Abstract
The CD44 gene encodes several protein isoforms due to alternative splicing and post translational modifications. Given that CD44 variant isoform 9 (CD44v9) is expressed within Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) glands during repair, CD44v9 may be play a functional role during the process of regeneration of the gastric epithelium. Here we hypothesize that CD44v9 marks a regenerative cell lineage responsive to infiltrating macrophages during regeneration of the gastric epithelium. Ulcers were induced in CD44-decient (CD44KO) and C57BL/6 (BL6) mice by a localized application of acetic acid to the serosal surface of the stomach. Gastric organoids expressing CD44v9 were derived from mouse stomachs and transplanted at the ulcer site of CD44KO mice. Ulcers, CD44v9 expression, proliferation and histology were measured 1, 3, 5 and 7-days post-injury. Human-derived gastric organoids were generated from stomach tissue collected from elderly (>55 years) or young (14–20 years) patients. Organoids were transplanted into the stomachs of NOD scid gamma (NSG) mice at the site of injury. Gastric injury was induced in NRG-SGM3 (NRGS) mice harboring human-derived immune cells (hnNRGS) and the immune profile analyzed by CyTOF. CD44v9 expression emerged within regenerating glands the ulcer margin in response to injury. While ulcers in BL6 mice healed within 7-days post-injury, CD44KO mice exhibited loss of repair and epithelial regeneration. Ulcer healing was promoted in CD44KO mice by transplanted CD55v9-expressing gastric organoids. NSG mice exhibited loss of CD44v9 expression and gastric repair. Transplantation of human-derived gastric organoids from young, but not aged stomachs promoted repair in NSG mouse stomachs in response to injury. Finally, compared to NRGS mice, huNRGS animals exhibited reduced ulcer sizes, an infiltration of human CD162+ macrophages and an emergence of CD44v9 expression in SPEM. Thus, during repair of the gastric epithelium CD44v9 emerges within a regenerative cell lineage t hat coincides with macrophage infiltration within the injured mucosa.
Keywords: gastric ulcer, repair, organoids, CD44-deficient mice
INTRODUCTION
Gastric ulcer repair is a complex process that involves tissue re-epithelialization and regeneration. During intestinal regeneration in the context of inflammatory bowel disease, epithelial stem cells from the crypt base at margin of ulcerations form tubes composed of an Ulcer-Associated Cell Lineages (UACL) [1, 2]. The UACL invade granulation tissue, proliferate and differentiate into intestinal crypts within the injured site, contributing to regeneration of the epithelium [1]. To maintain a barrier to protect the granulation tissue from further injury or bacterial infection, migration of epithelial cells from the ulcer margins restore epithelial continuity via a process known as re-epithelialization [1, 2]. Re-epithelialization requires epithelial cell migration and proliferation [1, 2]. Data from our laboratory demonstrate the emergence of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) glands localized to the base of the ulcer margin in stomach mucosa, in a position similar to where the UACL is initiated during repair in the intestine [1]. Our data suggest that SPEM represents the major reparative lineage responsible for wound healing after severe gastric injury.
SPEM is associated with increased expression of cell surface protein CD44, in particular variant isoform 9 (CD44v9) [3, 4]. Emergence of SPEM accompanied with CD44v9 expression is significant because CD44v9 is known to contribute to defense against reactive oxygen species (ROS) [3, 5, 6]. Within cancer cells, CD44v9 interacts with the glutamate-cysteine transporter xCT, stabilizes the protein and thereby potentiates defense against ROS, thus promoting tumor growth [6]. During mucosal injury and hypoxic-ischemic conditions, ROS are continuously produced. The resulting oxidative stress is crucial for the development of epithelial apoptosis and necrosis leading to mucosal injury and erosions [7]. We may hypothesize that the emergence of CD44v9 during gastric ulcer repair potentiates defense against ROS by stabilizing xCT and thus promoting effective regeneration in response to injury. The current study is the first to investigate the role of CD44v9 in the context of gastric epithelial regeneration in response to injury.
The immune system is also a crucial player for tissue repair, including the kidney [8, 9], and skin [10]. In particular, macrophages are key immune cells that secrete cytokines, chemokines and pro-angiogenic factors that are necessary for repair [11]. Typically, inflammatory cues within the regional microenvironment can prime macrophages into a reparative phenotype. Inflammatory monocytes are recruited in response to cytokine cues and undergo differentiation into two distinct subsets of macrophages that are typically categorized as either classically activated (M1) or alternatively activated (M2) [9]. M2 macrophages represent various phenotypes that can be further subdivided into M2a, M2b and M2c [12]. M2 macrophages may secrete trophic factors that suppress the pro-inflammatory response and promotes wound healing [9]. For example, during skeletal muscle regeneration, macrophages are first recruited as phagocytic cells with elevated release of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor (TNF-α), and then rapidly convert into an anti-inflammatory phenotype to promote myogenesis [13]. During regeneration in the injured kidney, macrophages produce Wnt7b to stimulate tissue repair [8]. Importantly, in the stomach, studies of mouse models and human metaplastic tissues indicate that M2 macrophages promote the advancement of SPEM in the presence of inflammation and parietal cell atrophy [14]. Given that CD44v9 marks SPEM glands, here we also address the question of whether macrophages are associated with the emergence of CD44v9 during gastric repair in response to injury.
Using a gastric organoid orthotopic transplantation model, the current study demonstrates that CD44v9 emerges at the ulcer margin in response to injury and contributes to the regeneration of the gastric epithelium. Moreover, in a mouse model expressing human-derived immune cells, macrophages infiltrate the injured gastric mucosa where these immune cells may induce the emergence of CD44v9 during regeneration of the epithelium.
MATERIALS AND METHODS
Mouse-derived gastric organoid cultures
Organoids were derived from C57/BL6 (The Jackson Laboratory, stock number: 000664) or B6.129(Cg)-Cd44tm1Hbg/J (CD44-deficient) mice (The Jackson Laboratory, stock number stock number: 005085) as previously described [15]. Briefly, mouse stomachs were dissected along the greater curvature, and washed in Ca2+/Mg2+-free Dulbecco’s Phosphate Buffered Saline (DPBS). The muscle layer was removed from the mucosa, tissue dissected into fragments and incubated at 4°C for 2 hours in DPBS with 5 mM EDTA with gentle rocking. Glands were harvested by shaking in a D-sorbitol/sucrose solution for 2 minutes, and centrifuged at 65×g for 5 minutes. Glands were re-suspended in Matrigel and supplemented with gastric organoid growth medium (Advanced DMEM/F12 medium (Thermo Fisher Scientific), 50% Wnt conditioned medium, 10% R-spondin conditioned medium, 1X Penicillin/Streptomycin, 1X B27, 1X N2, 1mM N-Acetylcysteine, supplemented with gastric growth factors including bone morphogenetic protein inhibitor, (Noggin, PeproTech), Gastrin I (Tocris), Epidermal grow factor (EGF, PeproTech), Y-27632 (Sigma), and Fibroblast growth factor 10 (FGF-10, PeproTech)) as previously described [15] and allowed to grow for 7 days prior to transplantation into CD44-deficient mice.
Human-derived gastric organoid cultures
Human organoids were derived from young (15–21 years old) and aged (>55 years old) patient stomach tissue collected from sleeve gastrectomies (IRB Protocol number 2015–4869) as previously described [16]. Briefly, stomachs were washed with DPBS, and mucosa was collected and cut into small fragments. Tissue fragments were incubated at 37°C supplemented with oxygen flow in DMEM/F12 with 1mg/mL Collagenase and 2mg/mL Bovine Serum Albumin. Glands were filtered and centrifuged at 65 × g for embedding into Matrigel. Human gastric organoid growth media was changed every 4 days (Advanced DMEM/F12 medium (Thermo fisher), 50% Wnt conditioned medium, 20% R-spondin conditioned medium, 1X Penicillin/Streptomycin, 1X Amphotericin/Gentamicin, 1X Kanamycin, 1X B27, 1X N2, 1mM N-Acetylcysteine, 10mM Nicotinamide, supplemented with growth factors including bone morphogenetic protein inhibitor, (Noggin, PeproTech), Gastrin I (Tocris), Epidermal grow factor (EGF, PeproTech), Y-27632 (Sigma), and Fibroblast growth factor 10 (FGF-10, PeproTech)) [16]. Human organoids were cultured at 37°C for 7 days prior to transplantation into NOD scid gamma (NSG) mice.
Cytometry-Time of Flight (CyTOF)
Mouse stomachs were collected from uninjured or injured areas of NRGS and huNRGS animals. Tissue was cut into fragments (approximately 2–5mm), and transferred to pre-warmed EDTA stripping buffer (5mM EDTA, 25mM HEPES, 10% Fetal Calf Serum in Hank’s Balanced Salt Solution (HBSS) (Corning, 12021CV)) for 10 minutes in a 37°C shaking incubator. Fragments were suspended in fresh EDTA stripping buffer for an additional 5 minutes in a 37°C shaking incubator. Tissue was washed with 1X HBSS, then re-suspended in collagenase digestion buffer (1.5mg/mL collagenase from Clostridium histolyticum (Sigma, C989), 20µg/mL hyaluronidase from bovine testes (Sigma, H3884), 1X Penicillin / Streptomycin in RPMI (Corning, 10041CV)) for approximately 30 minutes in a 37°C shaking incubator, or until tissue was digested. The digest was diluted in ice-cold DPBS and filtered through a 40µm filter. Cells were centrifuged at 1200 rpm for 5 minutes and re-suspended in 1mL PBS for viability staining. For 1mL of PBS for approximately 1×107 cells, 1µL (5µM) of Cell-ID™ Cisplatin was added for 5 minutes at room temperature, according to manufacturer’s instructions (DVS Sciences, 201064). For CyTOF analysis, the MaxPar® Cell Surface Staining Protocol was followed according to manufacturer’s instructions (Fluidigm, PRD012). Briefly, the Cisplatin reaction was quenched with 5X volume of MaxPar® Cell Staining Buffer, and cells were centrifuged at 400 × g for 5 minutes. The antibody cocktail was prepared with 1µL of each antibody for 3 million cells in a 100µL staining volume of MaxPar® Cell Staining Buffer, and cells were stained for 30 minutes at room temperature. Cells were washed twice in 1mL MaxPar® Cell Staining Buffer and centrifuged at 400 × g for 5 minutes. Cells were re-suspended in 1mL cell intercalation solution (125nM Cell-ID Intercalator-Ir in Maxpar Fix and Perm Buffer) for shipment overnight on ice to the University of Rochester Medical Center, New York where final washes with Maxpar water were performed according to manufacturer’s instructions and the samples were run on CyTOF machine. Data was analyzed using Premium CytoBank Software.
Material and Methods details for Immunofluorescence and Immunohistochemistry, Whole mount staining of gastric organoids, FACS and Statistical analyses can be found in Supporting Methods section.
RESULTS
CD44v9 expression emerges during gastric regeneration in response to injury
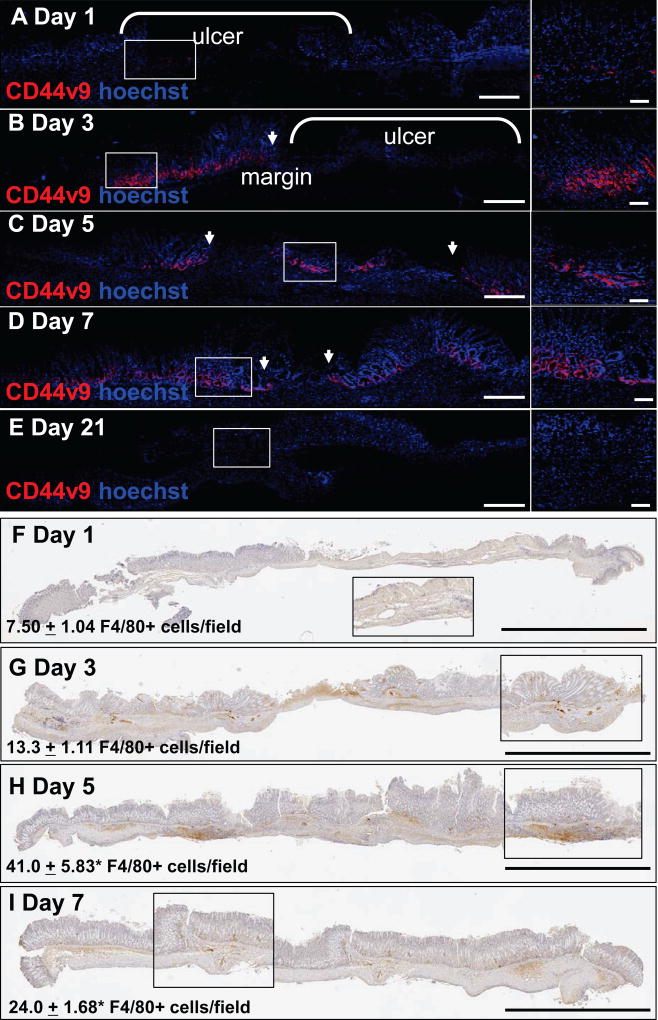
Previously, we identified CD44v9 as a marker of SPEM that emerges within the regenerating gastric epithelium during repair [4]. As such, we first investigated the pattern of CD44v9 emergence during repair in response to injury. CD44v9 expression was observed at the ulcer margin within 3 days after injury (Figure 1B), and most prominent 5 days post-injury (Figure 1C). As the gastric epithelium healed, CD44v9 expression was rarely expressed (Figure 1D, E). The emergence of CD44v9 correlated with the infiltration of macrophages (Figure 1F–I) that peaked 5 days post-injury (Figure 1H). These data show that the expression of CD44v9 is transient during repair of the gastric epithelium and correlates with the infiltration of macrophages.
Figure 1. CD44v9 expression during gastric regeneration.
(A–E) Immunofluorescent staining of CD44v9 (red) at 1, 3, 5, 7, and 21 days after ulcer injury in C57BL/6 mice. Arrows indicate ulcer margin. (F) Immunofluorescent staining of CD44v9 (red) in gastric organoids derived from BL6 mice. Scale bar tile scan = 200µm, scale bar higher magnification = 50µm. (F–I) Immunohistochemistry of F4/80 positive cells (brown) 1, 3, 5 and 7 days after ulcer injury in C57BL/6 mice. Higher magnifications are shown in insets. Scale bar = 2mm. Cell counts are recorded as F4/80+ cells/field. *P<0.05 compared to day 1 counts, n = 4 mice/group. Arrows indicate ulcer margin.
Loss of epithelial repair in CD44KO mice is restored by gastric organoid transplantation
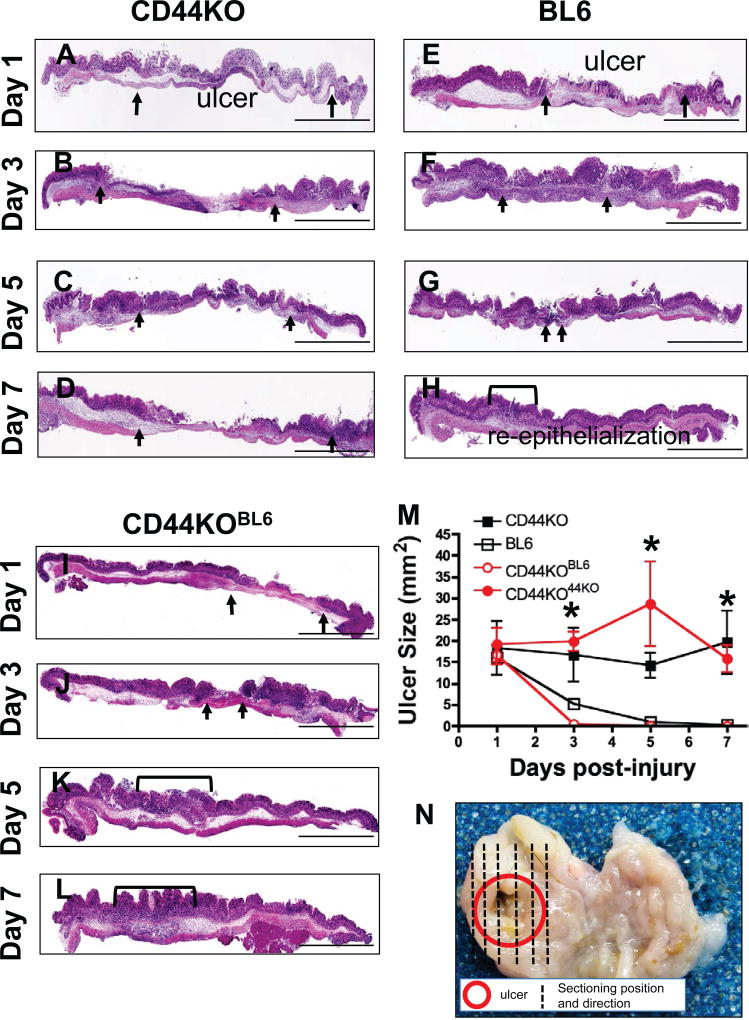
To study the functional role of CD44 in ulcer repair, CD44v9 expressing gastric cells were introduced into the gastric epithelium of CD44-deficient mice. This was achieved by transplantation of organoids derived from BL6 mice using the injury/orthotopic gastric organoid transplantation model (Supplemental Figure 1A, B). Four experimental groups were studied: 1) CD44-deificient (CD44KO) mice with induced ulcers, 2) BL6 mice with induced ulcers, 3) CD44KO mice with induced ulcers and transplanted with organoids derived from BL6 mice (CD44KOBL6), and 4) CD44KO mice with induced ulcers transplanted with organoids derived from CD44KO mice (CD44KO44KO) (Supplemental Figure 1C). Compared to BL6 mice, which exhibited re-epithelialization by 7 days post-injury (Figure 2E–H, M), CD44KO mice demonstrated a loss of epithelial repair (Figure 2A–D, M). However, CD44KO mice transplanted with CD44v9-expressing gastric organoids (CD44KOBL6) demonstrated epithelial repair that was comparable to the BL6 group (Figure 2I–L, M). Importantly, compared to CD44KOBL6 mice, the CD44KO44KO groups exhibited loss of normal repair (Figure 2M). Figure 2N shows representative gross morphology of ulcer size 3 days post-injury and the sectioning position and direction of tissues. High power magnifications of the ulcerated regions from all experimental mouse groups in Figure 2, are shown in Supplemental Figure 2. These data suggest that CD44 contributes to gastric ulcer repair.
Figure 2. Injury/orthotopic gastric organoid transplantations.
Hematoxylin and Eosin (H&E) stains of stomach tissue collected from (A–D) CD44KO, (E–H) BL6 and (I–L) CD44KOBL6 mice 1, 3, 5 and 7 days post-injury. Scale bar = 2mm. (M) Gastric ulcer sizes from CD44KO, BL6, CD44KOBL6 and CD44KO44KO mice. *P<0.05 compared to BL6 group, n = 4–8 mice/group. Representative gross morphology showing ulcerated area (red) and sectioning position and direction of stomach collected 3 days post-injury.
CD44v9 expression correlates with increased epithelial cell proliferation
CD44 is known to coordinate the proliferation of progenitor cells within the normal and metaplastic gastric epithelium [17]. This is of significance because epithelial proliferation plays a fundamental role in the regeneration of gastric tissue after injury [2]. Co-immunofluorescence staining for CD44v9 and BrdU incorporation was used to quantify the number of CD44v9/BrdU+ve proliferating cells at the ulcer margin of BL6, CD44KO and CD44KOBL6 mice at 1, 3, 5, and 7 days post-injury (Figure 3). As expected, there was no expression of CD44v9 in CD44KO mice (Figure 3A–D). However, there was a robust emergence of CD44v9 expression at 1, 3, 5, and 7 days post-ulcer injury in BL6 mice (Figure 3E–H). Importantly, there was engraftment of organoid-derived CD44v9-expressing cells in CD44KOBL6 mice (Figure 3I–L). Compared to CD44KO mice, BL6 mice exhibited a significant increase in the number of CD44v9/BrdU+ve proliferating cells (Figure 3M), that was consistent with an overall increase in proliferating cells that were negative for CD44v9 (Figure 3N). This significant induction in cell proliferation was rescued in the CD44KOBL6 mice (Figure 3M), suggesting that CD44v9 is expressed in proliferating cells.
Figure 3. CD44v9 expression and epithelial proliferation in response to gastric injury.
Immunofluorescence staining of CD44v9 (green) and BrdU (red) at the ulcer margin of (A–D) CD44KO, (E–H) BL6, and (I–L) CD44KOBL6 mice 1, 3, 5 and 7 days post-injury. (M) Quantification of CD44v9+/BrdU+ cells/gland from gastric ulcers 1, 3, 5 and 7 days post-injury from CD44KO, BL6 and CD44KOBL6 mice. (N) Quantification of BrdU+/CD44v9− cells/gland from gastric ulcers 1, 3, 5 and 7 days post-injury from CD44KO, BL6 and CD44KOBL6 mice. *P<0.05 compared to BL6 group, n=4. Scale bar = 50µm
To identify the regeneration and differentiation of organoid cells in vivo, immunofluorescent staining for CD44v9 organoid-derived cells, parietal cells (H+K+-ATPase) and surface mucous cell (UEAI) in CD44KO mice transplanted with BL6 organoids was performed (Supplemental Figure 3). These data demonstrated that CD44v9 was not expressed in parietal cells within the regenerating epithelium. Importantly, our data shows that 21 days post-injury and transplantation CD44v9 expression is decreased and rarely detected. These data suggest that the expression of CD44v9 is transient during regeneration.
Gastric organoids derived from young patients have an increased regenerative capacity compared to those from aged patients
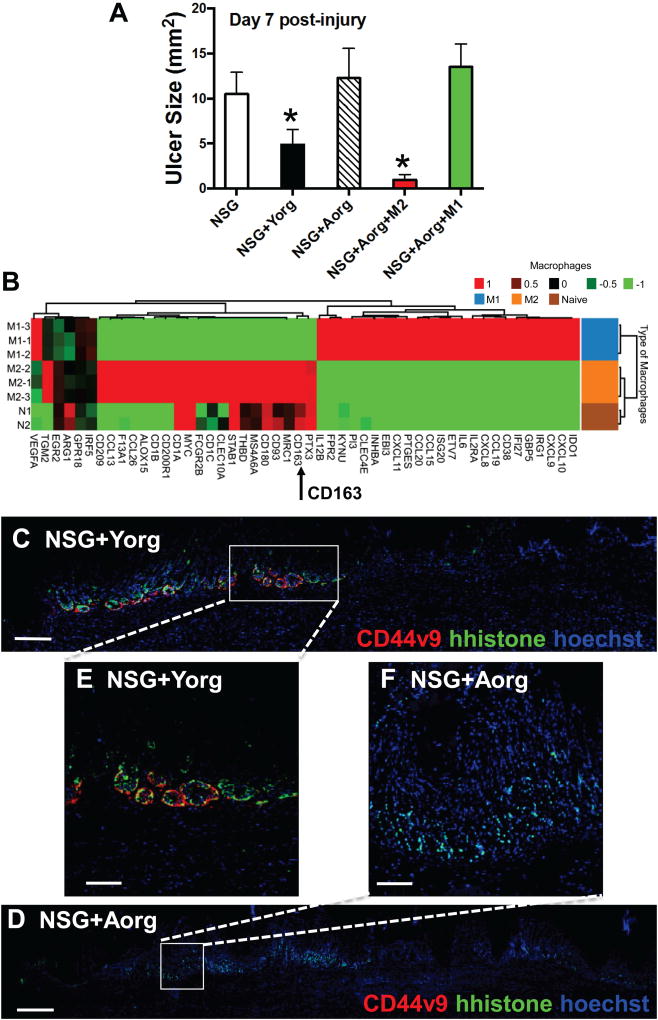
Aging is associated with a decreased regenerative capacity, which may suggest changes in epithelial stem cells [18, 19]. Indeed, compared to young mice (2–4 months), aged animals (>18 months) exhibit abnormal gastric morphology 9 months post-injury. While young mouse stomachs return to normal gastric gland morphology (Supplemental Figure 4A), aged animals develop severe foveolar hyperplasia (Supplemental Figure 4B). Moreover, human organoids derived from young patients exhibited CD44v9 expression and significantly increased epithelial cell proliferation when compared to organoids derived from aged patients (Supplemental Figure 4C–G). Organoids prior to immunostaining are shown in Supplemental Figure 4H and I. To determine the regenerative capacity of young and aged human-derived gastric organoids, orthotopic transplantations were performed after ulcer induction in NSG mice. Stomachs of NSG mice without transplantation of organoids did not repair 7 days post-injury (Figure 4A). In contrast to non-transplanted NSG mice, those transplanted with organoids derived from young patient stomachs exhibited significantly smaller ulcer sizes (Figure 4A). NSG mice transplanted with organoids derived from aged patient stomachs showed significantly larger ulcers 7 days post-injury, comparable to the non-transplanted group (Figure 4A). H&E stains of representative sections collected from NSG mouse experimental groups are shown in Supplemental Figure 5. To address the role of CD163+/CD45+ macrophages as a regulatory cell during repair, NSG mice transplanted with Aorg were co-injected with either M1 or M2 polarized macrophages isolated from humanized NRGS mice (Figure 4A, B). M1 and M2 polarized macrophages were aligned to human-specific genes that clearly showed unique gene profiles between the phenotypes (Figure 4B). In particular, CD163 was highly expressed in M2 polarized macrophages (Figure 4B). While co-injection of M1 macrophages did not drive repair in NSG+Aorg mice, M2 macrophages significantly reduced the ulcer size in these animals (Figure 4A).
Figure 4. Injury/orthotopic transplantation model using gastric organoids derived from young and aged patients.
(A) Gastric ulcer sizes 7 days post-injury in NSG, NSG mice transplanted with gastric organoids derived from young patients (NSG+Yorg) and NSG mice transplanted with gastric organoids derived from aged patients (NSG+Aorg), NSG+Aorg co-injected with M2 polarized macrophages or NSG+Aorg co-injected with M1 polarized macrophages 7 days post-injury. (B) Heat map generated from RNAsequencing data aligned to human-specific genes of macrophages isolated from huNRGS mice and polarized to either M1 or M2 phenotype. Immunofluorescence staining of CD44v9 (red) and human histone (hhistone, green) in NSG mice transplanted with (C) young or (D) aged human-derived gastric organoids 7 days post-injury. Higher magnification of day 7 images shown in (E, F). *P<0.05, n=4–8 mice per group. Scale bar tile scan = 200µm, scale bar higher magnification = 50µm.
Engraftment was documented by the expression of human-specific histone (hhistone) in mouse stomachs transplanted with either young (Figure 4C, E) or aged (Figure 4D, F) human-derived gastric organoids. Importantly, NSG mice transplanted with young human-derived gastric organoids expressed human-specific CD44v9 at the ulcer margin 7 days post-injury (Figure 4C, E). However, CD44v9 expression was absent in NSG mice transplanted with aged human-derived gastric organoids (Figure 4D, F). Representative image of an untransplanted NSG mouse stomach is shown in Supplemental Figure 5D. Collectively, these data show that human-derived gastric organoids engraft within the mouse epithelium and contribute to repair. In addition, macrophages with an M2 phenotype drive repair in the stomachs of NSG mice.
Macrophage infiltration decreases in the aged, as compared to young stomachs in response to injury
Macrophages are recruited to the ulcer site and are known to secrete growth factors and cytokines necessary for wound repair [2]. This is of significance given that it has been documented that M2 macrophages promote the advancement of SPEM in the presence of inflammation [14]. Indeed, we observed an increase in the myeloid cell number at the ulcer site of young mice 3 days after ulcer induction (Supplemental Figure 6A). By marked contrast to the young group, aged mice did not show recruitment of macrophages 3 days after ulcer induction (Supplemental Figure 6B). Tissue was collected from the ulcerated region, and the expression of TH2 cytokines was analyzed by qRT-PCR (Supplemental Figure 6C, D). Compared to the aged mice, within the injured tissue collected from young mouse stomachs 3 days post-injury there was a significant increase in IL-13, IL-4 and IL-33 (Supplemental Figure 6C, D) that is consistent with the expression of IL-33 and IL-13 as promoters of SPEM [20]. However, within the injured tissue of stomachs collected from aged mice these increases were not observed (Supplemental Figure 6D). Stomachs from both the uninjured and injured groups of mice were collected, enzymatically dissociated and F4/80+ macrophages isolated by FACS (Supplemental Figure 6E). Gene expression indicative of M1 and M2 phenotypes was examined from FACS sorted macropahges (Supplemental Figure 6F, G). We observed that while the expression of M1 markers (Supplemental Figure 6F) were low or undetected in macrophages isolated from injured tissue, these cells expressed significant amounts of M2 markers (Supplemental Figure 6G). These data demonstrate that there is a skewed cytokine microenvironment observed in the young versus aged animals, which correlates with an increase in M2 macrophage markers in the young injured tissue.
Gastric repair in a humanized immune cell mouse model correlates with an infiltration of CD163+ macrophages and CD44v9 expression
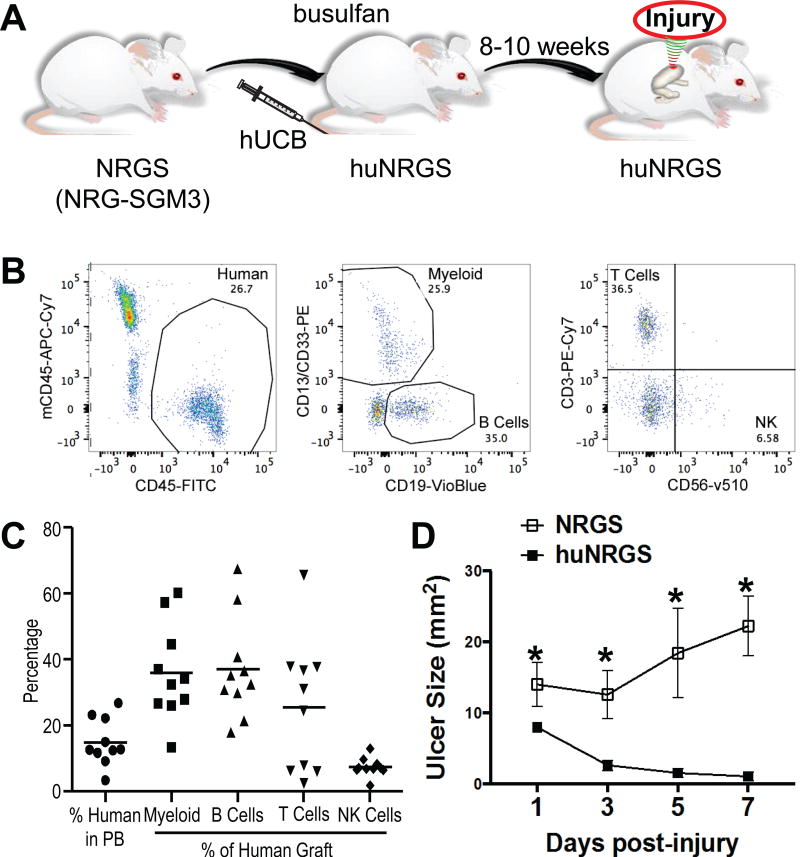
To identify the role of the immune response during repair, gastric regeneration in response to injury was studied in a mouse model expressing human immune cells [21]. NRGS mice were engrafted with human umbilical cord blood (UBC) and after 8–10 weeks the presence of human immune cells were confirmed by flow cytometric analysis (Figure 5A–B). Figure 5C demonstrates the percent of human cells in peripheral blood of NRGS mice 8–10 weeks after engraftment, and also shows the percent of human myeloid, B, T, and natural killer (NK) cells. Ulcers were induced in humanized (huNRGS, UBC engrafted) and non-engrafted (NRGS) mice and ulcer sizes measured after 1, 3, 5, and 7 days post-injury. Compared to NRGS mice, huNRGS animals exhibited significantly reduced ulcer sizes within 3 days post-injury (Figure 5D), suggesting a role for the immune response in repair.
Figure 5. Generation of humanized mice for the study of the human immune response during ulcer injury.
(A) Schematic representation of ulcer injury in NRG-SGM3 (NRGS) mice transplanted with human umbilical cord blood for the study of the human immune response. (B) Representative FACS analysis of cell composition of one sample obtained from an NRGS mouse 10 weeks after engraftment with 7.5 million cord blood cells. Human cells are detected by positive staining with human specific CD45 (FITC) and negative staining for mouse CD45 (APC-Cy7). (C) These cells contain various lineages of blood cells as determined by staining with CD13 and CD33 (myeloid cells), CD19 (B cells), CD3 (T cells), and CD56 (NK cells). This analysis was done on 10 separate mice shown as individual points. The bars represent averages. Lineages are shown as a percentage of the human cell population. (D) Ulcer sizes of non-engrafted NRGS and engrafted NRGS (huNRGS) mice 1, 3, 5, and 7 days post-injury.
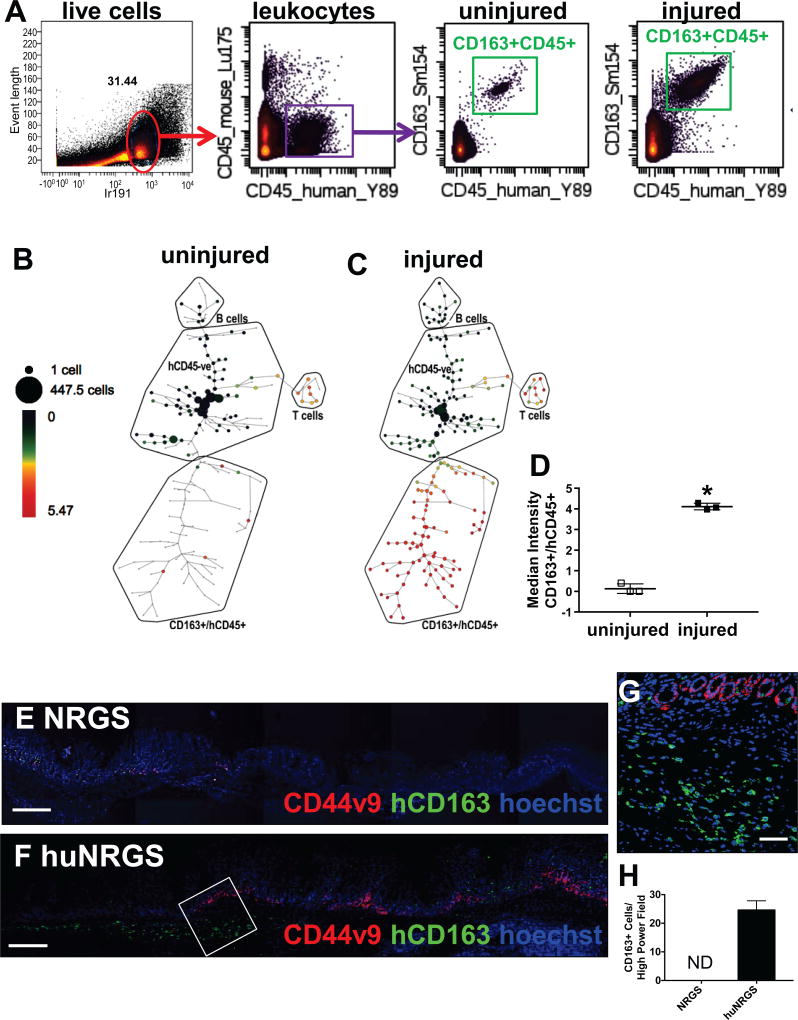
To determine the immune phenotype following ulcer injury in huNRGS mice, mass cytometry time-of-flight (CyTOF) analysis was performed on uninjured and injured stomachs 3 days after ulcer induction. Bivariate plots compare human-derived immune cells measured in mouse uninjured and injured stomach tissue (Figure 6A). Heat/intensity corresponds to cell abundance in a plot region for the population indicated above the plot (Figure 6A). Intact cells (31.44%, red gate) were defined using event length and an iridium based cell marker (Ir-191 intercalator) (Figure 6A). Among the intact cells, human leukocytes were defined as CD45+ events (Figure 6A). CyTOF analysis showed that compared to the uninjured huNRGS mouse stomachs, hCD45+ cells within the injured huNRGS mouse stomachs highly expressed CD163 (Figure 6A), a scavenger receptor expressed exclusively on cells of the monocyte/macrophage lineage in particular M2 macrophages [22]. In support of this, CD163+ cells were negative for T cell markers CD4 and CD3 (Supplemental Figure 3A, B). We identified cell subpopulations using spanning-tree progression analysis of density-normalized events (SPADE) [23] (Figure 6B, C). SPADE analysis is an unsupervised hierarchical clustering technique, in which the size of each node is the relative cell frequency, and the color is the median intensity of the expression of each marker (Figure 6B, C). Compared to the uninjured stomachs of huNRGS mice (Figure 6B, D), there was a significant increase in human-specific CD45+/CD163+ cells infiltrating the injured tissue (Figure 6C, D). Infiltration of CD163+ macrophages into the injured epithelium was confirmed by immunofluorescence (Figure 6F, H), that was not observed in the non-engrafted NRGS mice (Figure 6E, H). Importantly, macrophage infiltration correlated with elevated CD44v9 expression (Figure 6F,G). These data demonstrate a central role of the human immune response in ulcer repair, and identified the infiltration of CD163+ macrophages into the ulcer site. Moreover, macrophage infiltration correlated with increased expression of CD44v9 within the regenerating gastric epithelium.
Figure 6. Expression of human CD163+ macrophages in huNRGS mice post-injury.
(A) Bivariate plots compare human-derived immune cells measured in mouse uninjured and injured stomach tissue. Heat/intensity corresponds to cell abundance in a plot region for the population indicated above the plot. Among the intact cells (red gate), human leukocytes were defined as CD45+ events (purple gates). Expression of CD45+/CD163+ (green gate) cells in uninjured and injured stomach tissues. SPADE tree analysis of (B) uninjured and (C) injured stomach tissues collected from huNRGS mice 3 days post-injury. (D) Quantification of CD45+/CD163+ve cells in uninjured and injured huNRGS mouse stomachs. *P<0.05 compared to uninjured tissue, n = 4 mice/group. Immunofluorescence staining of CD44v9 (red) and human-specific CD163 (green) in gastric tissue collected from (E) NRGS and (F–G) huNRGS mice 3 days post-injury. (H) Quantification of CD163+ cells/high power field in NRGS and huNRGS mice. ND = not detected, n = 4 mice/group. Scale bar tile scan = 200µm, scale bar higher magnification = 50µm.
DISCUSSION
In the current study we show that CD44v9 emerges within the gastric epithelium in response to injury and may contribute to tissue regeneration. In mice lacking CD44 including CD44v9 (CD44KO), ulcer repair was impaired as indicated by the lack of re-epithelialization and epithelial gland regeneration, persistence of prominent ulcer sizes and loss of epithelial proliferation. Using an injury/orthotopic organoid transplantation model developed by our laboratory [4], CD44v9-expressing gastric organoids engrafted within the epithelium of CD44KO mice and subsequently resulted in induced epithelial cell proliferation and accelerated ulcer repair. Wound healing requires a complex process of biological and molecular events including angiogenesis, immune cell activation, tissue remodeling, and cell migration and proliferation [2, 24–26]. The emergence of CD44v9 at the ulcer margin correlated with increased epithelial cell proliferation, which is significant because CD44 is known to coordinate the proliferation of progenitor cells within the normal and metaplastic gastric epithelium [16, 17]. Importantly, CD44v9 is known to interact with xCT, a glutamate-cystine transporter, and regulates the intracellular level of reduced glutathione [6]. Human gastrointestinal cancer cells with a high level of CD44 expression showed an enhanced capacity for glutathione synthesis and defense against ROS [5, 6, 27]. Deletion of CD44 induced loss of xCT from the cell surface and suppressed gastric cancer tumor growth [6]. The role of CD44v9 in redox regulation is significant given that oxidative stress, caused in response to injury, is crucial for the development of epithelial apoptosis and necrosis leading to ulceration [7]. We may speculate that the emergence of CD44v9 during gastric ulcer repair potentiates defense against ROS by stabilizing xCT and thus promoting effective regeneration in response to injury. Collectively, our findings suggest that CD44 is required for normal repair of the gastric epithelium. In particular, the emergence of CD44v9 may have implications in protecting the regenerating epithelium against oxidative stress during repair. However, the precise role of CD44v9 during gastric regeneration requires further investigation.
Despite evidence showing that gastric ulcers and subsequent complications are a burden that is focused in the elderly, there is a lack of knowledge as to the molecular mechanisms that disrupt normal repair even after Helicobacter pylori (H. pylori) eradication within the aged stomach [28, 29]. While it is established that the incidence of gastric ulcers in the elderly is primarily due to increased use of medications, including NSAIDs, and increased H. pylori infection, repair from gastric injuries is also impaired [28, 29]. Evidence suggests that ulcers do not heal normally in the aged stomach. We demonstrate here that repair within the aged stomach is compromised whereby the gastric epithelium does not heal normally. In addition, we have reported that the aged epithelium exhibits an abnormal cell composition and decreased CD44v9 expression post-repair [4]. There are a number of mechanisms of action that prevent the gastric mucosa in the elderly from healing normally after being subjected to injury. For example, aging is associated with impaired gastric angiogenesis [30–32] and decreased regenerative capacity suggesting changes in epithelial stem cells [18, 19]. The capacity of organoids derived from aged human stomachs to facilitate repair in NSG mice was lost, when compared to organoids derived from young patients. This correlated with not only a loss of epithelial proliferation, but also a lack of CD44v9 expression within the organoid cultures. These data emphasize a critical role for the emergence of CD44v in normal ulcer repair in the human stomach. Here, we show that engraftment of cells through the transplantation of gastric organoids contributes to ulcer repair, and suggests a plausible role of CD44v9 during regeneration of the gastric epithelium that is compromised with aging.
Our data showing that aged mice lack an infiltration of macrophages to the ulcer injury site is consistent with studies describing impairment of the immune response as a result of aging [33]. CD163+/CD45+ macrophages are recruited to the gastric epithelium of young mice. Moreover, transplantation of CD163+ macrophages in NSG+Aorg mice facilitates rapid ulcer repair. Our group has published studies demonstrating that Hedgehog ligand, Sonic Hedgehog (Shh), is secreted from the gastric parietal cell in response to injury and subsequently contributes to epithelial repair using parabiosis experiments [34, 35]. Importantly, we have demonstrated that Shh acts as a macrophage chemoattractant during the initiation of H. pylori-induced gastritis using bone marrow chimera experiments [36]. Based on these studies, we may hypothesize that Hedgehog acts a chemoattractant for macrophages to the injured gastric epithelium.
We, and others have previously shown CD44v9 is a marker of SPEM [3, 4], and is lost in the human aged stomach after ulcer injury [4]. To identify a potential mechanism that results in induction of SPEM, we turned our attention to alternatively activated, or M2 macrophages. It was recently reported that M2 macrophages promote expansion and intestinalization of SPEM in the setting of parietal cell loss and inflammation [14]. Using a novel ‘humanized’ mouse model we demonstrated that engraftment of human immune cells was required for ulcer healing. Furthermore, CyTOF analysis of injured humanized mice revealed the specific upregulation of CD163, a scavenger receptor selectively expressed on M2 macrophages. Previous studies implicate CD163 in the clearance of dead cells following tissue injury [22, 37, 38]. Using these innovative approaches, we show that the immune response contributes to gastric ulcer repair. Our future goals are to identify the potential mechanism by which CD163+ macrophages induce the expression of CD44v9 during ulcer repair.
Supplementary Material
Acknowledgments
This work was supported by NIH 2 R01 DK083402-06 grant, College of Medicine Bridge Funding Program (Zavros), and the University of Cincinnati Graduate School Dean’s Fellowship, Albert J. Ryan Fellowship and 2T32GM105526-04 (Bertaux-Skeirik) and a Department of Veterans Affairs Merit Review Award (I01BX000930) and NIH R01 DK071590 (Goldenring). This project was supported in part by PHS Grant P30 DK078392 (Integrative Morphology Core) and NIH AR-47363 (Research Flow Cytometry Core in the Division of Rheumatology) of the Digestive Diseases Research Core Center in Cincinnati. The generation of humanized mice was supported by the National Cancer Institute of the National Institutes of Health Award Number R50CA211404.
Footnotes
Conflict of Interest;
The authors have nothing to declare
REFERENCE LIST
- 1.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- 2.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50:S24–S33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 3.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engevik AC, Feng R, Choi E, et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. CMGH. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191–5198. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 6.Ishimoto T, Nagano O, Yae T, et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc(-) and Thereby Promotes Tumor Growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahdavian Delavary B, van der Veer WM, van Egmond M, et al. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Tarnawski A, Stachura J, Durbin T, et al. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- 12.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen CP, Weis VG, Nam KT, et al. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146:1727–1738. e1728. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher MA, Aihara E, Feng R, et al. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–1827. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertaux-Skeirik N, Feng R, Schumacher MA, et al. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. 2015;11(2):e1004663. doi: 10.1371/journal.ppat.1004663. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khurana SS, Riehl TE, Moore BD, et al. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinsull SM. Effect of colloidal bismuth subcitrate on age related gastric lesions in the rat. Gut. 1991;32:355–360. doi: 10.1136/gut.32.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkwood TB. Intrinsic ageing of gut epithelial stem cells. Mech Ageing Dev. 2004;125:911–915. doi: 10.1016/j.mad.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut. 2017 doi: 10.1136/gutjnl-2016-312779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wunderlich M, Brooks RA, Panchal R, et al. OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues. Blood. 2014;123:e134–144. doi: 10.1182/blood-2014-02-556340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akahori H, Karmali V, Polavarapu R, et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat Commun. 2015;6:7792. doi: 10.1038/ncomms8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu P, Simonds EF, Bendall SC, et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol. 2011;29:886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright NA. Aspects of the biology of regeneration and repair in the human gastrointestinal tract. Philos Trans R Soc Lond B Biol Sci. 1998;353:925–933. doi: 10.1098/rstb.1998.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 26.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 27.Ju HQ, Lu YX, Chen DL, et al. Redox Regulation of Stem-like Cells Though the CD44v-xCT Axis in Colorectal Cancer: Mechanisms and Therapeutic Implications. Theranostics. 2016;6:1160–1175. doi: 10.7150/thno.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salles N. Is stomach spontaneously ageing? Pathophysiology of the ageing stomach. Best Pract Res Clin Gastroenterol. 2009;23:805–819. doi: 10.1016/j.bpg.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Jones JI, Hawkey CJ. Physiology and organ-related pathology of the elderly: stomach ulcers. Best Pract Res Clin Gastroenterol. 2001;15:943–961. doi: 10.1053/bega.2001.0251. [DOI] [PubMed] [Google Scholar]
- 30.Ahluwalia A, Jones MK, Deng X, et al. An imbalance between VEGF and endostatin underlies impaired angiogenesis in gastric mucosa of aging rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G325–332. doi: 10.1152/ajpgi.00127.2013. [DOI] [PubMed] [Google Scholar]
- 31.Ahluwalia A, Jones MK, Szabo S, et al. Aging impairs transcriptional regulation of vascular endothelial growth factor in human microvascular endothelial cells: implications for angiogenesis and cell survival. J Physiol Pharmacol. 2014;65:209–215. [PubMed] [Google Scholar]
- 32.Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol. 2014;29(Suppl 4):112–123. doi: 10.1111/jgh.12734. [DOI] [PubMed] [Google Scholar]
- 33.Pinti M, Appay V, Campisi J, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engevik AC, Feng R, Yang L, et al. The Acid-secreting parietal cell as an endocrine source of sonic hedgehog during gastric repair. Endocrinology. 2013;154:4627–4639. doi: 10.1210/en.2013-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao C, Feng R, Engevik A, et al. Sonic Hedgehog contributes to gastric mucosal restitution after injury. Lab Invest. 2013;93:96–111. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher MA, Donnelly JM, Engevik AC, et al. Gastric Sonic Hedgehog Acts as a Macrophage Chemoattractant During the Immune Response to Helicobacter pylori. Gastroenterology. 2012;142:1150–1159. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuluc F, Meshki J, Spitsin S, et al. HIV infection of macrophages is enhanced in the presence of increased expression of CD163 induced by substance P. J Leukoc Biol. 2014;96:143–150. doi: 10.1189/jlb.4AB0813-434RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.