Abstract
Rationale
The recruitment of the stress circuitry contributes to a shift from positive to negative reinforcement mechanisms sustaining long-term cocaine addiction. The kappa opioid receptor (KOPr) signaling is upregulated by stress and chronic cocaine exposure. While KOPr agonists induce anhedonia and dysphoria, KOPr antagonists display antidepressant and anxiolytic properties. Most of the knowledge on KOPr antagonism is based on drugs with unusual pharmacokinetic and pharmacodynamic properties, complicating interpretation of results. Here we characterized in vivo behavioral and neuroendocrine effects of the novel relatively short-acting KOPr antagonist LY2444296. To date, no study has investigated whether systemic KOPr blockade reduced anxiety-like and depressive-like behaviors in animals previously exposed to chronic extended access cocaine self-administration.
Objectives
We tested the effect of LY2444296 in blocking KOPr-mediated aversive and neuroendocrine effects. Then, we tested acute systemic LY2444296 in reducing anxiety- and depression-like behaviors, as well as releasing the stress hormone corticosterone (CORT), observed after chronic extended access (18 h/day for 14 days) cocaine self-administration.
Results
LY2444296 blocked both U69,593-induced place aversion and reduced motor activity, as well as U69,593-induced release of serum CORT, which confirmed its major site of action, without exerting an effect per se. Acute systemic administration of LY2444296 reduced anxiety-like and depressive-like behaviors, as well as CORT release, in rats tested after chronic extended access cocaine self-administration, but not in cocaine naïve rats.
Conclusions
Results suggest that acute blockade of KOPr by a relatively short-acting antagonist produces therapeutic-like effects selectively in rats with a history of chronic extended access cocaine self-administration.
Keywords: Anxiety, cocaine addiction, cocaine self-administration, cocaine withdrawal, corticosterone, depression, extended access self-administration, kappa opioid receptor OR KOR OR KOP OR KOP-r
Introduction
Cocaine addiction is a chronic relapsing disease characterized by compulsive drug intake and dysregulation of brain reward and stress systems. Addicted patients typically undergo a cycle with periods of active drug use, abstinence and relapse (Kreek and Koob 1998). Addiction is often comorbid with other psychiatric diseases, especially anxiety and depression, and considerable evidence supports the hypothesis that stress and depression are triggers for relapse and a reason for failure of patients to remain abstinent (Lalanne et al. 2014; Sinha et al. 2006). Indeed, the recruitment of stress-activated circuitries is thought to contribute to a “shift” from positive to negative reinforcement mechanisms, in the context of cocaine abuse (Kreek and Koob 1998; Picetti et al. 2013).
Currently, there are no approved medications for cocaine addiction, and classical antidepressants are usually ineffective treatments (Schmitz et al. 2001); therefore new pharmacological approaches are needed. A potential pharmacological target to improve quality of life and eventually reduce the vulnerability to relapse is the kappa opioid receptor (KOPr) (Butelman et al. 2012).
KOPr signaling tone is upregulated in depressive states, following both stress (Donahue et al. 2015; Land et al. 2008; Lucas et al. 2011; Reed et al. 2012) and chronic cocaine exposure, largely through increased mRNA transcription and activity of its endogenous peptides, the dynorphins (Fagergren et al. 2003; Sivam 1996; Spangler et al. 1996; Unterwald et al. 1994; Valenza et al. 2016). Activation of KOPr induces dysphoria in humans, as well as place aversion and depressive-like behaviors in preclinical models (Bruchas et al. 2009; Carlezon et al. 2006; Chefer et al. 2013; Land et al. 2008; Mucha and Herz 1985; Pfeiffer et al. 1986a; Todtenkopf et al. 2004). By contrast, KOPr antagonists alleviate anxiety-like and depressive-like behavior in rodents and can reduce stress-induced potentiation of cocaine conditioned place preference (CPP) as well as stress-induced reinstatement of cocaine CPP (Al-Hasani et al. 2013; Beardsley et al. 2005; Carr et al. 2010; Knoll et al. 2007; Mague et al. 2003; McLaughlin et al. 2003; Polter et al. 2014; Reed et al. 2012). However, most of the current knowledge on pharmacotherapeutic potential of KOPr antagonism is based on molecules (e.g. norBNI, JDTic) with extremely unusual pharmacokinetic and pharmacodynamic properties, including slow onset, delayed development of KOPr selectivity, and extended durations of action (approximately 2 months)(Butelman et al. 1993; Carroll et al. 2004; Zhou et al. 2015). These features complicate experimental designs, interpretation and translation of results into clinical investigations (Chavkin and Martinez 2015). Therefore, novel selective compounds with “medication-like” durations of action (<24 h) are useful to expand the understanding of dynorphin-KOPr involvement in the neurobiology of addiction (Carlezon and Krystal 2016; Rorick-Kehn et al. 2015; Rorick-Kehn et al. 2014). Several molecules have been recently synthetized for this purpose. Among them, LY2444296 ((S)3-fluoro-4-(4((2(3-fluorophenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide, known also as FP3FBZ) demonstrated high in vitro receptor binding affinity and selectivity for KOPr versus mu and delta opioid receptors (MOPr and DOPr, respectively). In the present study, we characterized in vivo, for the first time, the effectiveness of LY2444296 in blocking behavioral (aversion and locomotor activity) and neuroendocrine (serum corticosterone concentration) measurements induced by systemic administration of U69,593, a reference KOPr agonist.
KOPr signaling plays a crucial role in regulating reinforcing, dysphoric and anhedonic effects of chronic drugs of abuse, including cocaine. The long-acting KOPr antagonist norBNI, when administered during the self-administration period (sc, 30 min before test) attenuates intake and reduces progressive-ratio responding for cocaine in long-access sessions (Wee et al. 2009). However, in non-human primates, nor-BNI was ineffective in reducing cocaine intake in an extended access self-administration procedure of drug versus food choice (Hutsell et al. 2016). Chartoff et al. (2012) showed that in rats exposed to experimenter-administered “binge” cocaine (3 systemic cocaine injections/day for 14 days), intracerebroventricular (i.c.v.) pretreatment with norBNI attenuates the development of anhedonia, measured as increased intracranial self-stimulation threshold. In the same study, norBNI (i.c.v., 5 days before test) reduced the latency to immobility in the Forced Swim test.
To date, no study has demonstrated the effectiveness of systemic KOPr blockade administered as a pharmacotherapeutic-like intervention in tests performed after chronic extended access cocaine self-administration. Therefore, we tested the potential effect of LY2444296 in reducing anxiety-like and depressant-like responses, as well as its effect on stress hormone release (serum corticosterone), in rats previously exposed to chronic extended access cocaine self-administration (18 h/day for 14 days). Extended access self-administration protocols are translational models for the advanced stages of addiction seen in humans, where dysregulated drug intake and compulsivity drive behaviors (Ahmed and Koob 1998; Everitt and Robbins 2005; Kreek and Koob 1998). Rats that escalate cocaine intake over the 14 days of 18 h/day self-administration show higher striatal gene expression of Oprk1, encoding for KOPr, compared to their respective yoked-saline rats, and compared to rats that do not escalate intake (Valenza et al. 2016). Here, we also confirmed the activation of dyn/KOPr system in the dorsal striatum under these experimental conditions by gene expression analysis of prodynorphin.
Methods
Subjects
Adult male Sprague Dawley rats (240 – 250 g on arrival; Charles River, Wilmington, MA) were group-housed in a 12-h reverse light/dark cycle (lights OFF at 1PM, ON at 1AM) in an AAALAC-approved humidity-(60%) and temperature-(22°C) controlled stress-minimized behavioral facility. Rats had ad libitum access to food and water at all times. All experiments were carried out during the rodent active phase (dark). Rats were always group-housed (n=3/cage), except in self-administration experiments, in which they were single-housed after surgery until sacrifice, as well as in comparative experiments, in which cocaine naïve rats were single-housed for 8–9 weeks prior to behavioral experiments until sacrifice.
All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
Drugs
LY2444296 (kind gift of Eli Lilly and Co.) was dissolved in 5% ethanol/5% cremophor EL/saline and injected intraperitoneally (i.p; 1 ml/kg). U69,593 and cocaine hydrochloride (Sigma Aldrich, St. Louis, MO) were dissolved in sterile physiological saline. Doses were selected based on pilot studies and published data (Chefer et al. 2013; Melief et al. 2011; Picetti et al. 2010).
Conditioned Place Aversion
The conditioned place aversion assay was conducted in eight sound-attenuated three-compartment boxes (Med Associates, Georgia, VT). Each box consisted of two equal sized outer chambers (27.5×20.6×21.5 cm), differing in color and floor texture, connected by a smaller center chamber (11.9×20.6×21.5 cm), and separated by manual guillotine doors.
A 10-day unbiased procedure was used. Conditioning in the black or white compartment was counterbalanced among animals and treatment groups. On day 1 the pre-conditioning test was performed, allowing rats to freely explore the entire box for 30 min. During eight consecutive conditioning days rats received either U69,593 or vehicle and were confined respectively in the U69,593-paired compartment or vehicle-paired one for 30 min. LY2444296 (3 mg/kg or vehicle) was injected i.p. 30 min before U69,593 (0.32 mg/kg or vehicle; s.c.) and immediately placed in the testing chamber. On day 10 the post-conditioning test was performed just like the preconditioning day. Aversion was measured as the difference of time spent in the drug-paired compartment between post-conditioning and pre-conditioning tests.
During the four conditioning sessions, locomotor activity was measured by photocell beam breaks, and analyzed.
Intravenous extended-access cocaine self-administration
Surgery for cannula implantation
Construction of catheters and surgery were performed as previously published (Windisch et al. 2014) with minor modifications. The analgesic (NSAIDs) Rimadyl (5 mg/kg) and Cefazolin (20 mg/kg) were administered immediately before surgery and 24 h later. Rats were anesthetized with isoflurane (2–5% in air). An indwelling catheter was implanted in the right jugular vein and secured on the back of the rat. The catheter was flushed daily with 0.1 ml sterile saline solution containing 0.13 mg/ml Gentamicin and 20 U/ml Heparin.
Apparatus and Procedure
Operant conditioning chambers consisted of twelve standard sound-attenuated air-ventilated self-administration boxes (Med-Associates), equipped with retractable levers mounted on a side wall. Water and food were provided ad libitum at all times. Programming and data collection were performed using a computer system running MED-PC® IV software (Med-Associates).
The operant procedure was carried out as previously published with some modifications (Valenza et al. 2016). Rats were initially trained in the intravenous (i.v.) self-administration procedure (2 h/day). Then, the duration of sessions was increased to 18 h/day, and rats were run for 14 sessions. Rats were allowed to press two levers (one active and one inactive; evenly randomly assigned). Pressing the active lever resulted in i.v. delivery of a 0.5 mg/kg cocaine infusion in a fixed-ratio 1 schedule of reinforcement with a Time out = 40 s and the illumination of a light cue above the active lever. Sterile cocaine solution was delivered from syringes mounted on syringe pumps (PHM-100, Med-Associates) placed outside the box. Yoked-saline rats were similarly tested; they received sterile physiological saline instead of cocaine once their paired cocaine rat pressed the active lever. Volume and duration of infusion depended on the rat’s body weight, which was measured daily right before the session. Responding on the inactive lever was recorded, but had no consequences.
The operant session started 3 h after the onset of the dark cycle (at 4PM). At conclusion (10AM), rats were returned to the home cage for 6 h. The light/dark cycle in the operant chambers was synchronized with the housing room; therefore rats always lived in the same 12 h reverse light/dark cycle.
Behavioral experiments in rats exposed to chronic extended-access self-administration
All tests were performed at the same time of the day (≈4 PM). Due to the high amount of cocaine self-injected in the 18h/day protocol, we chose not to test rats in the hours immediately following their cocaine session, to avoid an effect of the residual cocaine-induced motor alteration on rats’ performance in the subsequent behavioral tests. Further, to avoid influence of altered circadian rhythm and minimize stress, we kept constant the time of testing. Therefore, we tested rats at a time in which they were typically placed in the operant boxes for self-administration, which corresponded to 30 h after their last self-administration session.
Two cohorts of cocaine self-administering rats (n=20) were tested in the EPM and FST as described below. After the last operant session, rats were returned to the home cage (day 14, 10AM). On the same day at 4PM, rats were exposed to the day 1 forced swimming session. After 15 min they were dried and returned to the home cage and left undisturbed until the following test day (day 15, at 4PM) on which they received LY2444296 (0, 3 mg/kg, i.p.) 30 min before being tested in the EPM, and then, 5 min later, in the FST. Separate groups of cocaine-naïve yoked-saline rats (n=5/treatment group), cocaine naïve group-housed and single-housed rats (n=8–12/treatment group) were tested in the EPM and FST under the same conditions.
A separate cohort of yoked-saline and cocaine rats (n=12) was used to measure baseline serum corticosterone (CORT) and striatal Pdyn gene expression as described in detail below. Rats were sacrificed 30 h after the end of the last cocaine self-administration session, without exposing them to any additional behavioral manipulations.
A further cohort of yoked-saline and cocaine rats (n=16) was administered LY2444296 (0, 3 mg/kg, i.p; treatment groups were matched for total cocaine intake) 30 h after the end of the last cocaine self-administration session and trunk blood was collected 30 min later, to measure serum CORT as described below, without exposing rats to any additional behavioral manipulations.
Elevated Plus Maze (EPM)
The apparatus was an elevated (50 cm) cross-shaped platform, in which two “closed” arms (50X10 cm) were protected by black walls and the other “open” arms were illuminated. The maze was placed at the center of a dark room, with two lights illuminating the open arms; Lux was measured at the beginning and end of the experiment as 0 lux in the dark compartments, 1±1 lux in the center, and 3±1 lux in the open arms. The test was carried out during the dark phase, approximately at the same time of the day at which cocaine rats usually initiated their daily operant sessions. LY2444296 was administered i.p. 30 min before test. Rats were placed at the center of the maze, facing one of the black walls. An anxiety-like response was measured as an inhibition of exploration behavior of the open arms; the number of entries in the two closed arms was counted to monitor for nonspecific locomotor responses.
Forced Swim Test (FST)
A two-day forced swimming test was performed (Iemolo et al. 2012; Porsolt et al. 1979). On day 1, rats were exposed to a 15-min pre-swim in 23 ±1 °C water in a clear Plexiglas cylinder (20X50 cm; Harvard Apparatus, MA), from which they could not escape and/or touch the bottom. This assay was carried out during the dark phase, approximately at the same time rats usually initiated their daily operant session. After 24 h, rats received LY2444296 (0 or 3 mg/kg), and 30 min later they were placed in the swimming cylinder for 5 min. Time spent immobile and latency to show immobility behavior were analyzed.
Quantitative Polymerase Chain Reaction
Procedures were carried out as previously published (Valenza et al. 2016; Valenza et al. 2015). About 30 h after the end of the last self-administration session, cocaine and yoked-saline rats were briefly anesthetized with carbon dioxide and decapitated. The brain was rapidly extracted, sliced on an ice-cold brain matrix, and both ventral and dorsal striata were dissected following a rat brain atlas (Paxinos and Watson 1986). Tissue collected was immediately frozen in dry ice and stored at −70 C.
All samples from a given brain region were always processed concurrently. Samples were homogenized in Qiazol solution (Qiagen, Valencia, CA) and the total mRNA extraction was performed using the miRNeasy Kit (Qiagen). Following RNA isolation, all samples were treated with the RNAse-free DNAse Set (Qiagen) and quantified with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Single-stranded cDNA was synthesized using the same amount of RNA (1 ug) for each sample of a given brain region, using the RT2 First Strand Kit (Qiagen), which includes an additional genomic DNA removal step. Samples were then stored at −70 C until further processing.
cDNA (1 μl) was amplified in a 10-μl solution containing RT2 SYBRGreen ROX qPCR Master Mix and 0.5 uM of RT2 qPCR Primer Assay for rat Pdyn (Qiagen). Gene sequences were amplified using a two-temperature protocol, which included an initial step at 10 min at 95 °C to activate the polymerase, followed by 40 denaturation cycles at 95 °C for 15 sec, annealing and extension at 60 °C for 1 min. Experimental samples were amplified simultaneously with a standard curve constructed using serial 10-fold dilutions of purified fragments (QIAquick PCR purification kit, Qiagen) of the target gene (Valenza et al. 2016). Standards and samples of both the target gene and the housekeeping gene of a given brain region were run in duplicate concurrently in the same plate, in the ABI Prism 7900HT Sequence Detection System (Applied Biosystem, Carlsbad, CA). The copy number of cDNA of the target and the housekeeping gene was determined by interpolating the threshold cycles (Ct) of an experimental sample to those in standard curves for the corresponding gene using the SDS 2.3 software (Applied Biosystem). Gene-specific amplification was determined by melting curve analysis corresponding to one peak at the expected melting temperature.
Results were analyzed as the ratio between copy numbers of the target gene and the housekeeping gene, beta-2-microglobulin (B2m). B2m was selected as the housekeeping gene for tissue normalization since it was not affected by Treatment in both brain regions analyzed (Bustin et al. 2010).
Corticosterone RadioImmunoAssay
Rat blood was collected approximately at the same time of the day in all experiments, 30 min after LY2444296 (or vehicle) and/or U69,593 (or saline) systemic administration. Serum was separated by centrifugation for 15 min at 2000 rpm at 4 C. Serum CORT samples were assayed in duplicate following a Corticosterone 125I-RIA kit protocol (MP Biomedicals, Solon, OH).
Statistics
A two-way ANOVA was performed in all data where co-administration of two treatments occurred (LY2444296 and U69,593; or yoked-saline/cocaine self-administration and LY2444296) as between-subject factors. A repeated-measures ANOVA was used when a within-subject factor was present (e.g. conditioning day or self-administration session). Upon detection of a significant main effect, post-hoc comparisons were carried out using the Student Newman-Keuls (SNK) test, while the Dunnett’s test was used to compare data to one control group. Comparisons between two groups were analyzed by Student’s t test. p value ≤ 0.05 was considered statistically significant. Correlation analysis was analyzed as Pearson’s correlation between the relative gene expression (mean Pdyn copy numbers/mean B2M copy numbers) and the sum of cocaine intake across 14 operant sessions.
The Statistical software used was Statistica 7.0 (StatSoft. Inc., Tulsa, OK). The graphical software used was SigmaPlot 12.5 (Systat Software Inc., San Jose, CA).
Results
LY2444296 prevents behavioral and neuroendocrine effects caused by the reference kappa agonist U69,593 in cocaine naïve rats
Systemic administration of 0.32 mg/kg U69,593 induced place aversion (Figure 1A) and reduced locomotor activity (Figure 1B). Pretreatment with 3 mg/kg LY2444296, 30 min before each U69,593 conditioning session, significantly prevented the development of U69,593-conditioned aversion and blocked the U69,593-induced reduction in motor activity.
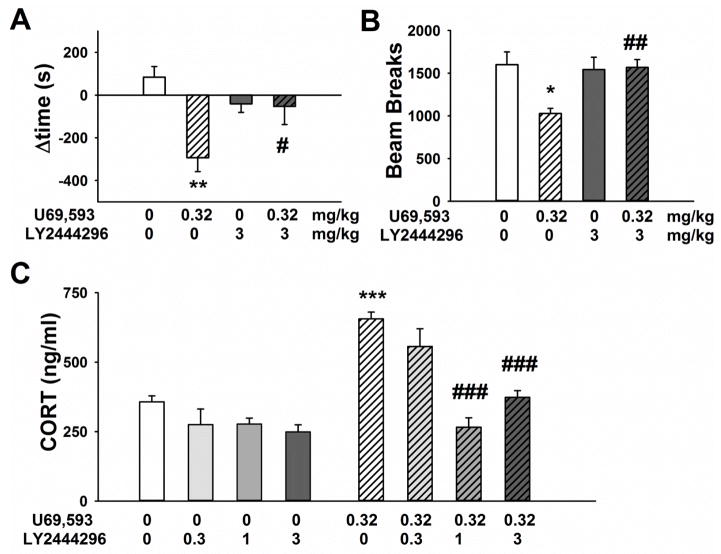
Figure 1.
Effect of acute systemic administration of LY2444296 (0, 3 mg/kg i.p.) on U69,593 (0, 0.32 mg/kg s.c.)-mediated A) conditioned place aversion (n= 6/group), B) mean locomotor activity over 4 conditioning days (n= 6/group). Panel C shows the effect of acute systemic administration of LY2444296 (0, 1, 3 mg/kg i.p.) on U69,593 (0, 0.32 mg/kg s.c.)-increased serum corticosterone (n= 8–10/group). All panels show the Mean ±SEM. *** p<0.001, ** p<0.01, * p<0.05 versus vehicle/vehicle group; ### p<0.001, ## p<0.01, # p<0.05 versus LY2444296 vehicle/U69,593 0.32 mg/kg (SNK test).
The two-way ANOVA on the conditioned-place aversion data revealed a significant main effect of U69,593 [F(1,20)=9.8, p<0.01], but not LY2444296 [F(1,20)=0.9, NS], and an interaction between the two drugs [F(1,20)=8.6, p<0.01]. Rats conditioned with LY2444296 alone did not establish either place aversion or preference.
A two-way repeated measures ANOVA on locomotor activity during the four conditioning days revealed a significant main effect of U69,593 treatment [F(1,20)=5.05, p<0.05] and an interaction between U69,593 and LY2444296 [F(1,20)=5.4, p<0.05]. No effect of the conditioning day was found [F(3,60)=2.5, NS; not shown], nor any interaction between either of the drugs and conditioning day. Post-hoc comparisons showed that U69,593 decreased rat locomotor activity, and that pretreatment with LY2444296 significantly blocked this U69,593-induced effect (Figure 1B). Of note, LY2444296 given alone did not alter rat motor activity.
Systemic administration of the KOPr agonist U69,593 induced an elevation in serum CORT level, measured 30 min after acute injection, compared to vehicle. Pretreatment with LY2444296 significantly attenuated the U69,593-induced increase in serum CORT (Figure 1C). A two-way ANOVA revealed a significant main effect of U69,593 [F(1,62)=47.7, p<0.0001], LY2444296 [F(3,62)=23.7, p<0.0001] and an interaction between U69,593 and LY2444296 [F(3,62)=9.0, p<0.0001]. Post-hoc comparisons showed that pretreatment with LY2444296 (1 mg/kg or 3 mg/kg) significantly prevented the U69,593-induced increase in serum CORT. Given alone, LY2444296 did not significantly affect serum CORT level. A separate one-way ANOVA on serum CORT data obtained after LY2444296 administration alone (0, 0.3, 1 and 3 mg/kg LY2444296; without U69,593 injection) revealed a main effect of Treatment [F(3,32)=3.2, p<0.05]. However, post-hoc analysis did not reveal a statistically significant difference of any LY2444296 dose compared to vehicle.
LY2444296 failed to block the KOPr agonist-induced rise in serum CORT level when injected 24 h before U69,593 [t(19)=1.9, NS, not shown], confirming a duration of action <24 h.
LY2444296 does not alter anxiety-like behaviors in cocaine naïve rats, whether group-housed or individually housed
Cocaine naïve rats singly housed during adulthood showed anxiety-like behavior, measured by shorter time spent in the open arms and longer latency to enter in the open arms of an EPM than group-housed rats (Figure 2). Acute systemic pretreatment with LY2444296 did not affect these measures of anxiety-like behavior in either single- or group-housed rats.
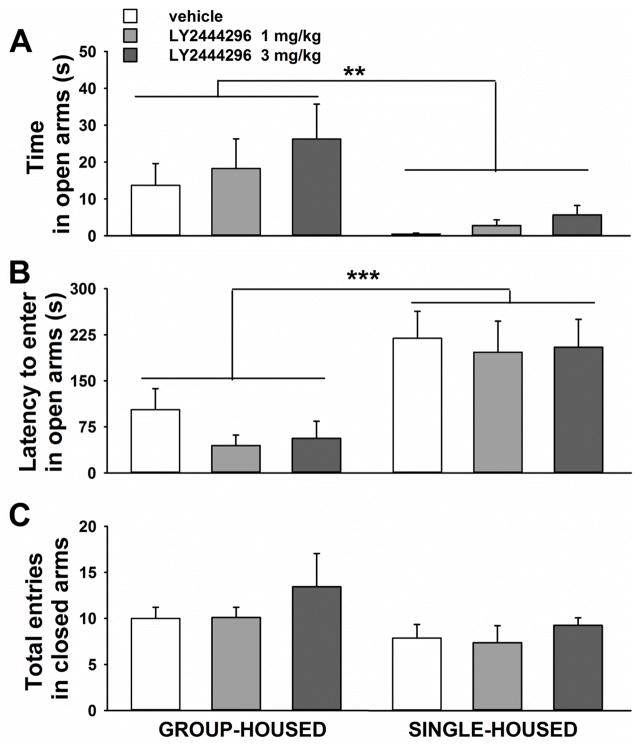
Figure 2.
Effect of acute systemic administration of LY2444296 (0, 1, 3 mg/kg) on A) time spent in the open arms, B) latency to the first entrance into an open arm, C) total number of entries in the closed arms, in cocaine naïve group-housed (n= 9–10/group; left graphs) and single-housed rats (n= 8/group; right graphs) tested in a 5-min EPM test. All panels show the Mean ±SEM. *** p<0.001, ** p<0.01 versus group-housed rats (ANOVA).
A two-way ANOVA on the time spent in the open arms revealed a main effect of the Housing Condition [F(1,48)= 9.9, p<0.003], no effect of the Treatment [F(2,48)=1.0, NS] and no interaction [F(2,48)=0.2, NS]. The two-way ANOVA on data of latency to enter in the open arms gave similar results [Housing Condition: F(1,48)=21.7, p<0.0001; Treatment: F (2,48)= 0.7, NS; Housing Condition* Treatment: F(2,48)=0.1, NS].
A two-way ANOVA on data on the number of entries in the closed arms showed a trend in the Housing Condition [F(1,48)=3.7, p=0.08], but no effect of Treatment [F(2,48)=1.0, NS], and no interaction between the two effects [F(2,48)=0.1, NS].
LY2444296 does not alter depressive-like behavior in cocaine naïve rats, whether group-housed or individually housed
Cocaine naïve rats singly housed during adulthood showed depressive-like behavior, measured by longer time spent immobile in the water and shorter latency to show immobility in a FST than group-housed rats (Figure 3). Systemic pretreatment with LY2444296 did not significantly affect these behavioral measures of depressive-like behavior in cocaine naïve rats, either group- or single-housed.
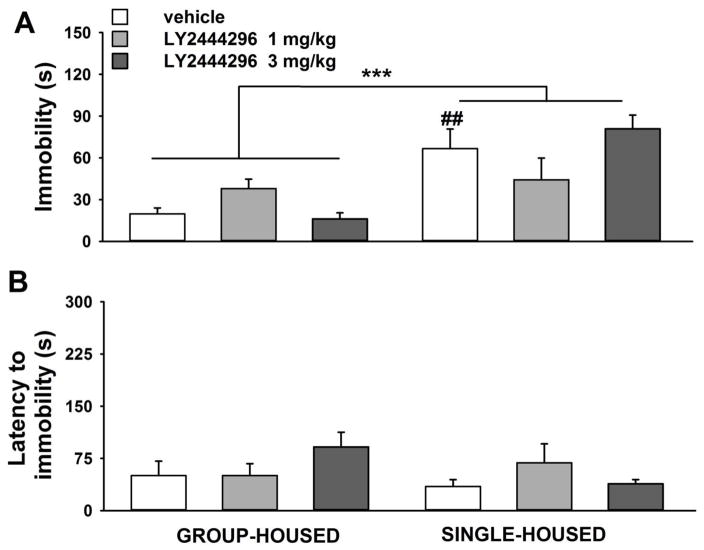
Figure 3.
Effect of acute systemic administration of LY2444296 (0, 1, 3 mg/kg) on A) immobility behavior, B) latency to show immobility, in cocaine naïve group-housed (n= 9–10/group; left graphs) and single-housed rats (n= 8–12/group; right graphs) tested in a 5-min FST. All panels show the Mean ±SEM. *** p<0.01 versus group-housed rats (ANOVA), ## p<0.01 versus vehicle group-housed rats (SNK test).
A two-way ANOVA on immobility data revealed a main effect of the Housing Condition [F(1,54)=24.3, p<0.0001], no effect of the Treatment [F(2,54)=0.3, NS], and a significant interaction Housing Condition*Treatment [F(2,54)=4.4, p<0.05]. Post-hoc comparisons showed a significant difference only between vehicle-injected rats in the two housing condition (p<0.01). Separate one-way ANOVAs revealed a significant main effect of Treatment in group-housed rats [F(2,26)=4.9, p<0.05], and not in single-housed rats [F(2,28)=1.9, NS]. However, post-hoc comparisons did not detect a statistically significant difference between the three doses in group-housed rats.
Analysis of latency data by two-way ANOVA did not show main effects of Housing Condition [F(1,54)=2.2, NS], Treatment [F(2,54)=1.0, NS] or an interaction [F(2,54)=2.6, NS].
Rats exposed to chronic 18 h/day cocaine self-administration progressively escalate intake and show increased striatal dynorphin expression
Rats trained for 2 h/day for six days acquired the operant behavior and reached a stable baseline intake of cocaine (percent coefficient of variation over the last 3 days =15%; Figure 4A). Rats progressively escalated cocaine intake over 14 sessions of extended access (18 h/day) intravenous self-administration (Figure 4B). The repeated measure one-way ANOVA analysis showed a significant main effect of the Session [F(13,273)=3.1, p<0.0005]. Post-hoc comparison by Dunnett’s test revealed a statistically significant increase in the amounts of cocaine self-injected from the 6th to the last day compared their first extended access session. By the 12th session, rats showed a mean 53 % increase of their cocaine intake compared to their first extended access session. Responses on the inactive lever were relatively infrequent and did not escalate [Mean 1stsession ±SEM=24.3 ±10.2; F(13,260)=0.9, NS; not shown].
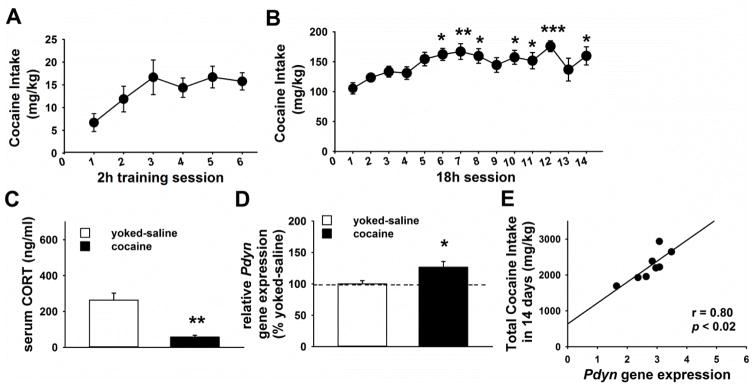
Figure 4.
Panel A and B show the cocaine intake of adult male Sprague Dawley rats (n= 22) during (A) the training and (B) the chronic (14 sessions) extended access (18 h/day) intravenous self-administration exposure. *** p<0.001, ** p<0.01, * p<0.05 versus the first session (Dunnett’s test). Panel C and Panel D show respectively the serum CORT concentration and the Pdyn gene expression of a separate cohort of rats exposed to chronic extended access cocaine self-administration (18 h/day for 14 days), and of their respective yoked-saline rats (n=6–8/group) sacrificed 30 h after their last operant session. * p<0.05 (Student’s t test). Panel E shows the Pearson’s correlation result between the Pdyn gene expression and the total cocaine intake that rats self-administered over 14 days. All panels show the Mean ±SEM.
The level of serum CORT measured 30 h after the last extended access self-administration session was lower in cocaine rats than in yoked-saline rats [t(11)=7.4, p<0.01; Figure 4C].
In the dorsal striatum, cocaine rats showed higher Pdyn gene expression [t(17)=2.5, p<0.03] than yoked-saline rats (Figure 4D). Moreover, Pdyn mRNA expression was positively correlated with the total amount (mg/kg) of cocaine self-injected over the 14 operant sessions (Figure 4E). No statistical difference in Pdyn mRNA was found between cocaine and yoked-saline rats in the ventral striatum [t(17)=0.3, NS; not shown].
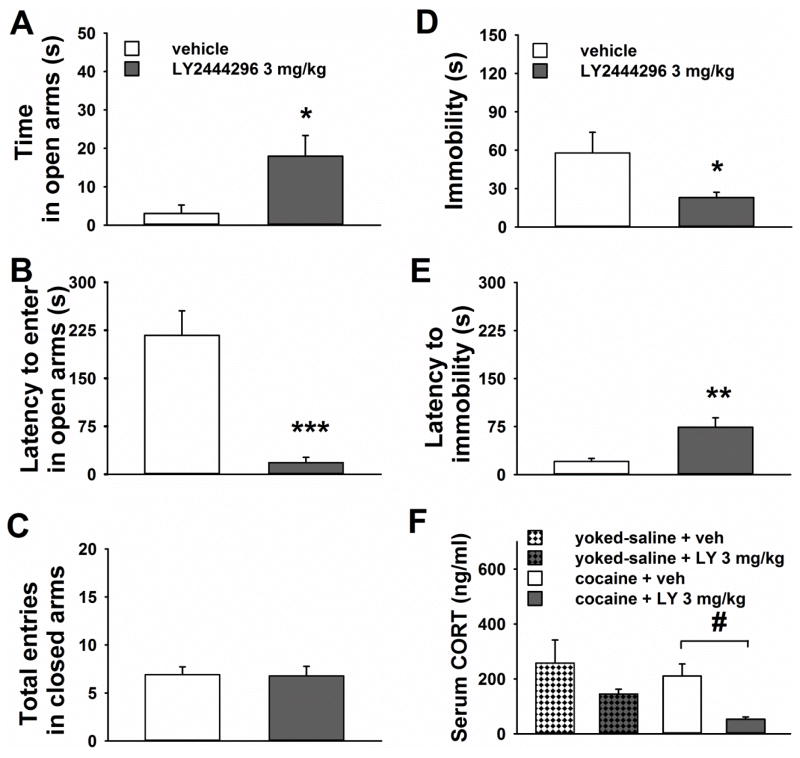
Behavioral and neuroendocrine effects of acute LY2444296 administration in rats exposed to chronic extended access cocaine self-administration
Acute pre-treatment with 3 mg/kg LY2444296, compared to vehicle, reduced measures of anxiety-like and depressive-like behaviors, as well as serum CORT concentration, in rats observed 30 h after their last cocaine self-administration session (Figure 5). Indeed, cocaine-exposed rats, acutely pre-treated with LY2444296 before exposure to an EPM, showed longer time spent in the open arms [t(17)=2.7, p<0.05; Figure 5A] and shorter latency to enter in the open arms [t(17)=4.8; p<0.001; Figure 5B] than vehicle-injected rats. LY2444296 treatment did not affect motor behavior, as measured by number of entries in the closed arms, compared to vehicle [t(17)=0.1, NS; Figure 5C].
Figure 5.
Panels A to E show the effect of acute systemic administration of LY2444296 (0, 3 mg/kg) on A) the time spent in the open arms, B) the latency to the first entrance in an open arm and C) the total number of entries in the closed arms in an 5-min EPM test, as well as D) the immobility, E) the latency to show immobility in a 5-min FST, performed in rats tested 30 h after their last extended access cocaine self-administration session (n= 9–11/group). All panels show the Mean ±SEM. *** p< 0.001, ** p< 0.01, * p< 0.05 versus vehicle-injected cocaine rats (Student’s t test). Panel F shows the Mean ±SEM of serum corticosterone concentration measured in rats pre-treated with acute systemic administration of LY2444296 (0, 3 mg/kg; n= 4/group) 30 h after their last extended access cocaine self-administration session, with no exposure to FST or EPM. # p<0.05 versus vehicle-injected cocaine exposed rats (LSD test).
In a separate group of rats exposed to chronic extended access yoked-saline self-administration, 3 mg/kg LY2444296 did not show any significant anxiolytic-like effects compared to vehicle [Time in open: t(8)=0.9, NS; Mean vehicle ±SEM=7.3 ±2.2; Mean LY ±SEM=11.5 ±3.7. Latency to open: t(8)=1.2, NS; Mean vehicle ±SEM=108.9 ±55.8; Mean LY ±SEM=193.5 ±42.0; not shown].
Results obtained in the forced swim test (FST), performed 30 h after the last operant session, revealed that cocaine-exposed rats pre-treated with acute LY2444296 spent less time immobile in the water [t(17)=2.2, p<0.05; Figure 5D] and showed longer latency to immobility [t(17)=3.3, p<0.01; Figure 5E] than vehicle-injected rats. LY2444296 did not show any significant effects in cocaine naïve rats exposed to yoked-saline extended access self-administration [Immobility: t(8)=0.3, NS; Mean vehicle ±SEM=29.9 ±14.9; Mean LY ±SEM=36.2 ±11.7. Latency to immobility: t(8)=1.5, NS; Mean vehicle ±SEM=24.8 ±6.4; Mean LY ±SEM=50.4 ±15.6; not shown].
A two-way ANOVA on serum CORT data, performed in a separate group of cocaine exposed rats and their respective yoked-saline counterparts pretreated with LY2444296, showed a significant main effect of LY2444296 treatment [F(1,12)=7.5, p<0.02]. Post-hoc tests revealed that cocaine-exposed rats acutely treated with LY2444296 had lower serum CORT level than vehicle-treated rats (p<0.05). No statistically significant difference was found between vehicle- and LY2444296-treated yoked-saline rats (Figure 5F).
Discussion
The main novel findings presented in this study show that I) acute systemic administration of the novel, selective, relatively short-acting kappa opioid receptor antagonist LY2444296 blocks KOPr agonist-induced conditioned place aversion and KOPr agonist-induced release of corticosterone. LY2444296 alone does not cause place aversion or preference, locomotor effects, nor CORT release; II) rats escalate cocaine intake over 14 days of 18 h/day cocaine self-administration and show an increased Pdyn gene expression in the dorsal striatum compared to yoked-saline rats; III) acute systemic pre-treatment with 3 mg/kg LY2444296 reduces anxiety-like and depressive-like behaviors in rats observed 30 h after the last extended access cocaine self-administration session, but not in cocaine naïve rats.
The recent characterization of novel selective KOPr antagonists with a shorter duration of action than compounds used so far (e.g. norBNI, JDTic) has opened new opportunities for studies of translational relevance addressing the neurobiology of dynorphin-KOPr signaling. Our results on the establishment of U69,593-conditioned place aversion are in line with several previous reports demonstrating that KOPr agonists administration induces dysphoria/aversion in animal models and humans (Butelman et al. 2012; Chefer et al. 2013; Mucha and Herz 1985; Pfeiffer et al. 1986a; Todtenkopf et al. 2004) and increases the release of stress hormones, including cortisol in non-human primates and humans (Adamson et al. 1991; Maqueda et al. 2016; Marco et al. 2005; Pascoe et al. 2008; Pfeiffer et al. 1986b; Ranganathan et al. 2012; Ur et al. 1997). Here we demonstrate for the first time, to the best of our knowledge, that acute pretreatment with LY2444296 blocks U69,593-induced place aversion and -reduced locomotor activity. We also show the first available data on the effectiveness of acute LY2444296 administration in blocking KOPr agonist-induced release of the stress hormone CORT. LY2444296 was effective when administered 30 minutes, but not 24 hours, prior to U69,593, demonstrating that it has a rapid onset and moderate duration of action (i.e. <24 h), in contrast to most reference KOPr antagonists studied to date. This is consistent with previous findings showing LY2444296 in vivo effect to have an onset of 30 min after oral administration in rats, and a duration of action < 24 h in an analgesia test in mice (Melief et al. 2011; Mitch et al. 2011). Of note, LY2444296 alone did not cause place aversion or preference and did not affect locomotor activity or corticosterone levels, suggesting limited dynorphin/KOPr baseline activity “tone” in neurobiological systems underlying these endpoints, in cocaine naïve rats.
In line with previous findings, cocaine naïve single-housed rats exhibited greater anxiety-like and depressive-like behaviors than group-housed rats (Das et al. 2015; Wallace et al. 2009), however acute LY2444296 administration (1 or 3 mg/kg) did not ameliorate their behavior. Previous studies showed a reduced latency to immobility and increased open arm exploration induced by long-acting KOPr antagonists, in cocaine naïve rats (Knoll et al. 2007; Mague et al. 2003; Pliakas et al. 2001). In line with our results, two recent studies reported that systemic administration of norBNI, but not 3 mg/kg LY2444296, reduced anxiety-like and depressive-like behaviors in cocaine naïve mice (Huang et al. 2016a; Huang et al. 2016b). Long-acting KOPr antagonists have complex pharmacokinetics and pharmacodynamic properties. Their timing of administration affects the selectivity of action, as well as the interpretation of results (Carlezon and Krystal 2016; Knoll and Carlezon 2010). Therefore, findings obtained through LY2444296 in vivo administration might not completely overlap with the ones obtained using norBNI. Of note, differential effects of KOPr antagonists in specific behavioral assays could be due to different downstream systems (Melief et al. 2011). These issues further underscore the need for studies on the neurobiology of dynorphin-KOPr signaling using selective relatively short-acting KOPr antagonists, like LY2444296. However, we cannot exclude that higher doses of LY2444296 would have been effective in our experimental condition, although this could also result in a potential loss of KOPr selectivity (Huang et al. 2016b; Melief et al. 2011).
Rats exposed to our protocol of chronic 18 h intravenous cocaine self-administration show a daily intake considerably greater than protocols already published, with peaks of intake reaching 150–200 mg/kg/session. Quantitative gene expression analysis revealed that rats exposed to this protocol show higher Pdyn mRNA in the dorsal striatum compared to their respective cocaine naïve yoked-saline rats. Moreover, Pdyn expression in the dorsal striatum was positively correlated with the total cocaine intake self-injected in the 14 days of exposure. By contrast, Pdyn mRNA levels in the ventral striatum were unchanged. This is consistent with findings from our laboratory and others, indicating that striatal Pdyn systems, dorsal rather than ventral, are changed by prolonged exposure to cocaine self-administration, potentially modeling a more advanced stage of addiction state (Everitt and Robbins 2005; Fagergren et al. 2003; Valenza et al. 2016). Previous work also reported that rats show a depressive-like state after extended access cocaine self-administration, proportional to the amount of cocaine intake (Markou and Koob 1991).
The present study shows for the first time that acute systemic pretreatment with the 3 mg/kg dose of the novel selective relatively short-acting KOPr antagonist LY2444296 exerts anxiolytic-like and antidepressant-like properties, in rats tested 30 h after the end of chronic exposure to extended access cocaine self-administration. No significant effect of LY2444296 administration was seen in a separate group of yoked-saline self-administering rats.
The selective effectiveness of LY2444296 in the EPM and FST in cocaine-exposed rats was not simply secondary to the individual housing condition (commonly used in self-administration experiments to prevent catheter disruption), since LY2444296 did not show such effects in cocaine naïve individually housed rats. Peters et al. (2011) showed that relatively short-acting KOPr antagonists, including a structural analog of LY2444296, displayed anxiolytic-like effects in a model of prenatal stress. Similarly, intracerebral administration of the long-acting KOPr antagonist norBNI reduced the latency to immobility in the Forced Swim Test in rats previously exposed to a experimenter-administered “binge” cocaine protocol, without exerting an effect in “binge” saline rats (Chartoff et al. 2012). Taken together, these results are consistent with the interpretation that a sustained stressful or cocaine exposure condition is necessary to activate the dynorphin-KOPr signaling enough to detect an anxiolytic/antidepressant-like effect of a relatively short-acting KOPr antagonist.
We report here that rats 30 h after the end of chronic extended access cocaine self-administration, (not exposed to either the EPM or FST), had significantly lower baseline serum CORT concentration than their respective yoked-saline rats. This is consistent with previous finding by Mantsch et al. (2007a), who assayed plasma CORT in rats 24 h after the end of cocaine self-administration (6 h/day for 14 days) and found that cocaine-exposed rats had a lower baseline, but a higher stress-induced increase in plasma CORT, than yoked-saline rats (Mantsch et al. 2007a; Mantsch et al. 2007b). Similarly, in the present study, rats tested 30 h after chronic cocaine self-exposure, given a single ip injection of vehicle, did not show a lower level of serum CORT than their respective yoked-saline rats (Fig. 5). Of note, cocaine-exposed rats, pretreated with LY2444296, showed a significant reduction of serum CORT compared to vehicle-injected rats. Taken together these data suggest that, in our condition, cocaine-exposed rats were more sensitive to a brief minor stressor (such as an i.p. vehicle injection) when compared to their control yoked-saline rats. The higher serum CORT seen in cocaine-exposed rats receiving an acute vehicle-injection could be mediated, at least in part, by endogenous KOPr activation. As a limitation, these studies had a relatively low “n”, due to the time and resources involved. However, hyper-responsiveness of signaling through the dynorphin/KOPr system after diverse stresses and chronic drug exposure appears to be a robust finding (Piras et al. 2010; Rose et al. 2016).
Preclinical data suggest a bidirectional interaction between CRF and dynorphin upon stress (although serum corticosterone level was not directly assayed) (Bruchas et al. 2009; Land et al. 2008; Valdez et al. 2007). This view is supported also by early studies reporting that KOPr activation by U50,488 evokes in vitro CRF release in isolated rat hypothalami (Buckingham and Cooper 1986). Also, dynorphin peptide is released in rat hypothalamic slices superfused with increasing doses of CRF (Nikolarakis et al. 1986). Additional studies are needed to explore the mechanisms through which the endogenous dynorphin-KOPr system increases CORT release after prolonged stress or cocaine exposure.
In conclusion, the present study shows for the first time that acute systemic administration of a novel, selective, relatively short-acting KOPr antagonist ameliorates in vivo both anxiety-like and depressant-like behaviors in rats observed after chronic extended access cocaine self-administration. Thus, LY2444296 is a valuable pharmacological tool to explore the neurobiology of the dynorphin-KOPr system and the consequences of KOPr blockade, and also to expand studies of behavioral and neuroendocrine status after chronic cocaine self-exposure. Future studies may investigate the effectiveness of such novel relatively short-acting KOPr antagonist in blocking drug taking and seeking in several animal models, with the long term goal of finding an effective pharmacotherapy for patients with cocaine addiction.
Acknowledgments
Funding
This work was supported by The Dorothea Dix Fellowship Fund (MV), the National Institute of Health grant RO1 DA018151 (ERB), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK). The authors declare not to have any conflict of interest to disclose.
The authors gratefully acknowledge the precious help received by: Dr. Linda Rorick-Kehn and Eli Lilly and Co. for providing the LY2444296 compound; Dr. Kyle A. Windisch, who modified the MedPC program and assembled catheters for the intravenous self-administration experiment; Dr. Joel M. Corrêa da Rosa for consultation on appropriate statistical analysis. We also thank Dr. Brian Reed for constructive discussion of data, as well as Ms. Lyla Bloom who proof-read the final manuscript.
References
- Adamson WT, Windh RT, Blackford S, Kuhn CM. Ontogeny of mu- and kappa-opiate receptor control of the hypothalamo-pituitary-adrenal axis in rats. Endocrinology. 1991;129:959–64. doi: 10.1210/endo-129-2-959. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Foshage AM, Bruchas MR. Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology. 2013;38:2484–97. doi: 10.1038/npp.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–26. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1986;44:36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–76. [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–96. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–7. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Krystal AD. Kappa-Opioid Antagonists for Psychiatric Disorders: From Bench to Clinical Trials. Depress Anxiety. 2016;33:895–906. doi: 10.1002/da.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–63. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–9. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–76. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Ebner SR, Sparrow A, Potter D, Baker PM, Ragozzino ME, Roitman MF. Relative Timing Between Kappa Opioid Receptor Activation and Cocaine Determines the Impact on Reward and Dopamine Release. Neuropsychopharmacology. 2016;41:989–1002. doi: 10.1038/npp.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Martinez D. Kappa Antagonist JDTic in Phase 1 Clinical Trial. Neuropsychopharmacology. 2015;40:2057–8. doi: 10.1038/npp.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Backman CM, Gigante ED, Shippenberg TS. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacology. 2013;38:2623–31. doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Nunes EV, Bisaga A, Carpenter KC, Mariani JP, Sullivan MA, Raby WN, Levin FR. A comparison of independent depression and substance-induced depression in cannabis-, cocaine, and opioid-dependent treatment seekers. Am J Addict. 2011;20:441–6. doi: 10.1111/j.1521-0391.2011.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Barhwal K, Hota SK, Thakur MK, Srivastava RB. Disrupting monotony during social isolation stress prevents early development of anxiety and depression like traits in male rats. BMC Neurosci. 2015;16:2. doi: 10.1186/s12868-015-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA., Jr Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol. 2015;26:654–63. doi: 10.1097/FBP.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–8. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Huang P, Tunis J, Parry C, Tallarida R, Liu-Chen LY. Synergistic antidepressant-like effects between a kappa opioid antagonist (LY2444296) and a delta opioid agonist (ADL5859) in the mouse forced swim test. Eur J Pharmacol. 2016a;781:53–9. doi: 10.1016/j.ejphar.2016.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Yakovleva T, Aldrich JV, Tunis J, Parry C, Liu-Chen LY. Two short-acting kappa opioid receptor antagonists (zyklophin and LY2444296) exhibited different behavioral effects from the long-acting antagonist norbinaltorphimine in mouse anxiety tests. Neurosci Lett. 2016b;615:15–20. doi: 10.1016/j.neulet.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict Biol. 2016;21:360–73. doi: 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, Sabino V, Cottone P. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23:593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–45. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: from addiction to depression, and back. Front Psychiatry. 2014;5:170. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–14. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Dragisic T, Duwaerts CC, Swiatkowski M, Suzuki H. Effects of recovery from immobilization stress on striatal preprodynorphin- and kappa opioid receptor-mRNA levels of the male rat. Physiol Behav. 2011;104:972–80. doi: 10.1016/j.physbeh.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–30. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007a;1167:101–11. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett. 2007b;415:269–73. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda AE, Valle M, Addy PH, Antonijoan RM, Puntes M, Coimbra J, Ballester MR, Garrido M, Gonzalez M, Claramunt J, Barker S, Lomnicka I, Waguespack M, Johnson MW, Griffiths RR, Riba J. Naltrexone but Not Ketanserin Antagonizes the Subjective, Cardiovascular, and Neuroendocrine Effects of Salvinorin-A in Humans. Int J Neuropsychopharmacol. 2016:19. doi: 10.1093/ijnp/pyw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Llorente R, Perez-Alvarez L, Moreno E, Guaza C, Viveros MP. The kappa-opioid receptor is involved in the stimulating effect of nicotine on adrenocortical activity but not in nicotine induced anxiety. Behav Brain Res. 2005;163:212–8. doi: 10.1016/j.bbr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–83. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA, Jr, Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C. Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol. 2011;80:920–9. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch CH, Quimby SJ, Diaz N, Pedregal C, de la Torre MG, Jimenez A, Shi Q, Canada EJ, Kahl SD, Statnick MA, McKinzie DL, Benesh DR, Rash KS, Barth VN. Discovery of aminobenzyloxyarylamides as kappa opioid receptor selective antagonists: application to preclinical development of a kappa opioid receptor antagonist receptor occupancy tracer. J Med Chem. 2011;54:8000–12. doi: 10.1021/jm200789r. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–80. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Res. 1986;399:152–5. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko MC. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33:478–86. doi: 10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MF, Zacco A, Gordon J, Maciag CM, Litwin LC, Thompson C, Schroeder P, Sygowski LA, Piser TM, Brugel TA. Identification of short-acting kappa-opioid receptor antagonists with anxiolytic-like activity. Eur J Pharmacol. 2011;661:27–34. doi: 10.1016/j.ejphar.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986a;233:774–6. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Knepel W, Braun S, Meyer HD, Lohmann H, Brantl V. Effects of a kappa-opioid agonist on adrenocorticotropic and diuretic function in man. Horm Metab Res. 1986b;18:842–8. doi: 10.1055/s-2007-1012453. [DOI] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 2010;211:313–23. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Schlussman SD, Zhou Y, Ray B, Ducat E, Yuferov V, Kreek MJ. Addictions and stress: clues for cocaine pharmacotherapies. Curr Pharm Des. 2013;19:7065–80. doi: 10.2174/13816128113199990610. [DOI] [PubMed] [Google Scholar]
- Piras AP, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Acute withdrawal from chronic escalating-dose binge cocaine administration alters kappa opioid receptor stimulation of [35S] guanosine 5′-O-[gamma-thio]triphosphate acid binding in the rat ventral tegmental area. Neuroscience. 2010;169:751–7. doi: 10.1016/j.neuroscience.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC, Kauer JA. Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol Psychiatry. 2014;76:785–93. doi: 10.1016/j.biopsych.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57:201–10. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the kappa opioid agonist Salvinorin A in humans. Biol Psychiatry. 2012;72:871–9. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B, Fang N, Mayer-Blackwell B, Chen S, Yuferov V, Zhou Y, Kreek MJ. Chromatin alterations in response to forced swimming underlie increased prodynorphin transcription. Neuroscience. 2012;220:109–18. doi: 10.1016/j.neuroscience.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witcher JW, Lowe SL, Gonzales CR, Weller MA, Bell RL, Hart JC, Need AB, McKinzie JH, Statnick MA, Suico JG, McKinzie DL, Tauscher-Wisniewski S, Mitch CH, Stoltz RR, Wong CJ. Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–44. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol. 2016:19. doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Whitfield TW, Jr, Park PE, Crawford EF, George O, Vendruscolo LF, Koob GF. Long-term antagonism of kappa opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci. 2013;33:19384–92. doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–14. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Dopaminergic regulation of postnatal development of dynorphin neurons in rat striatum. Neuropeptides. 1996;30:103–7. doi: 10.1016/s0143-4179(96)90062-1. [DOI] [PubMed] [Google Scholar]
- Spangler R, Ho A, Zhou Y, Maggos CE, Yuferov V, Kreek MJ. Regulation of kappa opioid receptor mRNA in the rat brain by “binge’ pattern cocaine administration and correlation with preprodynorphin mRNA. Brain Res Mol Brain Res. 1996;38:71–6. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–70. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–6. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol. 1997;120:781–4. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–33. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Valenza M, Picetti R, Yuferov V, Butelman ER, Kreek MJ. Strain and cocaine-induced differential opioid gene expression may predispose Lewis but not Fischer rats to escalate cocaine self-administration. Neuropharmacology. 2016;105:639–50. doi: 10.1016/j.neuropharm.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Steardo L, Cottone P, Sabino V. Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology (Berl) 2015;232:3215–26. doi: 10.1007/s00213-015-3981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–9. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–9. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–75. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Schlosburg JE, Wee S, Gould A, George O, Grant Y, Zamora-Martinez ER, Edwards S, Crawford E, Vendruscolo LF, Koob GF. kappa Opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J Neurosci. 2015;35:4296–305. doi: 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch KA, Kosobud AE, Czachowski CL. Intravenous alcohol self-administration in the P rat. Alcohol. 2014;48:419–25. doi: 10.1016/j.alcohol.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;173:146–52. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- Zhou L, Stahl EL, Lovell KM, Frankowski KJ, Prisinzano TE, Aube J, Bohn LM. Characterization of kappa opioid receptor mediated, dynorphin-stimulated [35S]GTPgammaS binding in mouse striatum for the evaluation of selective KOR ligands in an endogenous setting. Neuropharmacology. 2015;99:131–41. doi: 10.1016/j.neuropharm.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]