly(ε-caprolactone)-graft-poly(2-(dimethylamino) ethyl methacrylate) (PCL-g-PDMAEMA) was able to load PTX and condense DNA/siRNA [145, 146]. It was found that the amphiphilic properties facilitate the cellular uptake of DNA and PCL-g-PDMAEMA NPs/DNA polyplex to escape from the endosome. Other similar systems based on different cationic blocks (such as polyethyleneimine, polylysine and dendrimer) and hydrophobic copolymers were also synthesized for combination delivery of hydrophobic drugs and nucleic acids [147]. Further, Wang et al. developed a cationic core shell nanoparticle system using biodegradable amphiphilic copolymers for DNA/siRNA and PTX co-delivery [127]. The polymer namely, poly (N-methyldietheneamine sebacate)-co-((cholesteryl oxocaronylamidoethyl) methyl bis(ethylene) ammonium bromide) sebacate formed self assembling core–shell NPs encapsulating the drug and gene payload. These core–shell NPs demonstrated superior tumor suppression both in the in vitro cell cultures and in vivo animal models [127]. Natural polymers such as dextran, chitosan, gelatin and hyaluronic acid have several advantages compared to synthetic polymers in terms of biological safety of the engineered nano-constructs. Along these lines, a novel lipid-modified dextran-based self-assembling polymeric nanosystem was designed to encapsulate MDR-1 siRNA as well as anticancer drugs such as DOX [128,129].
4.2.2 Lipid and liposomal nanoparticles
Researchers from Minko’s group have also developed liposomal-based nano-carrier systems for the co-delivery of siRNA and DTX to treat MDR small cell lung cancer (SCLC)[130]. Series of lipid-like materials have been synthesized by Anderson’s lab using combinatory design [63, 141, 143]. These materials allow efficient delivery of siRNA systemically. Due to the liposomal structure of the particles, the lipidoid particles also have the potential to co-deliver siRNA with small-molecule drugs for the purpose of chemotherapy sensitizing.
4.2.3 Inorganic nanoparticles
Due to their unique structure and ease of surface functionalization, MSNs have also been applied for co-delivery of drugs with nucleic acid. Usually, cationic polyethylenimine (PEI) polymers were absorbed onto the surface of MSNs for condensation of DNA and siRNA. Meanwhile, the polymer attachment leaves the porous interior free for drug binding and delivery, thus establishing the rationale for simultaneous drug and nucleic acid delivery [26, 27]. Indeed, Meng et al have reported that MSN can be functionalized to effectively deliver the chemotherapeutic agent DOX together with anti-P-gp siRNA to a drug-resistant cancer cell line (KB-V1 cells) to accomplish cell killing in an additive or synergistic fashion [49]
4.2.4 Drug/siRNA co-encapsulation considering the interaction
When designing drug/siRNA combinations, the interaction between small-molecule drugs and siRNA is of crucial importance (Figure 2). The mechanism of most DNA intercalating agents (e.g. cisplatin) functions through their non-reversible binding with nucleotides in the genomic DNA, interfering with cell division. Since siRNA also bears a nucleotide structure similar to that of DNA, unintended intercalation between the intercalating agent and therapeutic siRNA will diminish the efficacy of both. Recently, a polymeric NP platform composed of PLGA-PEG and formulated with a cationic, lipid-like structure, (G0-C14) was proposed for the co-encapsulation of siRNA with cisplatin [60]. Three major components define the structure of the novel platform: an aqueous inner core, a cationic and hydrophobic layer composed of PLGA and G0-C14, and a hydrophilic PEG corona. The G0-C14 compound is synthesized with cationic head groups that can efficiently bind siRNA via electrostatic interactions and flexible hydrophobic tails for self-assembly with PLGA-PEG to form Pt(IV)-prodrug encapsulating NPs. No direct interaction exists between the siRNA and the Pt(IV)-prodrug. The prodrug was released before siRNA. By using this delivery system, it was demonstrated that a combination of the cisplatin prodrug with REV1/REV3L-specific siRNAs, which suppress gene targets crucial to translesion synthesis (TLS) pathways, could synergistically result in tumor cell sensitization to cisplatin and tumor inhibition in a mouse model. The co-delivery of cisplatin and siRNA with minimal interactions was also achieved by our laboratory. We have developed lipid-coated calcium phosphate nanocores with efficient encapsulation of siRNA [30]. Separately, lipid-coated cisplatin nanocores were also formulated [9]. Inspired by the successful encapsulation of oleic acid-coated iron oxide NPs and quantum dots into PLGA NPs [148, 149], it was expected that these two lipid-coated, hydrophobic nanocores could be loaded simultaneously into PLGA NPs with minimal interaction between the two types of the cores. Thus, this formulation is expected to result in the minimal possible interaction between the encapsulated cisplatin and siRNA.
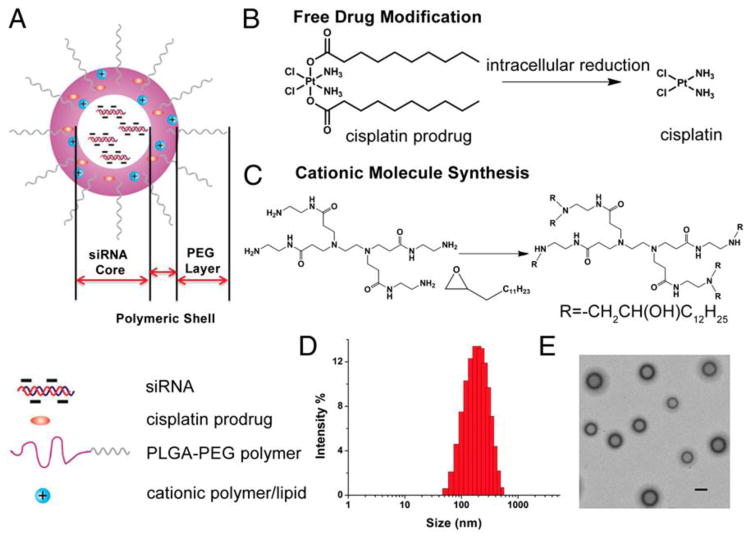
Figure 2. A novel platinum and siRNA combination formulation.
A. Schematic illustration of PLGA-PEG/G0-C14 NPs. The NPs are composed of three components: (i) a PEG layer, (ii) a PLGA/G0-C14 layer that encapsulate the hydrophobic cisplatin prodrug and protect siRNA from retention in the NP core, preventing the release and interaction, (iii) an aqueous inner core loaded with siRNA. (B) Chemical structure of the hydrophobic platinum (IV) prodrug and the mechanism by which the active drug cisplatin is released intracellularly. C. The chemistry of G0-C14 core synthesis. D. Size distribution of the NPs containing both platinum prodrug and siRNA. E. TEM image of the NPs. (Scale bar, 200 nm.). Copyright of PNAS [60].
While interactions between cisplatin and siRNA result in diminished efficacy of both therapeutics, other drug/siRNA interactions prove beneficial to the encapsulation of both agents. Specifically, a DOX/siRNA liposome-polycation-DNA complex (LPD) reported this mutualistic relationship. Mechanistically, Chen et al. reported that DOX forms a physical complex with nucleic acids, further promoting incorporation into PEGylated liposomes to form LPD particles (Figure 3). The non-covalent intercalation between siRNA and DOX furthered the co-encapsulation of both therapeutics. Additionally, therapy was augmented as well due to synergy between DOX and c-Myc siRNA [74, 150].
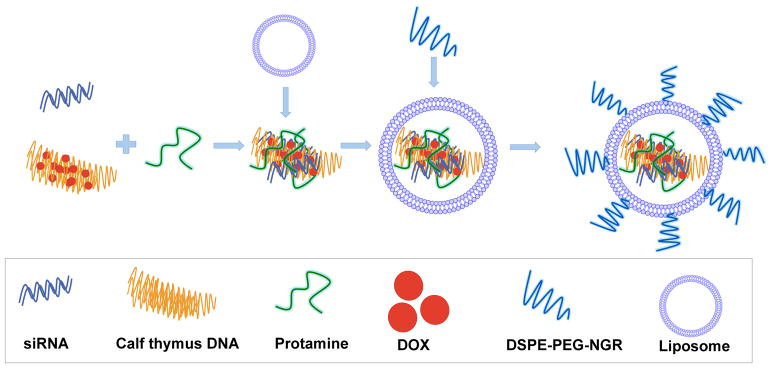
Figure 3. Illustration of preparation of LPD-PEG-NGR containing siRNA and DOX.
DOX was intercalated with DNA and used to construct PEGylated LPD (liposome-polycation-DNA) nanoparticle for siRNA delivery. Copyright 2010 Nature Group. [74]
4.2.5 siRNA and drug as a carrier
Drugs with cationic properties can also be utilized as carriers to condense and deliver siRNA. For instance, our lab has recently developed polymerized metformin (PolyMet), which functions through a similar therapeutic mechanism to metformin. The cationic structure of PolyMet can also be utilized to deliver siRNA (e.g. VEGF and Bcl-2) [71]. Based on this, metformin has been further exploited to conjugate with hydrophobic lipid chains to form cationic lipid-like drugs, and thus co-encapsulating siRNA or DNA for combinatory or synergistic purposes [135, 140] (Figure 4).
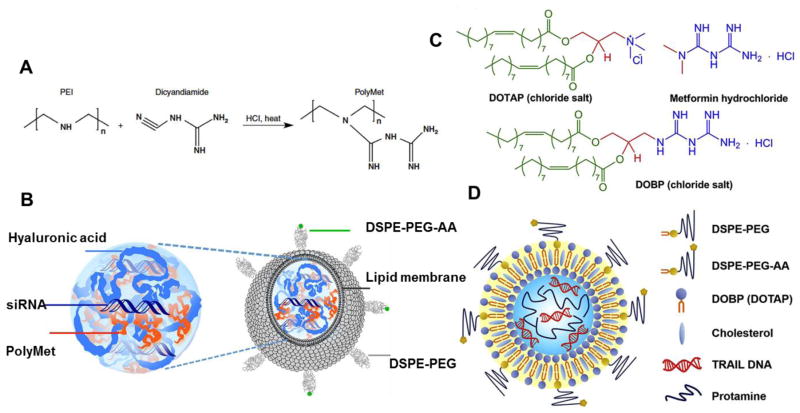
Figure 4. Metformin related drug carriers for gene delivery.
A. Chemical structure of PolyMet as both a drug and a gene carrier. B. Schematic illustration of PolyMet NPs. Anionic HA and siRNA were condensed by cationic PolyMet into a negatively charged PolyMet/(HA.siRNA) complex. The complex was further coated with DOTAP/cholesterol cationic liposomes. DSPE-PEG and DSPE-PEG-anisamide (AA) were post-inserted onto the particles to form the LPH-PolyMet NPs. C. Chemical structures of DOTAP, metformin and DOBP. D. Schematic illustration of LPD-TRAIL NPs using metformin-lipid as a carrier for gene delivery. Copyright of 2016 Nature group [71] and Elsevier [140].
4.2.6 Co-delivery of siRNA with miRNA
In addition to combining with small-molecule drugs, siRNA has also been applied together with microRNA (miRNA) to induce synergy. Two recent studies focused on restoring tumor suppressor genes and silencing of oncogenes. For example, lipidoid NPs 7C1 was formulated to encapsulate miR-34a and anti-Kras siRNA in an equal molar ratio to treat lung adenocarcinoma since lung adenocarcinoma is often associated with mutations in Kras and p53 [63]. The targeted inhibition of Kras expression and the stimulation of p53 effector functions are attractive strategies for lung adenocarcinoma [63]. The direct targeting of Kras and p53 using small molecules are elusive, yet promising targets for small RNA-based therapy. The miR-34 family of miRNAs are direct transcriptional targets of p53 and can stimulate the tumor suppressor pathway downstream of p53 [151]. Since most of the miRNA and siRNA have similar structures, the non-viral carrier forms complexes with miRNA/siRNA independent of nucleotide sequence, and thus the small RNA combination advances other therapy as a modular and flexible approach [61]. Another example is the combination of siEphA2, targeting the ovarian cancer oncogene siEphA2, with miR-520d-3p, as a tumor suppressor upstream of EphA2, using 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) liposomes. This combination showed synergistic antitumor efficiency and greater therapeutic efficacy than either of the mono-therapy alone [142]. Recently, a multiple siRNA/miRNA combination therapy has shown enhanced anti-cancer efficacy in the clinical trials [99, 152]. The combination of miRNA and siRNA technology is a significant step toward realizing the promising potential of small RNA therapies.
5. Lipid-coated, drug-loaded calcium phosphate nanoparticles for multi-purpose combinatory delivery
The lipid-coated calcium phosphate NP (LCP) platform developed by our lab is a platform with the capability to deliver disparate modalities with high efficiency and efficacy. The versatility and broad applicability of this platform lends itself well to act as an ideal carrier for multi-purpose combinatory applications. The LCP formulations can be applied in mono- or co- delivery cases of macromolecules (e.g. DNA, siRNA, peptide), or small-molecule drugs (gemcitabine, cisplatin). The nanocores developed during the first step of LCP synthesis could be incorporated into PLGA NPs for further combination with hydrophobic drugs, providing a broader platform for combination therapy.
5.1 Development of lipid-coated calcium phosphate nanoparticle platform
During the last two decades, calcium phosphate (CaP) has been widely used as a non-viral method for in vitro transfection of a wide variety of mammalian due to its biocompatibility, low cost, and easy preparation. CaP forms a complex with the nucleic acid backbone and imparts a stabilizing function to certain DNA structures. The complex can then be internalized into cells. However, the CaP/DNA complex suffers from instability due to the uncontrollable precipitation process, resulting in aggregation and rapid clearance from the blood by the reticuloendothelial system (RES) system.
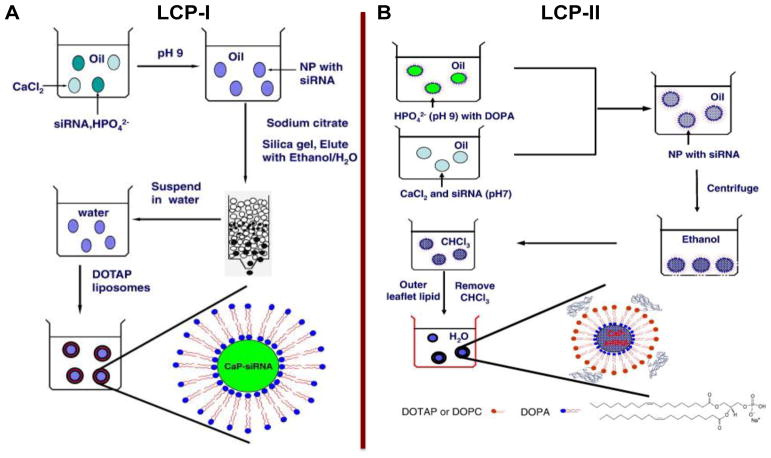
We developed a nanoparticulate CaP/siRNA delivery system using a microemulsion system with controlled size and improved stability for use in vitro and in vivo [153]. Calcium chloride/siRNA and NaHPO4 were separately dispersed into a reverse water-in-oil microemulsion. After mixing two microemulsions, sodium citrate was added dropwise to the micro-emulsion. Sodium citrate will adsorb onto the surface of CaP/siRNA NPs and enable the NPs negatively charged for further modification and preventing aggregation during purification. CaP/siRNA NPs was further purified using silica column chromatography. For intravenous administration, CaP/siRNA NPs were then coated with a dense layer of lipid and PEG. After being taken up by the cell, the CaP composite is dissolved in the acidic environment of the endosome and the encapsulated nucleic acid is released into the cytoplasm. This was the LCP-I formulation [154].
Since CaP cores in LCP-I are highly polar and require an un-scalable column method for purification, dioleoylphosphatydic acid (DOPA), an amphiphilic anionic lipid with strong binding affinity to calcium is further adapted to stabilize CaP NPs in microemulsion [155]. DOPA coating enables CaP NPs to disperse after centrifugation and dissolve in CHCl3. DOPA coated CaP NPs (nanocore) are quite homogeneous and approximately 15 nm in size. A hydrophobic layer of DOPA not only furthers surface-functionalized with amphiphilic lipids to ensure successful evasion of RES and efficient tumor targeting, but also allows versatile coating and surface modification for a variety of purposes, in a manner similar to oleic acid coated inorganic nanoparticles [148]. To further disperse the hydrophobic DOPA-coated CaP NPs into aqueous solution, additional lipids composed of DOTAP, cholesterol, DSPE-mPEG and DSPE-PEG-AA were used. These lipids self-assembled in water into the outer leaflet of the bilayer through a hydrophobic interaction with DOPA-coated CaP NPs to form LCP-II NPs, while the DOPA layer served as the inner leaflet of the asymmetric bilayer coating the CaP core (Figure 5).
Figure 5. Preparation of LCP-I (A) and LCP-II (B).
For LCP-I, phosphate salt (pH = 9.0) and siRNA are mixed and distributed in one emulsion, and sodium citrate was used to stabilize CaP cores; for LCP-II, calcium ion and siRNA are mixed and distributed in one emulsion, dioleoylphosphatydic acid (DOPA) was used to stabilize CaP cores. Copyright 2010 and 2012 Elsevier.
5.2 Delivery of macromolecular drug
5.2.1 Delivery of nucleic acid (siRNA and DNA)
LCP NPs were designed and synthesized for the delivery of siRNA from the beginning. LCP-I NPs encapsulated siRNA was found to dissolve followed by rapid release of payload in the endosomes as detected by the concentration of calcium released into the cytoplasm via Fura-2 AM assay [153]. LCP-I NPs loaded with anti-luciferase siRNA and modified with targeting ligand showed increased silencing efficiency compared to LPD formulations and non-targeted LCP-I NPs in vitro. In vivo, silencing efficiency was further confirmed in an H460-luc subcutaneous xenograft tumor model. An LCP-II delivery system with DOPA as a CaP stabilizer was developed as mentioned above [155]. Compared to LPD formulations loaded with anti-luc siRNA, LCP-II modified with the anisamide ligand showed significantly improved silencing efficiency.
To test the anticancer effects, LCP-II was evaluated in a subcutaneous, non-small cell lung cancer (NSCLC) model and a lung metastasis model [155, 156]. In the NSCLC model, LCP exhibited a 9-fold higher siRNA silencing efficiency compared to non-targeted LCP in vitro. To maximize anti-cancer effects, a combination of three siRNAs targeting HDM2, c-Myc and VEGF oncogenes was co-encapsulated into LCP-II NPs. LCP-II NPs efficiently silenced the target genes, significantly inhibiting tumor cell growth in A549 tumors. In the metastasis model, anti-luciferase siRNA formulated in LCP-II NP significantly decreased luciferase activity in metastatic B16F10 tumor-loaded lungs.
We also attempted to deliver plasmid DNA (pDNA) to hepatocytes using LCP-II NPs [31]. To enhance delivery of pDNA into hepatocytes and prolong circulation of NPs in blood, a dense layer of polyethylene glycol tethered to galactose was coated onto LCP-II NPs. It is demonstrated that 111In-labeled LCP distributed rapidly and primarily to liver. CaP itself is not very efficient in transfecting cells due to low efficiency of pDNA transport into the nucleus. To overcome this barrier, we included CaP cores, cysteine-flanked octaarginine peptides (Cys-(Arg)8-Cys, CR8C) which efficiently condensed pDNA. Inclusion of such peptides in LCP was sufficient to promote a high degree of nuclear translocation of pDNA. Notably, our results present a step toward the outperformance of hydrodynamic injection.
5.2.2 Co-delivery of phosphorylated peptide and ODN
Tyrosinase-related protein 2 (Trp2) has been identified as a melanoma-associated antigen that is also expressed by normal melanocytes. To break immune tolerance and improve the efficiency of a Trp2 peptide vaccine, our strategy is to co-deliver the antigen along with CpG ODN as a potent adjuvant [157]. To efficiently encapsulate the Trp2 peptide, we have introduced two phosphate groups into serine residues at the N-terminus. The encapsulation efficiency of the p-Trp2 peptide was ~50% and the efficiency of CpG ODN encapsulation was about 40%. Vaccination with LCP-II encapsulating p-Trp2 and CpG exhibited superior inhibition of tumor growth in both B16F10 subcutaneous and lung metastasis models [158].
5.2.3 Delivery of small-molecule drugs
To load small-molecule drugs using LCP-II NP, phosphorylation modification is a prerequisite for successful loading. Gemcitabine is a nucleoside analog prodrug and undergoes sequential phosphorylation to gemcitabine triphosphate (GTP) within cells and inhibits DNA synthesis. Taking advantage of this mechanism, gemcitabine was phosphorylated to GTP and encapsulated into LCP-II NPs with 25% efficiency [32]. Therapeutic studies showed that GTP-loaded LCPs triggered effective apoptosis of tumor cells and significant reduction of tumor cell proliferation, leading to dramatic inhibition of tumor growth with little toxicity. Similarly, another nucleoside analog, acyclovir, with phosphorylation modification has been successfully encapsulated into LCP-II NPs [159]. Remarkably, this anti-herpes simplex virus (HSV) drug, when loaded into LCP-II NPs, showed significant anticancer effect on H460 xenograft tumors.
5.2.4 Co-delivery of a small-molecule drug and a macromolecular drug
Gemcitabine monophosphate (GMP) and anti-c-Myc siRNA were co-encapsulated into single LCP-II NPs to treat aggressive non-small-cell lung cancer (NSCLC) [32, 160]. Anti-c-Myc siRNA and GMP were encapsulated with 55% and 75% efficiency, respectively. The combination did not alter the encapsulation efficiency of either drug. In subcutaneous and orthotopic lung cancer models, (anti-c-Myc siRNA+GMP) loaded LCP NPs showed superior tumor inhibition to GMP-LCP, c-Myc-LCP-AA and their mixture. Similarly, anti-VEGF siRNA was co-encapsulated into LCP NPs with GMP to treat NSCLC and exhibited anti-angiogenesis effects by decreasing the density of tumor microvessels (MVD) and showed potent anti-cancer effects on subcutaneous and orthotopic lung cancer models. These studies demonstrate the importance of formulating synergistic drug combinations into a single carrier, and LCPs show a great potential in this regard.
5.2.5 Lipid-coated platinum drug nanoparticle
Cisplatin is an inorganic compound widely used as first-line chemotherapy in the clinic. Poor aqueous solubility and insolubility in organic solvents complicate the development of nanoparticulate formulations to overcome these issues. Inspired by the synthesis of LCP-II NP and taking advantage of the poor aqueous solubility of cisplatin, lipid-coated cisplatin nanoparticles (LPC NP) were developed [161, 162]. Different from LCP-II NPs, the core of LPC NP is composed of pure cisplatin, synthesized by reacting a cisplatin precursor with potassium chloride in a microemulsion. Similar to the calcium ion, the platinum cation has a strong binding affinity for the phosphate groups of the lipid, DOPA. Therefore, DOPA is used to stabilize cisplatin cores during synthesis and purification. Drug loading of LPC NPs is 82 wt%. Hydrophobic DOPA-coated CDDP cores with a size of 12 nm were further coated with outer leaflet lipids to form LPC NPs. LPC NPs exhibited significant antitumor therapeutic activity without inducing nephrotoxicity. The flexibility of LPC NPs may allow for encapsulation of other insoluble drugs, such as etoposide[163].
5.3 Combination of diagnostic nanoparticles and drugs in PLGA NPs
Oleic acid-coated iron oxide nanoparticles (IONPs) and quantum dots (QDs) have been successfully loaded into PLGA NPs along with hydrophobic drugs for theranostic application [148, 164]. For example, Préat et al. co-encapsulated PTX and IONPs into PLGA NPs, which inhibited the growth of CT26 tumors [165]. In our lab, we have synthesized a series of DOPA coated drugs containing CaP cores and DOPA-coated CDDP cores (nanocores) [32, 155, 161]. Drugs encapsulated into CaP cores include siRNA, DNA, ODN, GTP, GMP and peptide. Inspired by the successful encapsulation of INOPs in PLGA NPs, we have showed that cisplatin nanocores and gemcitabine nanocores, or hydrophobic drugs (e.g. rapamycin) could be efficiently encapsulated into PLGA NPs with high efficiency[9, 33]. This provides an approach to load drugs with controlled ratio and another strategy to co-deliver hydrophilic drugs with hydrophobic drugs.
6. Tumor microenvironment and sequential/cascade or separate anti-cancer therapy
6.1 Rationale and significance of targeting tumor microenvironment for combination therapy
The aforementioned traditional combination therapy (either small-molecule, or NP-based combination) mainly focuses on overcoming unicellular drug resistance, with limited cases solving the issues related with de novo drug resistance. However, recent studies have demonstrated that specific niches within the TME may provide a sanctuary for subpopulations of tumor cells, leading to a survival advantage following initial drug exposure and facilitating the acquisition of both innate and acquired drug resistance (Figure 6) [16, 18, 166]. TME refers to non-malignant stroma cells that co-exist with the neoplastic epithelial cells, a diverse milieu of cytokines, growth factors, hormones and components of the ECM [18, 167]. The TME-induced drug resistance can be attributed to multiple reasons. Firstly, limited and compressed vasculatures impair blood supply, resulting in diffusion-limited hypoxia and low interstitial pH within the tumor region, which further aids the progression and metastasis of remaining cells by inducing genetic instability, angiogenesis, inflammation, autophagy and altered metabolism [168–171]. Secondly, soluble factors within the TME, such as TGF-β, nitric oxide (NO), vascular endothelial growth factor (VEGF), etc, negatively regulate drug response [18]. In addition, the interaction among tumor, stroma cells and the ECM contribute to both the innate and acquired drug resistance. For example, the host environment provides a chemo-protective niche through cell adhesion to the ECM, which is known as cell adhesion mediated drug resistance (CAM-DR) [172, 173]. This process is usually mediated though ECM-derived adhesive signals such as the integrin family to modulating the apoptosis and proliferating of tumor cells [174–176]. Furthermore, crosstalk between tumor cells and stroma cells, especially tumor-associated fibroblasts (TAFs) can induce acquired or innate resistance of tumor cells through paracrine secretion of survival factors such as CXCL12[177], Wnt16[178], HGF[171], FAK[179], and so on.
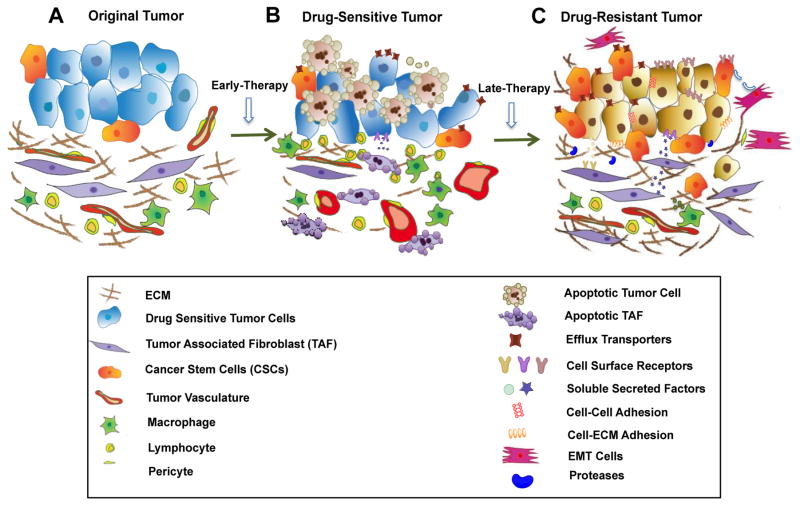
Figure 6. Drug resistance mechanism.
In original tumors, tumor cells remain intimately with surrounding microenvironment. High interstitial fluid pressure and compression of blood vessels limit penetration of drugs. Small subpopulation of CSCs serves as an intrinsic resistant component (A). Efficient chemotherapy leads to apoptosis of TAF and tumor cells and normalizing of tumor vasculature. However, elevated expression of efflux receptors, activated tumor cellular pathways and solute factors secreted from damaged fibroblast start to induce initial drug resistance (B). In late treatment, protective microenvironment facilitates development of acquired drug resistance through cell-ECM adhesion, cellular interaction, secreted factors or proteases. EMT and CSCs also add to the permanent resistance and failure of chemotherapy.
In addition to initiating multidrug resistance and supporting the tumorigenic process, a permissive microenvironment also acts as a physical barrier that limits the penetration of therapeutic agents, thus inhibiting anti-cancer efficacy [172]. While the perfusion of irregular and compressed tumor vasculature affects the vascular transport and trans-vascular delivery of drugs and therapeutic NPs, the organization and composition of the stroma cells and the ECM limits interstitial penetration of the extravasated drug or NPs [172]. The high IFP and increased solid stress further inhibits successful NP extravasation. Consequently, the limited NP perfusion within tumor compromises the therapeutic efficacy of the nanocarriers, leading to the ultimate failure of the treatment. Furthermore, non-specifically delivering chemotherapy to non-tumor stroma cells underlines the potential of inducing chemotherapeutic resistance due to their disparate response towards treatment [180].
Therefore, the complicated TME suggests that NP-based co-delivery of multiple agents for tumor cell-specific targeting and killing is not sufficient to overcome microenvironment barriers. Combination therapy with one regimen targeting the TME-associated drug resistance and another regimen targeting the tumor holds higher promise to synergistically improve the therapeutic outcome of cancer treatment. To this end, the TME modulating agent can be a small-molecule agent with low systemic toxicity and greater intratumoral permission, or therapeutic NPs/monoclonal antibodies specifically targeting a particular subpopulation of stroma cells. To improve the efficiency of combination therapy, the pair of combinatory agents is often loaded in separate carriers with disparate properties and targets. Cascade or sequential delivery is also performed with the first administration designed to modulate or prime the TME for higher perfusion and lower resistance before administration of the second-wave to achieve more efficient cancer-specific killing [22].
6.2 Targeting blood vessels/endothelia and cascade/sequential/separate nanoparticle therapy
Vascular and trans-vascular transport are the initial steps for intratumoral delivery of small- molecule drugs or NPs. Unlike normal vessels, which are orderly, tumor vessels are tortuous and chaotic in their organization [19]. Blood perfusion in tumors is significantly lower than surrounding normal tissue due to the compression. The impaired and heterogeneous perfusion not only limits oxygen supply, resulting in a hypoxic TME, but also lowers the perfusion of systemic drugs and the infiltration of effector immune cells [173]. Therefore, modifying vessel diameter or permeability would be efficient strategy to achieve optimal drug perfusion, and is considered as a promising combination strategy. Stylianopoulos et al. summarized that in tumors with hypopermeable and compressed vessels (e.g., desmoplastic pancreatic ductal adenocarcinomas), solid stress alleviation is more beneficial (discussed later in the review) to achieve better NP penetration; whereas, vascular normalization is more effective for tumors with hyperpermeable but largely uncompressed vessels (e.g., glioblastomas) [181]. Proangiogenic molecules, including VEGF, fibroblast growth factor (FGF) and PDGF are often overexpressed in tumors and favor angiogenesis [172, 173]. In the meantime, the angiogenetic process is counterbalanced by antiangiogenic molecules, such as thrombospondins [182]. However, during cancer progression, this balance is tipped in favor of new vessel formation, but the resulting vessels are highly abnormal. Jain et al. proposed that through mopping up excess VEGF using mAb or blocking VEGF signaling, they could restore this balance [19]. For example, bevacizumab (Avastin), the first approved anti-angiogenic mAb, and its derivative, ranibizumab have been used in the treatment of metastatic colorectal cancer. VEGF receptor blocking antibody DC101 was also applied to prune immature vessels and resize the remaining vessels, leading to a normalized vasculature. We further hypothesized, that the normalized blood vessels with better function would enhance both the delivery and effectiveness of concurrent therapies, which was also supported by Jain’s preclinical and clinical study [21, 183]. For example, bevacizumab increased survival when used in conjunction with chemotherapy or immunotherapy for clinically metastatic lung, colorectal and kidney cancers [20, 184–187]. Furthermore, siRNA against VEGF has been loaded with chemotherapy (e.g. gemcitabine, PTX) simultaneously in one NPs for combinatory angiogenesis effect and antitumor efficacy. This co-delivery is reasonable to an extent that both tumor endothelial cells (TECs) and epithelial malignant tumor cells overexpressing VEGF could respond to the combinatory treatment simultaneously. However, recent study suggests that delivery of a chemotherapeutic agent within the blood vessel normalization window would be more beneficial for anticancer treatment, providing the rationale for sequential or separate delivery of blood vessel remodeling agent together with chemotherapeutic NPs [19]. Normalization windows were analyzed to achieve the optimal combinatory effect [19].
During angiogenesis, the cytoskeletons of newly formed tumor vasculature are composed of microtubules. It has been hypothesized that when microtubule polymerization is inhibited by combretastatin A4 phosphate (CA4P), a microtubule inhibitor, the flat structure of TECs is compromised, causing them to occlude the lumen of the blood vessel [9]. Destruction of blood vessel integrity has been shown to cause hemorrhage and subsequent necrosis of the surrounding region. Recently, we have found that intratumoral hemorrhage induced by CA4P allows for an increased accumulation of NPs throughout the tumor, suggesting another potential of combination therapy by inducing vascular hemorrhage. Satterlee et al. observed that NPs dosed 3 h after CA4P treatment allows increased amount of particles penetrating into the tumor region due the initiation of vascular hemorrhage. In consistent with the hypothesis, sequential delivery of CA4P with 177Lutetium (177Lu)-loaded Lipid-Calcium-Phosphate NPs (177Lu-LCP NPs) synergistically inhibited the progression of desmoplastic bladder cancer xenografts [188]. Based on the similar rationale, Sengupta et al have proposed a nanoshell drug delivery system for the sequential release of combretastatin A4 and anticancer drug DOX. The release of combretastatin A4 from the pegylated-lipid outer shell induced vascular hemorrhage, which facilitated the delivery and trapping of the cores containing DOX within the tumor [189].
TECs are therapeutic targets for both anti-angiogenic and vascular hemorrhage therapy. Under most circumstances, they also are one of the first stromal cells within TME that encounter systemic delivered therapeutic drugs and NPs. Akiyama et al. demonstrated that PTX treatment of TECs induced up-regulation of MDR1 in neighbored malignant cells, suggesting the role of TECs in contributing to MDR after a chemotherapy induced phenotypic shift. To this end, a combination of VEGFR kinase inhibitor, Ki8751, or a phosphatidylinositol 3-kinase–Akt inhibitor, LY294002 with PTX was utilized to block the MDR-related interaction between TECs and tumor cells, synthetizing tumor cells to PTX treatment [190].
Collectively, for the purpose of improving the penetration and efficacy of a sequentially delivered therapeutic drug or NPs, tumor blood vessels or TECs could be normalized using normalization agents, e.g. VEGFR inhibitors, or destroyed entirely using the small-molecule drugs such as CA4P and combretastatin A4. The normalization and destruction windows should be carefully monitored to achieve an optimal microenvironment for the additional agent. During the chemotherapy-mediated treatment, the effect of chemotherapy to TECs should also be evaluated in case of generating TECs-mediated acquired MDR.
6.3 Degradation of ECM proteins enhances nanoparticle and drug delivery
Biological and physical interactions exist between the ECM and cells. With the gradual secretion of ECM proteins, the physical tension between the cell and the ECM is increased. On one hand, increases in matrix stiffness are likely to induce transformation of mammary epithelial cells [17]. Epithelial cells sense force through mechanoreceptors and respond by clustering integrins, activating the Rho/Rock-dependent mechanical tension in their actin cytoskeleton and furthering adhesions to ECM. These changes further drive the nuclear translocation of β-catenin, perturbing tissue polarity and ultimately lead to enhanced progression [175, 191]. The increased stiffness has also been implicated in the modulation of chemotherapeutic resistance [176]. Therefore, a decrease in tissue stiffness, such as utilizing selective ROCK inhibitor Y27632, or the inhibition of collagen crosslinking can synergistically prevent malignant growth when combining with chemotherapeutics [174, 191].
Furthermore, the matrix stiffness also contributes to solid stress within the tumor mass, compressing the intratumoral vessels, producing hypoxia and limiting blood perfusion. Likewise, it also increases IFP, impairing the delivery of drug and NPs. As mentioned in the previous section, in hypo-vascular tumor with compressed blood vessels, degradation of the ECM is an optimal strategy to improve the perfusion of NPs. Since collagen is the major component of the ECM, cascade/sequential or concurrent delivery of collagenase alongside nano-therapeutics have been reported to enhance the intratumoral transport of NPs. For example, local intratumoral injection of bacterial collagenase with HSV vector enhanced the intratumoral distribution of the HSV vector in a melanoma xenograft [192]. In another case, intravenous injection of collagenase-I led to the enhanced gene expression of lipoplex in xenograft tumors, further demonstrating the synergy between collagen degradation and improved NP penetration [193]. However, systemic injection of collagenase suffers from fast clearance and immune-toxicity. Hormone relaxin was utilized as a substitute of bacterial collagenase for in vivo application due to the better safety and longer systemic circulation [172, 173]. Enhanced diffusion was observed by using hormone relaxin [194], due to the stimulation of collagenase synthesis and inhibition of collagen production [195, 196].
Similar to collagen, hyaluronan (HA) is also a key matrix element that induces vascular destruction [197]. In pancreatic ductal carcinoma (PDA), where HA constitutes 70% of the ECM, degradation of HA sensitizes tumors to chemotherapy [198]. In a genetically engineered mouse model of PDA, a PEGylated recombinant human PH20 hyaluronidase (PEG-PH20), significantly improved the vascular perfusion of small-molecule drugs, such as DOX and gemcitabine [198]. In another case, the perfusion of DOX was improved by almost 4-fold when hyaluronidase was applied in a human osteosarcoma xenograft [172, 173, 199]. Therefore, degradation of HA is another promising combination strategy to sensitize the therapeutic efficacy of both small-molecule drugs and therapeutic NPs [198].
6.4 Targeting perivascular fibroblasts and adipocytes to improve the cascade/sequential/separate nanoparticle therapy
Pericytes are a major constituent of non-tumor stroma cells covered microvessels. For hyperpermeable tumors with uncompressed vessels and low pericyte-coverage (e.g. melanoma, colon cancer and ovarian cancer), NP extravasation can be enhanced via decreasing the nonfunctional vessels while increasing the pericyte coverage of the functional vessels. VEGF-A is a negative regulator of pericyte function and vessel maturation. As expected, Kano et al showed that Sorafenib, a VEGF-A inhibitor, increased the extravasation of 2MDa dextran in a CT26 colon cancer model. The combination therapy also effectively enhanced the penetration and therapeutic efficacy of Doxil. For desmoplastic tumors with extensively high coverage of pericytes (pancreatic, diffuse-type gastric cancer), low-dose TGF-β inhibitor inhibits pericyte proliferation, and thus improves the efficacy of Doxil. In another case, Meng et al. has used a two-wave therapy to treat BXPC3 pancreatic cancer. They showed that the first wave using TGF-β inhibitor LY364947 loaded MSN reduced the pericyte-coverage of blood vessels. And then, the second wave therapy with gemcitabine loaded MSN exhibited significant anticancer efficacy as compared to either facet of mono-therapy [22].
Fibroblasts are another group of peri-vascular cells, especially in stroma-vessel desmoplastic tumors where blood vessels are embedded in the stroma area aligning the fibroblasts [200]. Due to the anatomical architecture and localization of fibroblasts in the stroma-vessel tumors, we demonstrated that fibroblasts are the major cell population that trap NPs that are extravasated from blood vessels [200]. This labels the fibroblast as a major binding site barrier for NP delivery. In addition, direct or paracrine interaction between TAFs and tumor cells also enhance cancer cell proliferation, invasion, and contribute to immune suppression and innate resistance to chemotherapy [201]. Therefore, direct elimination of fibroblasts was hypothesized to block the tumor-TAF interaction and increase the interstitial transport of NPs. In this light, the delivery of lipid coated cisplatin NP and docetaxel conjugates to fibroblast through the binding site barrier effected great therapeutic outcomes in the treatment of desmoplastic bladder and breast cancer xenografts through depletion of fibroblasts [202, 203]. Since TAFs are overexpressing the fibroblast activation protein (FAP) on the surface, the depleting of fibroblasts could also be achieved using FAP substrate conjugated drugs and FAP antibody conjugated immuno-liposomes [204, 205]. In a recent study, DNA vaccines targeting FAP are also developed to deplete TAFs [206].
However, recent investigations into desmoplastic cancers showed that depletion of fibroblasts led to tumor progression, indicating a paradoxical effect of TAF depletion [172, 173, 207]. Depletion of fibroblasts runs the risk of eliminating key stromal components that secreted from TAFs and required for tissue homeostasis, and thus facilitating the outgrowth of tumor cells [172, 173]. Meanwhile, chronic exposure of fibroblasts to chemotherapy leads to acquired resistance, affecting the survival of neighboring tumor cells. A recent study by Girotti et al. supported this hypothesis [208]. Intravital imaging of BRAF-mutant melanoma cells containing an ERK/MAPK biosensor revealed the paradoxical activation of melanoma-associated fibroblasts by a BRAF inhibitor, PLX4720. Their study suggested that PLX4720 promoted matrix production and fibroblasts remodeling, leading to elevated integrin β1/FAK/Src signaling in melanoma cells owing to the crosstalk between melanoma and stroma cells. To this end, they proposed a combination of PLX4720 with either integrin β1 or FAK depletion, to prevent ERK/MAPK re-activation, synergistically inducing efficient cell death [179, 208, 209]. Furthermore, the off-target distribution of DNA damaging agents to TAFs may facilitate paracrine secretion of survival factors such as Wnt16, eliciting drug resistance in neighboring cells [178, 210]. Consistent with this observation, we showed that a combination of siRNA against Wnt16 NPs with cisplatin NPs sensitized desmoplastic bladder cancer to cisplatin NPs treatment [210]. In addition, the novel concept of reprograming of TAFs to the quiescent state has become another promising strategy to improve the second-wave NP delivery [211, 212]. Recent studies by Diop-Frimpong et al. have utilized an angiotensin receptor inhibitor, Losartan, to block TGF-β and decrease the synthesis of collagen I, and thus normalizing the TAFs to quiescent stage while improving the intratumoral delivery of small-molecule drugs and NPs [213]. The success of this formulation has led to a phase II clinical trial of Losartan together with FOLFIRINOX in patients [214, 215]. Another approach for fibroblast reprogramming is to target the Vitamin D Receptor (VDR). Sherman et al. demonstrated that the VDR ligand acts as a transcriptional regulator of fibroblasts to revert the stroma to quiescent state, reducing the ECM content and increasing vascular perfusion [211]. We have also combined the method of reprogramming TAFs with the engineering of TAFs. Recently, the binding site barrier effect of fibroblasts was used to deliver plasmids encoding secretable TNF-related apoptosis-inducing ligand (TRAIL) to fibroblasts [216]. The anisamide targeted NP showed enhanced accumulation in fibroblasts due to overexpression of the sigma receptor. To this end, fibroblasts can be engineered to act as a reservoir for protein production. The TRAIL protein secreted from fibroblasts efficiently diminished neighboring tumor cells. Interestingly, the apoptotic tumor cells reciprocally reprogrammed TAFs to normal fibroblasts, further facilitating the delivery of second wave chemotherapy. On the basis of engineering fibroblasts for protein generation, future studies should focus on modifying fibroblasts to macrophage like cells, providing us hope for in situ engineering using NP delivered agents to convert fibroblasts into natural killers [217].
6.5 Combining nanoparticle-based cancer vaccines with TME modulating agents
6.5.1 Rationales and significance of combination therapy
The immune system plays a critical role in fighting against tumors. Cancer immunotherapy can be developed with more effective and safer engineering strategies using approaches based on NPs. Vaccines are one of the major strategies in cancer immunotherapy. Cancer vaccines stimulate cytotoxic T cells to kill malignancies overexpressing cancer-associated or related antigens while establishing immunologic memory of the antigens. The use of NPs in vaccine formulations allows not only improved antigen stability and immunogenicity, but also targeted delivery and slow release [218]. In addition to abnormal blood vessels and stroma cells, there are a variety of innate and adaptive immune cells within TME that constitute a vaccine suppressive microenvironment [17, 219]. Major immune-suppressive cells include regulatory T cells (Treg) and myeloid suppressor cells (MDSC) [29]. These cells function together to develop an immune-suppressive TME that limits the activity and penetration of effective T cells, compromising the efficacy of cancer vaccines. Thus, the combination of NP-based vaccines with TME modulating agents facilitates better penetration of NPs into the tumor site, infiltration of T cells through multiple barriers and ultimately stronger therapeutic outcome. With enhanced targeted delivery to lymphocytes as well as tumors, NPs offer the potential limit off-target toxicities often associated with immune modulators [220].
6.5.2 Combining NP-based cancer vaccines with TME modulating agents
Antigen-presenting cells (APC), especially dendritic cells (DCs), play a crucial role in the response to vaccines. Specifically, they govern the activation of CD4+ and CD8+ T cells. In nano-vaccination, NPs deliver not only antigens of interest but also co-adjuvants, such as poly(I:C), CpG, and MPL, to act specifically on DC activation. The key application of vaccine NP encapsulating either peptides, mRNA or DNA is to modulate APC function. By themselves, NPs may also work as intrinsic adjuvants, immune potentiators, or to promote multivalent receptor cross-linking through intracellular uptake. NP also functions for cytosolic processing and synergistic co-delivery of antigens and drugs. Through size-based delivery of vaccines to lymph nodes, more effective antigen presentation is achieved than through distal administration to peripheral tissues [220]. Moreover, NPs may also penetrate mucosal epithelial and skin barriers for vaccines and immunomodulation. In pre-clinical studies, our lab has co-delivered the melanoma-associated antigen Tyrosinase-related protein 2 (Trp2) and CpG oligonucleotides (ODN) adjuvant as vaccine NPs [29]. Two phosphate groups were introduced into serine residues in the N-terminus of Trp2 peptide to achieve an encapsulation efficiency of over 50%. The LCP NPs were further modified with mannose to enhance and prolong cargo deposit into lymph nodes for antigen presenting post subcutaneous injection. Vaccination with LCP NPs encapsulating p-Trp2 and CpG exhibited superior inhibition of tumor growth in both B16F10 subcutaneous and lung metastasis models. However, poor tumor growth inhibition has been observed in more advanced models due to the development of an immune-suppressive TME. TME modulating agents, such as immunomodulatory antibodies, small-molecule drugs, and recombinant cytokines, could be used to block or remold negative regulatory factors within the tumor nest [221]. For instance, the use of immunomodulatory antibodies, or checkpoint blockade antibodies such as Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), would block negative regulatory receptors on T cells, allowing stronger endogenous immune response against tumors brought up by therapeutic vaccines [222]. In achievement of synergistic therapy with Trp2 peptide vaccine, we have successfully co-delivered: a) multi-target receptor tyrosine kinase inhibitor sunitinib base into polymeric micelles (SUNb-PM), [223] b) Anti-inflammatory triterpenoid methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate (CDDO-Me) into PLGA NPs, [224] c) siRNA silencing immune-suppressive cytokines TGF-β into liposome-protamine-hyaluronic acid (LPH) NPs, [29] and d) Curcumin–polyethylene glycol conjugate into an amphiphilic micelle [225]. The targeted delivery of these TME modulating agents to tumors would enhance the therapeutic efficacy of the existing vaccine, leading to increased T cell activation and proliferation, decreased MDSCs and Tregs in the TME and shifted cytokine expression from the Th2 to Th1 type, thus achieving a more profound inhibitory effect on tumor growth and metastasis, improving overall survival. Recently, a plasmid DNA encoding engineered C-X-C Motif Chemokine Ligand 12 protein (CXCL12 trap) was engineered into LCP NPs for local and transient gene therapy of trapping pro-metastatic TME chemokine CXCL12, thus inhibit the metastasis of colorectal cancer the liver [226]. The “trapping” with high binding affinity would further boost vaccination, as well as evade systemic toxicity compared to conventional protein antibodies, thus facilitates immunotherapy. In addition to the co-delivery of antigens and immune-modulators, modifying the surfaces of NPs can contribute to the delivery of antigens specifically to relevant immune cells [227]. For instance, Imiquimod (TLR7 agonist)-entrapped PLGA NPs coated with a chitosan derivative could improve the protective response generated by mucosal immunization [228]. Multifunctional NPs-based vaccines significantly increase the immune response generated by the target-specific, effective, and stable delivery of an antigen [228]. In combination with targeted modification of TME, immunotherapy offers a flexible and powerful platform for both the mechanism study and clinical pharmaceutical applications.
7. Light based combination therapy methods
Although NP-mediated co/concurrent or sequential/cascade delivery of multiple chemotherapeutic agents or genes have been extensively explored and summarized above, the NP-based combination therapy of chemotherapy with other modalities, such as photodynamic (PDT) or photothermal therapy (PTT) is still a burgeoning research area. PDT, a light-triggered therapeutic method, is an effective modality against multidrug resistant tumors. In PDT, the photosensitizer (PS) generates reactive oxygen species in response to light, inducing direct lipid and protein damage, leading to mitochondrial-induced apoptosis that bypasses classical MDR mechanisms. It also disrupts the membrane of endo-lysosome, releasing trapped chemo-drug into cytoplasm, protecting them from enzyme-induced drug degradation or elimination. PDT has been applied to clinical trials due to its specificity to malignancy [132, 229, 230]. Therefore, “PDT+chemotherapy” has been hypothesized and shown to be a powerful combination strategy in destroying cancer tissues. So far, versatile materials have been applied to encapsulate chemotherapy with photosensitizers, such as synthetic polymers [231], lipids, inorganic materials [136] or biomacromolecule scaffolds. However, due to the particular structure of photosensitizers (i.e. strong ππ stacking interaction), the drug loading capacities of such systems are comparatively low (typically less than 10 %), not only reducing effective drug accumulation, but also leading to undesirable effects due to overdose of the carrier. Interestingly, Zhang et al. reported a carrier-free photosensitizer doped Forster Resonance Energy Transfer (FRET) NP to achieve the desired function with high drug loading capacity. Curcumin constitutes the matrix of the NP with approximately 77.6% of loading. Curcumin is then doped with the hydrophobic photosensitizer H2TPyP together with perylene as a FRET pair [229]. High drug loading can also be achieved by covalently conjugating photosensitizers with the polymeric carrier to improve the hydrophobicity [230]. Chemotherapy can also be combined with PTT, which converts light energy into heat that kills the tumor. The FDA-approved drug, indocyanine green (ICG), is an attractive PTT agent to absorb near infrared light to generate heat. With the ability of photosensitizer, ICG allows the combination of PTT with PDT. ICG has been encapsulated into, or coated onto inorganic particles, polymeric micelles for multiple purposes [232]. Metals such as gold (Au) or silver (Ag) can also generate heat through the so-called “surface plasmon” phenomenon. Combining chemotherapy with the metal carriers have also been frequently investigated. Despite the more localized and lesser off-target toxic effect of PTT and PDT compared to the systemic delivery of chemotherapy, the low penetration depth of the light source is one major limitation of the application. So far, these two strategies are limited to topical application.
Carrier-mediated combination therapy is not only limited to two components (e.g. chemodrug/siRNA, chemodrug/chemodrug, chemodrug/PDT or PTT, chemodrug tumor microenvironment agent, vaccine/tumor microenvironment modulating agent, etc.). In a recent study, Conde and his co-workers have reported a local-triple combination of gene, drug and phototherapy (chemodrug/siRNA/PTT) through a prophylactic hydrogel patch leads (Figure 7) [233]. They have observed complete remission when applying patches to non-resected colorectal tumors.
Figure 7.
Triple components loaded in implantable hydrogels for local treatment of colon cancer. A. scheme of the drug, gold nanorods and siRNA–gold nanospheres doped implantable hydrogels. b, Transmission electron micrographs of gold nanorods with an aspect ratio of 4.1 and nanospheres. Copyright of 2016 Nature Group [233].
Summary and expectations
Despite these promising pre-clinical results of NP-mediated combination therapy, clinically applied NP-based combination therapy is still rare. Combined formulation of drugs with distinct chemical and physical properties remains a challenge. The capacity of NP formulations to penetrate the tumor is still limited by the TME, and this contributes to MDR in several ways. The heterogeneity within tumor and the microenvironment, the mutation of tumor during the process as well as the individual disparities are all overarching problems limiting the overall therapeutic performance and clinical application of the NP-mediated combination therapy. So far, there are no optimal ways to solve the aforementioned issues, thus tumor relapse and metastasis occurs after chronic combination therapy despite the latency time being longer than that of mono-therapy. The incorporation of cancer immunotherapy within the combination regimens still poses as one of the most promising approaches to improve the therapeutic outcome and prolong the patient survival. However, recent preclinical and clinical studies underline the potential of inducing immunogenicity or autoimmune diseases due to the application of cancer immunotherapy. In addition to treatment, another more plausible application of combination therapy is for efficient and high throughput screening of lead compounds or lead formulations. Combination of RNAs with different sequences, together with model drugs has the potential for screening of optimal drugs using the RNA as a specific barcode. This approach would very likely to offer a flexible and powerful platform for both mechanistic studies and clinical screening.
Acknowledgments
The work was supported by NIH grants CA151652, CA151455 and CA149363.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jabr-Milane LS, van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer treatment reviews. 2008;34:592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochemical pharmacology. 2012;83:1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Kohli M, Smith A. Nanoparticles for combination drug therapy. ACS nano. 2013;7:9518–9525. doi: 10.1021/nn405674m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer DS, Puranik AS, Peppas NA. Intelligent nanoparticles for advanced drug delivery in cancer treatment. Curr Opin Chem Eng. 2015;7:84–92. doi: 10.1016/j.coche.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo ST, Miao L, Wang YH, Huang L. Unmodified drug used as a material to construct nanoparticles: delivery of cisplatin for enhanced anti-cancer therapy. Journal of Controlled Release. 2014;174:137–142. doi: 10.1016/j.jconrel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo S, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnology advances. 2014;32:778–788. doi: 10.1016/j.biotechadv.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urologic oncology. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Valencia PM, Pridgen EM, Perea B, Gadde S, Sweeney C, Kantoff PW, Bander NH, Lippard SJ, Langer R, Karnik R, Farokhzad OC. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine (Lond) 2013;8:687–698. doi: 10.2217/nnm.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Lin CM, Xu Z, Miao L, Wang Y, Huang L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS nano. 2014;8:4996–5009. doi: 10.1021/nn5010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu CM, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeutic delivery. 2010;1:323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 11.Jia J, Zhu F, Ma X, Cao Z, Li Y, Chen YZ. Mechanisms of drug combinations: interaction and network perspectives. Nature reviews. Drug discovery. 2009;8:111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 12.Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Advanced drug delivery reviews. 2016;98:19–34. doi: 10.1016/j.addr.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman RM, Bouvet M. Nanoparticle albumin-bound-paclitaxel: a limited improvement under the current therapeutic paradigm of pancreatic cancer. Expert opinion on pharmacotherapy. 2015;16:943–947. doi: 10.1517/14656566.2015.1016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang NJ, Chao TY, Hsieh RK, Wang CH, Wang YW, Yeh CG, Chen LT. A phase I dose-escalation study of PEP02 (irinotecan liposome injection) in combination with 5-fluorouracil and leucovorin in advanced solid tumors. BMC cancer. 2016;16:907. doi: 10.1186/s12885-016-2933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanai O, Fujita K, Nakatani K, Mio T. Repetitive responses to nanoparticle albumin-bound paclitaxel and carboplatin in malignant pleural mesothelioma. Respirology case reports. 2016;4:28–31. doi: 10.1002/rcr2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlehurst LA, Landowski TH, Dalton WS. Role of the tumor microenvironment in mediating de novo resistance to drugs and physiological mediators of cell death. Oncogene. 2003;22:7396–7402. doi: 10.1038/sj.onc.1206943. [DOI] [PubMed] [Google Scholar]
- 17.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer research. 2012;72:2473–2480. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. The New England journal of medicine. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 21.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 22.Meng H, Zhao Y, Dong J, Xue M, Lin YS, Ji Z, Mai WX, Zhang H, Chang CH, Brinker CJ, Zink JI, Nel AE. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS nano. 2013;7:10048–10065. doi: 10.1021/nn404083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. The Journal of clinical investigation. 2013;123:1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug discovery today. 2012;17:1044–1052. doi: 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG, Zhong Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 26.Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin S, Wang X, Zhao Y, Ji Z, Zink JI, Nel AE. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanafi-Bojd MY, Ansari L, Malaekeh-Nikouei B. Codelivery of anticancer drugs and siRNA by mesoporous silica nanoparticles. Therapeutic delivery. 2016;7:649–655. doi: 10.4155/tde-2016-0045. [DOI] [PubMed] [Google Scholar]
- 28.Mo R, Jiang T, Gu Z. Recent progress in multidrug delivery to cancer cells by liposomes. Nanomedicine (Lond) 2014;9:1117–1120. doi: 10.2217/nnm.14.62. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS nano. 2014;8:3636–3645. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. Journal of controlled release: official journal of the Controlled Release Society. 2012;158:108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Haynes MT, Wang Y, Liu F, Huang L. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS nano. 2013;7:5376–5384. doi: 10.1021/nn4012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Peng L, Mumper RJ, Huang L. Combinational delivery of c-myc siRNA and nucleoside analogs in a single, synthetic nanocarrier for targeted cancer therapy. Biomaterials. 2013;34:8459–8468. doi: 10.1016/j.biomaterials.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao L, Guo S, Zhang J, Kim WY, Huang L. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Advanced functional materials. 2014;24:6601–6611. doi: 10.1002/adfm.201401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy - Strategies and perspectives. Journal of controlled release: official journal of the Controlled Release Society. 2016;240:489–503. doi: 10.1016/j.jconrel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyer AK, Singh A, Ganta S, Amiji MM. Role of integrated cancer nanomedicine in overcoming drug resistance. Advanced drug delivery reviews. 2013;65:1784–1802. doi: 10.1016/j.addr.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 37.Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Current drug targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda Y, Schuetz JD. ABC transporters and their role in nucleoside and nucleotide drug resistance. Biochemical pharmacology. 2012;83:1073–1083. doi: 10.1016/j.bcp.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kale Mohana Raghava Srivalli PKL. Overview of P-glycoprotein inhibitors: a rational outlook. Brazilian Journal of Pharmaceutical Sciences. 2012;48:15. [Google Scholar]
- 40.Xiao B, Ma L, Merlin D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert opinion on drug delivery. 2016:1–9. doi: 10.1080/17425247.2016.1205583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubensack M, Muller C, Hocherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. Journal of cancer research and clinical oncology. 2008;134:597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PubMed] [Google Scholar]
- 42.Song XR, Cai Z, Zheng Y, He G, Cui FY, Gong DQ, Hou SX, Xiong SJ, Lei XJ, Wei YQ. Reversion of multidrug resistance by co-encapsulation of vincristine and verapamil in PLGA nanoparticles. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2009;37:300–305. doi: 10.1016/j.ejps.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Neerati P, Sudhakar YA, Kanwar JR. Curcumin Regulates Colon Cancer by Inhibiting P-Glycoprotein in In-situ Cancerous Colon Perfusion Rat Model. Journal of cancer science & therapy. 2013;5:313–319. [PMC free article] [PubMed] [Google Scholar]
- 44.Chavanpatil MD, Patil Y, Panyam J. Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoprotein-mediated drug efflux. International journal of pharmaceutics. 2006;320:150–156. doi: 10.1016/j.ijpharm.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 45.Lee CK, Ki SH, Choi JS. Effects of oral curcumin on the pharmacokinetics of intravenous and oral etoposide in rats: possible role of intestinal CYP3A and P-gp inhibition by curcumin. Biopharmaceutics & drug disposition. 2011;32:245–251. doi: 10.1002/bdd.754. [DOI] [PubMed] [Google Scholar]
- 46.Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer letters. 2008;259:111–118. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, Hicklin D, Kerbel RS. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:221–232. [PubMed] [Google Scholar]
- 48.Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Molecular pharmaceutics. 2009;6:928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 49.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalyoncu E, Olmez TT, Ozkan AD, Sarioglu OF. Biosystems Engineering of Prokaryotes with Tumor-Killing Capacities. Current pharmaceutical design. 2016;22:1521–1528. doi: 10.2174/1381612822666151210123752. [DOI] [PubMed] [Google Scholar]
- 51.Yanase K, Tsukahara S, Asada S, Ishikawa E, Imai Y, Sugimoto Y. Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Molecular cancer therapeutics. 2004;3:1119–1125. [PubMed] [Google Scholar]
- 52.Saunders NA, Simpson F, Thompson EW, Hill MM, Endo-Munoz L, Leggatt G, Minchin RF, Guminski A. Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO molecular medicine. 2012;4:675–684. doi: 10.1002/emmm.201101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moufarij MA, Phillips DR, Cullinane C. Gemcitabine potentiates cisplatin cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Molecular pharmacology. 2003;63:862–869. doi: 10.1124/mol.63.4.862. [DOI] [PubMed] [Google Scholar]
- 54.Besancon OG, Tytgat GA, Meinsma R, Leen R, Hoebink J, Kalayda GV, Jaehde U, Caron HN, van Kuilenburg AB. Synergistic interaction between cisplatin and gemcitabine in neuroblastoma cell lines and multicellular tumor spheroids. Cancer letters. 2012;319:23–30. doi: 10.1016/j.canlet.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Xie K, Doles J, Hemann MT, Walker GC. Error-prone translesion synthesis mediates acquired chemoresistance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20792–20797. doi: 10.1073/pnas.1011412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. The EMBO journal. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright JA, Keegan KS, Herendeen DR, Bentley NJ, Carr AM, Hoekstra MF, Concannon P. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and abrogate cell cycle checkpoint control. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7445–7450. doi: 10.1073/pnas.95.13.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vendetti FP, Lau A, Schamus S, Conrads TP, O’Connor MJ, Bakkenist CJ. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget. 2015;6:44289–44305. doi: 10.18632/oncotarget.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moufarij MA, Phillips DR, Cullinane C. Gemcitabine potentiates cisplatin cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Mol Pharmacol. 2003;63:862–869. doi: 10.1124/mol.63.4.862. [DOI] [PubMed] [Google Scholar]
- 60.Xu XY, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, Shi JJ, Wu J, Kantoff PW, Lippard SJ, Langer R, Walker GC, Farokhzad OC. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18638–18643. doi: 10.1073/pnas.1303958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. Journal of controlled release: official journal of the Controlled Release Society. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond) 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue W, Dahlman JE, Tammela T, Khan OF, Sood S, Dave A, Cai W, Chirino LM, Yang GR, Bronson R, Crowley DG, Sahay G, Schroeder A, Langer R, Anderson DG, Jacks T. Small RNA combination therapy for lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3553–3561. doi: 10.1073/pnas.1412686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, Yuan YW, Zhang JR, Zhou DY. Up-regulation of c-myc expression in MCF-7/Adr human breast cancer cells and its association with resistance against doxorubicin. Di 1 jun yi da xue xue bao = Academic journal of the first medical college of PLA. 2002;22:124–126. [PubMed] [Google Scholar]
- 65.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Molecular pharmacology. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 66.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Down-regulation of pregnane X receptor contributes to cell growth inhibition and apoptosis by anticancer agents in endometrial cancer cells. Molecular pharmacology. 2007;72:1045–1053. doi: 10.1124/mol.107.037937. [DOI] [PubMed] [Google Scholar]
- 67.Lee E, Oh C, Kim IS, Kwon IC, Kim S. Co-delivery of chemosensitizing siRNA and an anticancer agent via multiple monocomplexation-induced hydrophobic association. Journal of controlled release: official journal of the Controlled Release Society. 2015;210:105–114. doi: 10.1016/j.jconrel.2015.05.262. [DOI] [PubMed] [Google Scholar]
- 68.Lima RT, Martins LM, Guimaraes JE, Sambade C, Vasconcelos MH. Specific downregulation of bcl-2 and xIAP by RNAi enhances the effects of chemotherapeutic agents in MCF-7 human breast cancer cells. Cancer gene therapy. 2004;11:309–316. doi: 10.1038/sj.cgt.7700706. [DOI] [PubMed] [Google Scholar]
- 69.Godwin P, Baird AM, Heavey S, Barr MP, O’Byrne KJ, Gately K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Frontiers in oncology. 2013;3:120. doi: 10.3389/fonc.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Wang Q, Qin L, Fu H, Fang Y, Han B, Duan Y. EGF-modified mPEG-PLGA-PLL nanoparticle for delivering doxorubicin combined with Bcl-2 siRNA as a potential treatment strategy for lung cancer. Drug delivery. 2016;23:2936–2945. doi: 10.3109/10717544.2015.1126769. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Wang W, Guo S, Wang Y, Miao L, Xiong Y, Huang L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nature communications. 2016;7:11822. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ganesh S, Iyer AK, Weiler J, Morrissey DV, Amiji MM. Combination of siRNA-directed Gene Silencing With Cisplatin Reverses Drug Resistance in Human Non-small Cell Lung Cancer. Molecular therapy. Nucleic acids. 2013;2:e110. doi: 10.1038/mtna.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng C, Zheng M, Gong P, Deng J, Yi H, Zhang P, Zhang Y, Liu P, Ma Y, Cai L. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials. 2013;34:3431–3438. doi: 10.1016/j.biomaterials.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Wu JJ, Huang L. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:828–834. doi: 10.1038/mt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borah A, Raveendran S, Rochani A, Maekawa T, Kumar DS. Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis. 2015;4:e177. doi: 10.1038/oncsis.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 77.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 79.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 80.Zhang C, An T, Wang D, Wan G, Zhang M, Wang H, Zhang S, Li R, Yang X, Wang Y. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(beta-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. Journal of controlled release: official journal of the Controlled Release Society. 2016;226:193–204. doi: 10.1016/j.jconrel.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 81.Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Ji Z, Chang CH, Nel AE. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS nano. 2015;9:3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Tian Y, Zhu Z, Xu H, Li X, Zheng D, Sun W. Efficient antitumor effect of co-drug-loaded nanoparticles with gelatin hydrogel by local implantation. Scientific reports. 2016;6:26546. doi: 10.1038/srep26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiang CT, Yeh PY, Gao M, Chen CW, Yeh LC, Feng WC, Kuo SH, Hsu CH, Lu YS, Cheng AL. Combinations of mTORC1 inhibitor RAD001 with gemcitabine and paclitaxel for treating non-Hodgkin lymphoma. Cancer letters. 2010;298:195–203. doi: 10.1016/j.canlet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Konings IR, Verweij J, Wiemer EA, Sleijfer S. The applicability of mTOR inhibition in solid tumors. Current cancer drug targets. 2009;9:439–450. doi: 10.2174/156800909788166556. [DOI] [PubMed] [Google Scholar]
- 85.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer letters. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Xiao B, Si X, Han MK, Viennois E, Zhang M, Merlin D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. Journal of materials chemistry. B, Materials for biology and medicine. 2015;3:7724–7733. doi: 10.1039/c5tb01245g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang H, Geng D, Liu H, Li Z, Cao J. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors. Drug delivery. 2016:1–9. doi: 10.1080/10717544.2016.1217954. [DOI] [PubMed] [Google Scholar]
- 88.Kang X, Zhao C, Yan L, Qi R, Jing X, Wang Z. Sensitizing nanoparticle based platinum(IV) drugs by curcumin for better chemotherapy, Colloids and surfaces. B. Biointerfaces. 2016;145:812–819. doi: 10.1016/j.colsurfb.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 89.Sreekanth CN, Bava SV, Sreekumar E, Anto RJ. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene. 2011;30:3139–3152. doi: 10.1038/onc.2011.23. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Yang C, Wang W, Liu J, Liu Q, Huang F, Chu L, Gao H, Li C, Kong D, Liu Q, Liu J. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Scientific reports. 2016;6:21225. doi: 10.1038/srep21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duan J, Mansour HM, Zhang Y, Deng X, Chen Y, Wang J, Pan Y, Zhao J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. International journal of pharmaceutics. 2012;426:193–201. doi: 10.1016/j.ijpharm.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 92.Dasanu CA, Herzog TJ, Alexandrescu DT. Carboplatin-gemcitabine in the therapy of advanced ovarian cancer: dose reduction consideration. Journal of oncology pharmacy practice: official publication of the International Society of Oncology Pharmacy Practitioners. 2010;16:63–66. doi: 10.1177/1078155209105396. [DOI] [PubMed] [Google Scholar]
- 93.Goette DK. Topical chemotherapy with 5-fluorouracil. A review. Journal of the American Academy of Dermatology. 1981;4:633–649. doi: 10.1016/s0190-9622(81)80196-x. [DOI] [PubMed] [Google Scholar]
- 94.Aryal S, Hu CM, Zhang L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Molecular pharmaceutics. 2011;8:1401–1407. doi: 10.1021/mp200243k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang T, Sun W, Zhu Q, Burns NA, Khan SA, Mo R, Gu Z. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Advanced materials. 2015;27:1021–1028. doi: 10.1002/adma.201404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaspar VM, Moreira AF, Costa EC, Queiroz JA, Sousa F, Pichon C, Correia IJ. Gas-generating TPGS-PLGA microspheres loaded with nanoparticles (NIMPS) for co-delivery of minicircle DNA and anti-tumoral drugs, Colloids and surfaces. B. Biointerfaces. 2015;134:287–294. doi: 10.1016/j.colsurfb.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Pacardo DB, Ligler FS, Gu Z. Programmable nanomedicine: synergistic and sequential drug delivery systems. Nanoscale. 2015;7:3381–3391. doi: 10.1039/c4nr07677j. [DOI] [PubMed] [Google Scholar]
- 98.Dong Z, Feng L, Zhu W, Sun X, Gao M, Zhao H, Chao Y, Liu Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60–70. doi: 10.1016/j.biomaterials.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 99.Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends in molecular medicine. 2015;21:223–232. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Batist G, Gelmon KA, Chi KN, Miller WH, Jr, Chia SK, Mayer LD, Swenson CE, Janoff AS, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 102.Wang B, Yu XC, Xu SF, Xu M. Paclitaxel and etoposide co-loaded polymeric nanoparticles for the effective combination therapy against human osteosarcoma. Journal of nanobiotechnology. 2015;13:22. doi: 10.1186/s12951-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishra GP, Nguyen D, Alani AW. Inhibitory effect of paclitaxel and rapamycin individual and dual drug-loaded polymeric micelles in the angiogenic cascade. Molecular pharmaceutics. 2013;10:2071–2078. doi: 10.1021/mp400122m. [DOI] [PubMed] [Google Scholar]
- 104.Liao L, Liu J, Dreaden EC, Morton SW, Shopsowitz KE, Hammond PT, Johnson JA. A convergent synthetic platform for single-nanoparticle combination cancer therapy: ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. Journal of the American Chemical Society. 2014;136:5896–5899. doi: 10.1021/ja502011g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y, Chen F, Pan Y, Li Z, Xue X, Okeke CI, Wang Y, Li C, Peng L, Wang PC, Ma X, Liang XJ. Nanodrug Formed by Coassembly of Dual Anticancer Drugs to Inhibit Cancer Cell Drug Resistance. ACS applied materials & interfaces. 2015;7:19295–19305. doi: 10.1021/acsami.5b05347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao H, Song H, Yang Q, Cai H, Qi R, Yan L, Liu S, Zheng Y, Huang Y, Liu T, Jing X. A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials. 2012;33:6507–6519. doi: 10.1016/j.biomaterials.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 107.Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, Li Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS nano. 2013;7:5858–5869. doi: 10.1021/nn4010796. [DOI] [PubMed] [Google Scholar]
- 108.Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci U S A. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Valencia PM, Pridgen EM, Perea B, Gadde S, Sweeney C, Kantoff PW, Bander NH, Lippard SJ, Langer R, Karnik R, Farokhzad OC. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine. 2012;8:687–698. doi: 10.2217/nnm.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vong LB, Nagasaki Y. Combination Treatment of Murine Colon Cancer with Doxorubicin and Redox Nanoparticles. Molecular pharmaceutics. 2016;13:449–455. doi: 10.1021/acs.molpharmaceut.5b00676. [DOI] [PubMed] [Google Scholar]
- 111.Ma Y, Liu D, Wang D, Wang Y, Fu Q, Fallon JK, Yang X, He Z, Liu F. Combinational delivery of hydrophobic and hydrophilic anticancer drugs in single nanoemulsions to treat MDR in cancer. Molecular pharmaceutics. 2014;11:2623–2630. doi: 10.1021/mp400778r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao P, Xia G, Dong S, Jiang ZX, Chen M. An iTEP-salinomycin nanoparticle that specifically and effectively inhibits metastases of 4T1 orthotopic breast tumors. Biomaterials. 2016;93:1–9. doi: 10.1016/j.biomaterials.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, Hennink WE, Storm G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30:3466–3475. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 114.Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 115.Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Advanced drug delivery reviews. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 116.Langer CJ, O’Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, Ciuleanu TE, Orlov SV, Dediu M, Heigener D, Eisenfeld AJ, Sandalic L, Oldham FB, Singer JW, Ross HJ. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2008;3:623–630. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 117.Dai W, Jin W, Zhang J, Wang X, Wang J, Zhang X, Wan Y, Zhang Q. Spatiotemporally controlled co-delivery of anti-vasculature agent and cytotoxic drug by octreotide-modified stealth liposomes. Pharm Res. 2012;29:2902–2911. doi: 10.1007/s11095-012-0797-2. [DOI] [PubMed] [Google Scholar]
- 118.Zucker D, Andriyanov AV, Steiner A, Raviv U, Barenholz Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: relating structure and pharmacokinetics to therapeutic efficacy. J Control Release. 2012;160:281–289. doi: 10.1016/j.jconrel.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 119.Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li F, Zhao X, Wang H, Zhao R, Ji T, Ren H, Anderson GJ, Nie G, Hao J. Multiple Layer-by-Layer Lipid-Polymer Hybrid Nanoparticles for Improved FOLFIRINOX Chemotherapy in Pancreatic Tumor Models. Advanced functional materials. 2015;25:788–798. [Google Scholar]
- 121.Liu D, Bimbo LM, Makila E, Villanova F, Kaasalainen M, Herranz-Blanco B, Caramella CM, Lehto VP, Salonen J, Herzig KH, Hirvonen J, Santos HA. Co-delivery of a hydrophobic small molecule and a hydrophilic peptide by porous silicon nanoparticles. J Control Release. 2013;170:268–278. doi: 10.1016/j.jconrel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 122.Ashley CE, Carnes EC, Phillips GK, Padilla D, Durfee PN, Brown PA, Hanna TN, Liu J, Phillips B, Carter MB, Carroll NJ, Jiang X, Dunphy DR, Willman CL, Petsev DN, Evans DG, Parikh AN, Chackerian B, Wharton W, Peabody DS, Brinker CJ. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10:389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao Y, Jaskula-Sztul R, Javadi A, Xu W, Eide J, Dammalapati A, Kunnimalaiyaan M, Chen H, Gong S. Co-delivery of doxorubicin and siRNA using octreotide-conjugated gold nanorods for targeted neuroendocrine cancer therapy. Nanoscale. 2012;4:7185–7193. doi: 10.1039/c2nr31853a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials. 2010;31:3694–3706. doi: 10.1016/j.biomaterials.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 125.Wilson KD, de Jong SD, Kazem M, Lall R, Hope MJ, Cullis PR, Tam YK. The combination of stabilized plasmid lipid particles and lipid nanoparticle encapsulated CpG containing oligodeoxynucleotides as a systemic genetic vaccine. The journal of gene medicine. 2009;11:14–25. doi: 10.1002/jgm.1267. [DOI] [PubMed] [Google Scholar]
- 126.Liu D, Bimbo LM, Makila E, Villanova F, Kaasalainen M, Herranz-Blanco B, Caramella CM, Lehto VP, Salonen J, Herzig KH, Hirvonen J, Santos HA. Co-delivery of a hydrophobic small molecule and a hydrophilic peptide by porous silicon nanoparticles. Journal of controlled release: official journal of the Controlled Release Society. 2013;170:268–278. doi: 10.1016/j.jconrel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 127.Tian J, Rodgers Z, Min Y, Wan X, Qiu H, Mi Y, Tian X, Wagner KT, Caster JM, Qi Y, Roche K, Zhang T, Cheng J, Wang AZ. Nanoparticle delivery of chemotherapy combination regimen improves the therapeutic efficacy in mouse models of lung cancer. Nanomedicine: nanotechnology, biology, and medicine. 2016 doi: 10.1016/j.nano.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao B, Han MK, Viennois E, Wang L, Zhang M, Si X, Merlin D. Hyaluronic acid-functionalized polymeric nanoparticles for colon cancer-targeted combination chemotherapy. Nanoscale. 2015;7:17745–17755. doi: 10.1039/c5nr04831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aditya NP, Aditya S, Yang H, Kim HW, Park SO, Ko S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food chemistry. 2015;173:7–13. doi: 10.1016/j.foodchem.2014.09.131. [DOI] [PubMed] [Google Scholar]
- 130.Wu J, Tang C, Yin C. Co-delivery of doxorubicin and interleukin-2 via chitosan based nanoparticles for enhanced antitumor efficacy. Acta biomaterialia. 2017;47:81–90. doi: 10.1016/j.actbio.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 131.Ding Y, Su S, Zhang R, Shao L, Zhang Y, Wang B, Li Y, Chen L, Yu Q, Wu Y, Nie G. Precision combination therapy for triple negative breast cancer via biomimetic polydopamine polymer core-shell nanostructures. Biomaterials. 2017;113:243–252. doi: 10.1016/j.biomaterials.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 132.Zhang D, Wu M, Zeng Y, Wu L, Wang Q, Han X, Liu X, Liu J. Chlorin e6 Conjugated Poly(dopamine) Nanospheres as PDT/PTT Dual-Modal Therapeutic Agents for Enhanced Cancer Therapy. ACS applied materials & interfaces. 2015;7:8176–8187. doi: 10.1021/acsami.5b01027. [DOI] [PubMed] [Google Scholar]
- 133.Wang FZ, Xing L, Tang ZH, Lu JJ, Cui PF, Qiao JB, Jiang L, Jiang HL, Zong L. Codelivery of Doxorubicin and shAkt1 by Poly(ethylenimine)-Glycyrrhetinic Acid Nanoparticles To Induce Autophagy-Mediated Liver Cancer Combination Therapy. Molecular pharmaceutics. 2016;13:1298–1307. doi: 10.1021/acs.molpharmaceut.5b00879. [DOI] [PubMed] [Google Scholar]
- 134.Yin T, Wang L, Yin L, Zhou J, Huo M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials. 2015;61:10–25. doi: 10.1016/j.biomaterials.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 135.Xiong Y, Zhao Y, Miao L, Lin CM, Huang L. Co-delivery of polymeric metformin and cisplatin by self-assembled core-membrane nanoparticles to treat non-small cell lung cancer. Journal of controlled release: official journal of the Controlled Release Society. 2016;244:63–73. doi: 10.1016/j.jconrel.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang W, Shen J, Su H, Mu G, Sun JH, Tan CP, Liang XJ, Ji LN, Mao ZW. Co-Delivery of Cisplatin Prodrug and Chlorin e6 by Mesoporous Silica Nanoparticles for Chemo-Photodynamic Combination Therapy to Combat Drug Resistance. ACS applied materials & interfaces. 2016;8:13332–13340. doi: 10.1021/acsami.6b03881. [DOI] [PubMed] [Google Scholar]
- 137.Daglioglu C, Okutucu B. Synthesis and Characterization of AICAR and DOX Conjugated Multifunctional Nanoparticles as a Platform for Synergistic Inhibition of Cancer Cell Growth. Bioconjugate chemistry. 2016;27:1098–1111. doi: 10.1021/acs.bioconjchem.6b00080. [DOI] [PubMed] [Google Scholar]
- 138.Daglioglu C, Okutucu B. Therapeutic Effects of AICAR and DOX Conjugated Multifunctional Nanoparticles in Sensitization and Elimination of Cancer Cells via Survivin Targeting. Pharmaceutical research. 2016 doi: 10.1007/s11095-016-2053-7. [DOI] [PubMed] [Google Scholar]
- 139.Hernandez-Gil J, Cobaleda-Siles M, Zabaleta A, Salassa L, Calvo J, Mareque-Rivas JC. An Iron Oxide Nanocarrier Loaded with a Pt(IV) Prodrug and Immunostimulatory dsRNA for Combining Complementary Cancer Killing Effects. Advanced healthcare materials. 2015;4:1034–1042. doi: 10.1002/adhm.201500080. [DOI] [PubMed] [Google Scholar]
- 140.Luo C, Miao L, Zhao Y, Musetti S, Wang Y, Shi K, Huang L. A novel cationic lipid with intrinsic antitumor activity to facilitate gene therapy of TRAIL DNA. Biomaterials. 2016;102:239–248. doi: 10.1016/j.biomaterials.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dong Y, Eltoukhy AA, Alabi CA, Khan OF, Veiseh O, Dorkin JR, Sirirungruang S, Yin H, Tang BC, Pelet JM, Chen D, Gu Z, Xue Y, Langer R, Anderson DG. Lipid-like nanomaterials for simultaneous gene expression and silencing in vivo. Advanced healthcare materials. 2014;3:1392–1397. doi: 10.1002/adhm.201400054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nishimura M, Jung EJ, Shah MY, Lu C, Spizzo R, Shimizu M, Han HD, Ivan C, Rossi S, Zhang X, Nicoloso MS, Wu SY, Almeida MI, Bottsford-Miller J, Pecot CV, Zand B, Matsuo K, Shahzad MM, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Sood AK, Calin GA. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer discovery. 2013;3:1302–1315. doi: 10.1158/2159-8290.CD-13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nature reviews. Drug discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu P, Yu H, Sun Y, Zhu M, Duan Y. A mPEG-PLGA-b-PLL copolymer carrier for adriamycin and siRNA delivery. Biomaterials. 2012;33:4403–4412. doi: 10.1016/j.biomaterials.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 145.Guo ST, Qiao Y, Wang WW, He HY, Deng LD, Xing JF, Xu JQ, Liang XJ, Dong AJ. Poly(epsilon-caprolactone)-graft-poly(2-(N, N-dimethylamino) ethyl methacrylate) nanoparticles: pH dependent thermo-sensitive multifunctional carriers for gene and drug delivery. J Mater Chem. 2010;20:6935–6941. [Google Scholar]
- 146.Yue XY, Qiao Y, Qiao N, Guo ST, Xing JF, Deng LD, Xu JQ, Dong AJ. Amphiphilic Methoxy Poly(ethylene glycol)-b-poly(epsilon-caprolactone)-b-poly(2-dimethylaminoethyl methacrylate) Cationic Copolymer Nanoparticles as a Vector for Gene and Drug Delivery. Biomacromolecules. 2010;11:2306–2312. doi: 10.1021/bm100410m. [DOI] [PubMed] [Google Scholar]
- 147.Creixell M, Peppas NA. Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today. 2012;7:367–379. doi: 10.1016/j.nantod.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nehilla BJ, Allen PG, Desai TA. Surfactant-free, drug-quantum-dot coloaded poly(lactide-co-glycolide) nanoparticles: towards multifunctional nanoparticles. ACS nano. 2008;2:538–544. doi: 10.1021/nn700281b. [DOI] [PubMed] [Google Scholar]
- 149.Prashant C, Dipak M, Yang CT, Chuang KH, Jun D, Feng SS. Superparamagnetic iron oxide--loaded poly(lactic acid)-D-alpha-tocopherol polyethylene glycol 1000 succinate copolymer nanoparticles as MRI contrast agent. Biomaterials. 2010;31:5588–5597. doi: 10.1016/j.biomaterials.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 150.Chen Y, Bathula SR, Li J, Huang L. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. The Journal of biological chemistry. 2010;285:22639–22650. doi: 10.1074/jbc.M110.125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hermeking H. The miR-34 family in cancer and apoptosis. Cell death and differentiation. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 152.Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M, Gounder MM, Falzone R, Harrop J, White AC, Toudjarska I, Bumcrot D, Meyers RE, Hinkle G, Svrzikapa N, Hutabarat RM, Clausen VA, Cehelsky J, Nochur SV, Gamba-Vitalo C, Vaishnaw AK, Sah DW, Gollob JA, Burris HA., 3rd First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer discovery. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 153.Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. Journal of controlled release: official journal of the Controlled Release Society. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158:108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang Y, Hu Y, Wang Y, Li J, Liu F, Huang L. Nanoparticle Delivery of Pooled siRNA for Effective Treatment of Non-Small Cell Lung Caner. Mol Pharm. 2012;9:2280–2289. doi: 10.1021/mp300152v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Z, Ramishetti S, Tseng YC, Guo S, Wang Y, Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J Control Release. 2013;172:259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 158.Xu Z, Ramishetti S, Tseng YC, Guo S, Wang Y, Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. Journal of controlled release: official journal of the Controlled Release Society. 2013;172:259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 159.Yao J, Zhang Y, Ramishetti S, Wang Y, Huang L. Turning an antiviral into an anticancer drug: nanoparticle delivery of acyclovir monophosphate. J Control Release. 2013;170:414–420. doi: 10.1016/j.jconrel.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Y, Schwerbrock NM, Rogers AB, Kim WY, Huang L. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther. 2013;21:1559–1569. doi: 10.1038/mt.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guo S, Wang Y, Miao L, Xu Z, Lin CM, Zhang Y, Huang L. Lipid-coated Cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS nano. 2013;7:9896–9904. doi: 10.1021/nn403606m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo S, Miao L, Wang Y, Huang L. Unmodified drug used as a material to construct nanoparticles: delivery of cisplatin for enhanced anti-cancer therapy. J Control Release. 2014;174:137–142. doi: 10.1016/j.jconrel.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Srinivas R, Satterlee A, Wang Y, Zhang Y, Wang Y, Huang L. Theranostic etoposide phosphate/indium nanoparticles for cancer therapy and imaging. Nanoscale. 2015;7:18542–18551. doi: 10.1039/c5nr04509f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Y, Ng YW, Chen Y, Shuter B, Yi J, Ding J, Wang Sc, Feng SS. Formulation of Superparamagnetic Iron Oxides by Nanoparticles of Biodegradable Polymers for Magnetic Resonance Imaging. Adv Funct Mater. 2008;18:308–318. [Google Scholar]
- 165.Schleich N, Sibret P, Danhier P, Ucakar B, Laurent S, Muller RN, Jerome C, Gallez B, Preat V, Danhier F. Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int J Pharm. 2013;447:94–101. doi: 10.1016/j.ijpharm.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 166.Curtis LT, Frieboes HB. The Tumor Microenvironment as a Barrier to Cancer Nanotherapy. Advances in experimental medicine and biology. 2016;936:165–190. doi: 10.1007/978-3-319-42023-3_9. [DOI] [PubMed] [Google Scholar]
- 167.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer research. 2013;73:4965–4977. doi: 10.1158/0008-5472.CAN-13-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ostman A. The tumor microenvironment controls drug sensitivity. Nat Med. 2012;18:1332–1334. doi: 10.1038/nm.2938. [DOI] [PubMed] [Google Scholar]
- 169.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 170.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature reviews Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 171.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends in pharmacological sciences. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Miao L, Lin CM, Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:192–204. doi: 10.1016/j.jconrel.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miao L, Huang L. Exploring the tumor microenvironment with nanoparticles. Cancer treatment and research. 2015;166:193–226. doi: 10.1007/978-3-319-16555-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 176.Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, Benten D, Forbes SJ, Wells RG, Iredale JP. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Frame MC, Serrels A. FAK to the Rescue: Activated Stroma Promotes a “Safe Haven” for BRAF-Mutant Melanoma Cells by Inducing FAK Signaling. Cancer cell. 2015;27:429–431. doi: 10.1016/j.ccell.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 180.Li Y, Wang J, Wientjes MG, Au JL. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Advanced drug delivery reviews. 2012;64:29–39. doi: 10.1016/j.addr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jain RK. Taming vessels to treat cancer. Scientific American. 2008;298:56–63. doi: 10.1038/scientificamerican0108-56. [DOI] [PubMed] [Google Scholar]
- 184.Berretta M, Lleshi A, Zanet E, Bearz A, Simonelli C, Fisichella R, Nasti G, Berretta S, Tirelli U. Bevacizumab plus irinotecan-, fluorouracil-, and leucovorin-based chemotherapy with concomitant HAART in an HIV-positive patient with metastatic colorectal cancer. Onkologie. 2008;31:394–397. doi: 10.1159/000132360. [DOI] [PubMed] [Google Scholar]
- 185.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England journal of medicine. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 186.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 187.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 188.Song W, Tang Z, Zhang D, Yu H, Chen X. Coadministration of Vascular Disrupting Agents and Nanomedicines to Eradicate Tumors from Peripheral and Central Regions. Small. 2015;11:3755–3761. doi: 10.1002/smll.201500324. [DOI] [PubMed] [Google Scholar]
- 189.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 190.Akiyama K, Ohga N, Hida Y, Kawamoto T, Sadamoto Y, Ishikawa S, Maishi N, Akino T, Kondoh M, Matsuda A, Inoue N, Shindoh M, Hida K. Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment. The American journal of pathology. 2012;180:1283–1293. doi: 10.1016/j.ajpath.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 191.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, Barker N, Clevers H, Sansom OJ, Anderson KI, Weaver VM, Olson MF. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer research. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 193.Kato M, Hattori Y, Kubo M, Maitani Y. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. International journal of pharmaceutics. 2012;423:428–434. doi: 10.1016/j.ijpharm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 194.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. Journal of controlled release: official journal of the Controlled Release Society. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 195.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 196.Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265:10681–10685. [PubMed] [Google Scholar]
- 197.Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophysical journal. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eikenes L, Tari M, Tufto I, Bruland OS, de Lange Davies C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. British journal of cancer. 2005;93:81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miao L, Newby JM, Lin CM, Zhang L, Xu F, Kim WY, Forest MG, Lai SK, Milowsky MI, Wobker SE, Huang L. The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS nano. 2016 doi: 10.1021/acsnano.6b02776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 202.Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer research. 2013;73:4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 203.Zhang J, Miao L, Guo S, Zhang Y, Zhang L, Satterlee A, Kim WY, Huang L. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. Journal of controlled release: official journal of the Controlled Release Society. 2014;182:90–96. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.LeBeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther. 2009;8:1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Baum P, Muller D, Ruger R, Kontermann RE. Single-chain Fv immunoliposomes for the targeting of fibroblast activation protein-expressing tumor stromal cells. J Drug Target. 2007;15:399–406. doi: 10.1080/10611860701453034. [DOI] [PubMed] [Google Scholar]
- 206.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. The Journal of clinical investigation. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Girotti MR, Lopes F, Preece N, Niculescu-Duvaz D, Zambon A, Davies L, Whittaker S, Saturno G, Viros A, Pedersen M, Suijkerbuijk BMJM, Menard D, McLeary R, Johnson L, Fish L, Ejiama S, Sanchez-Laorden B, Hohloch J, Carragher N, Macleod K, Ashton G, Marusiak AA, Fusi A, Brognard J, Frame M, Lorigan P, Marais R, Springer C. Paradox-Breaking RAF Inhibitors that Also Target SRC Are Effective in Drug-Resistant BRAF Mutant Melanoma. Cancer cell. 2015;27:85–96. doi: 10.1016/j.ccell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Miao L, Wang Y, Lin CM, Xiong Y, Chen N, Zhang L, Kim WY, Huang L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. Journal of controlled release: official journal of the Controlled Release Society. 2015;217:27–41. doi: 10.1016/j.jconrel.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O’Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 213.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bild W, Hritcu L, Stefanescu C, Ciobica A. Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:79–88. doi: 10.1016/j.pnpbp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 215.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Miao L, Liu Q, Lin CM, Luo C, Wang Y, Liu L, Yin W, Hu S, Kim WY, Huang L. Targeting Tumor-associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer research. 2016 doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, Graf T. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 219.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nature reviews. Immunology. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical reviews. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Van der Jeught K, Bialkowski L, Daszkiewicz L, Broos K, Goyvaerts C, Renmans D, Van Lint S, Heirman C, Thielemans K, Breckpot K. Targeting the tumor microenvironment to enhance antitumor immune responses. Oncotarget. 2015;6:1359–1381. doi: 10.18632/oncotarget.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. Journal of leukocyte biology. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huo M, Zhao Y, Satterlee AB, Wang Y, Xu Y, Huang L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. Journal of controlled release: official journal of the Controlled Release Society. 2016;245:81–94. doi: 10.1016/j.jconrel.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao Y, Huo M, Xu Z, Wang Y, Huang L. Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials. 2015;68:54–66. doi: 10.1016/j.biomaterials.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lu Y, Miao L, Wang Y, Xu Z, Zhao Y, Shen Y, Xiang G, Huang L. Curcumin Micelles Remodel Tumor Microenvironment and Enhance Vaccine Activity in an Advanced Melanoma Model. Molecular therapy: the journal of the American Society of Gene Therapy. 2016;24:364–374. doi: 10.1038/mt.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Goodwin TJ, Zhou Y, Musetti SN, Liu R, Huang L. Local and transient gene expression primes the liver to resist cancer metastasis. Science translational medicine. 2016;8:364ra153. doi: 10.1126/scitranslmed.aag2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular pharmaceutics. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jia F, Liu X, Li L, Mallapragada S, Narasimhan B, Wang Q. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. Journal of controlled release: official journal of the Controlled Release Society. 2013;172:1020–1034. doi: 10.1016/j.jconrel.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 229.Zhang J, Liang YC, Lin X, Zhu X, Yan L, Li S, Yang X, Zhu G, Rogach AL, Yu PK, Shi P, Tu LC, Chang CC, Zhang X, Chen X, Zhang W, Lee CS. Self-Monitoring and Self-Delivery of Photosensitizer-Doped Nanoparticles for Highly Effective Combination Cancer Therapy in Vitro and in Vivo. ACS nano. 2015;9:9741–9756. doi: 10.1021/acsnano.5b02513. [DOI] [PubMed] [Google Scholar]
- 230.Jiang D, Gao X, Kang T, Feng X, Yao J, Yang M, Jing Y, Zhu Q, Feng J, Chen J. Actively targeting D-alpha-tocopheryl polyethylene glycol 1000 succinate-poly(lactic acid) nanoparticles as vesicles for chemo-photodynamic combination therapy of doxorubicin-resistant breast cancer. Nanoscale. 2016;8:3100–3118. doi: 10.1039/c5nr07724a. [DOI] [PubMed] [Google Scholar]
- 231.Chang JE, Cho HJ, Yi E, Kim DD, Jheon S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancer. Journal of photochemistry and photobiology. B, Biology. 2016;158:113–121. doi: 10.1016/j.jphotobiol.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 232.Cao M, Wang P, Kou Y, Wang J, Liu J, Li Y, Li J, Wang L, Chen C. Gadolinium(III)-Chelated Silica Nanospheres Integrating Chemotherapy and Photothermal Therapy for Cancer Treatment and Magnetic Resonance Imaging. ACS applied materials & interfaces. 2015;7:25014–25023. doi: 10.1021/acsami.5b06938. [DOI] [PubMed] [Google Scholar]
- 233.Conde J, Oliva N, Zhang Y, Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nature materials. 2016;15:1128–1138. doi: 10.1038/nmat4707. [DOI] [PMC free article] [PubMed] [Google Scholar]