Abstract
Patients with overgrowth and complex vascular malformation syndromes, including Proteus syndrome have an increased risk of thromboembolism. Proteus syndrome is a mosaic, progressive overgrowth disorder involving vasculature, skin, and skeleton, and caused by a somatic activating mutation in AKT1. We conducted a comprehensive review of the medical histories and hematologic evaluations of 57 patients with Proteus syndrome to identify potential risk factors for thrombosis. We found that six of ten patients, who were deceased, died secondary to deep venous thrombosis and/or pulmonary embolism. Of the remaining 47 living patients, six had thromboembolic events that all occurred postoperatively and in an affected limb. Eleven of 21 patients had an abnormal hypercoagulable panel including Factor V Leiden heterozygotes, antithrombin III deficiency, positive lupus anticoagulant, or Protein C or S deficiencies. We observed that eight of 17 patients had an abnormal D-dimer level >0.5 mcg/dl, but deep venous thromboses occurred in only four of those with D-dimer >1.0 mcg/dl. We conclude that the predisposition to thrombosis is likely to be multifaceted with risk factors including vascular malformations, immobility, surgery, additional prothrombotic factors, and possible pathophysiologic effects of the somatic AKT1 mutation on platelet function or the vascular endothelium. The D-dimer test is useful as a screen for thromboembolism, although the screening threshold may need to be adjusted for patients with this disorder. We propose developing a registry to collect d-dimer and outcome data to facilitate adjustment of the D-dimer threshold for Proteus syndrome and related disorders, including PIK3CA-Related Overgrowth Spectrum.
Keywords: Proteus syndrome, Thrombosis, Deep vein thrombosis (DVT), Pulmonary embolism (PE)
INTRODUCTION
Proteus syndrome is a highly variable disorder with asymmetric and disproportionate overgrowth, connective tissue nevi, epidermal nevi, dysregulated adipose tissue, and vascular malformations [Figure 1] [Biesecker et al., 1999; Biesecker et al., 2006] caused by a somatic activating mutation in AKT1 [Lindhurst et al., 2011]. Patients with Proteus syndrome have an increased risk for deep vein thrombosis (DVT) and pulmonary embolism (PE) [Figure 2] [Skovby et al., 1993; Eberhard, 1994; Slavotinek et al., 2000; Staub et al., 2006]. Other disorders with vascular malformations, including Klippel-Trenaunay syndrome (KTS) and CLOVES syndrome, which are both in the spectrum of PIK3CA-Related Overgrowth Spectrum (PROS), also have an increased risk for DVT and PE [Mulak et al., 1995; Huiras et al., 2005; Douma et al., 2012; Sapp et al., 2007; Alomari et al., 2010; Keppler-Noreuil et al., 2015], although the magnitude of DVT/PE risk may not be the same in Proteus syndrome and PROS. PIK3CA-Related Overgrowth Spectrum has phenotypic overlap with Proteus syndrome, but is caused by somatic mutations in PIK3CA, which is in the same signaling pathway as AKT1 [Kurek et al., 2012; Lindhurst et al., 2012; Keppler-Noreuil et al., 2014]. Furthermore, there are multiple reports of patients with isolated congenital vascular malformations not associated with an underlying syndrome that have increased risk of DVT and PE [Enjolras et al, 1997; Mason et al, 2001; Mazoyer et al, 2002; Merli, 2005; Dompmartin et al, 2008; Mazoyer et al., 2008; Oduber et al, 2009]. However, in all these conditions, the underlying mechanism for increased risk of thromboembolism remains unknown. To better understand the natural history of DVT and PE and potential causative factors, we set out to evaluate the largest cohort of patients with Proteus syndrome for these complications. We present the clinical findings in 57 individuals with Proteus syndrome, including relevant history, physical examination, radiologic, and laboratory results of the hematologic evaluations. The purposes of this study were to identify potential risk factors for thrombosis in Proteus syndrome, which would form the basis for future etiologic investigations of DVT and PE.
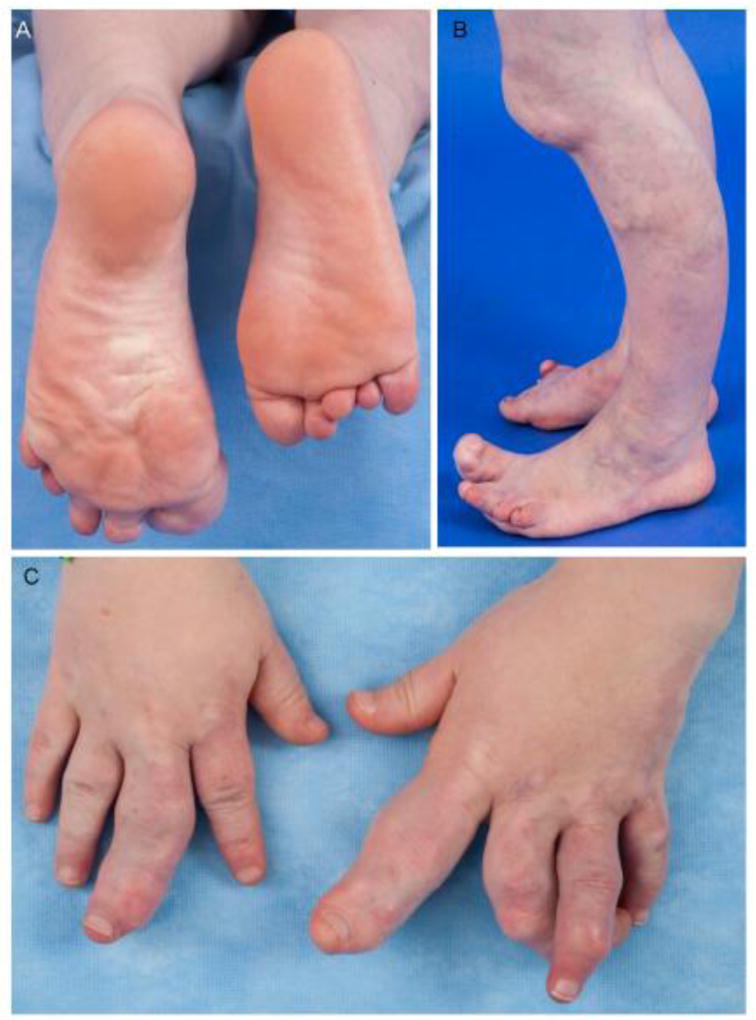
Figure 1.
6.5-year-old boy with Proteus syndrome, (A) developing cerebriform connective tissue nevus of the left foot, and bony overgrowth of bilateral feet and toes, (B) venous malformations with bony overgrowth of legs and feet, (C) bony overgrowth and distortion of the fingers, and capillary malformations of the fingers and hands.
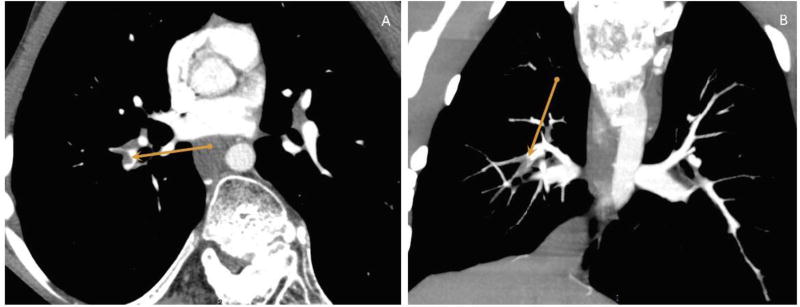
Figure 2.
16-year-old boy with Proteus syndrome, (A) Axial and (B) Coronal, Maximum intensity projection (MIP) contrast enhanced CT scan images of the chest showing an intraluminal filling defect in the descending branch of the right pulmonary artery diagnostic of a pulmonary embolus.
METHODS
Fifty-seven patients with Proteus syndrome were evaluated at the National Institutes of Health between 1997 and 2012, as part of a natural history study approved by the National Human Genome Research Institute (NHGRI) IRB (study # 04-HG-0132). Written informed consent from participants or their guardians was obtained. All met clinical diagnostic criteria for Proteus syndrome [Biesecker et al., 1999; Biesecker, 2006]. Fifty-two of the 57 patients had the somatic activating mutation, c.49G>A, p.Glu17Lys in AKT1 confirmed from the affected tissue samples (data not shown). For the ten of the 57 patients who were deceased, the cause of death was recorded. Of the remaining 47 patients with Proteus syndrome, their medical records were reviewed for relevant findings, medical course, history of superficial and deep venous thromboembolic events, use of anticoagulants, comorbidity, and whether they had a hematology consultation including D-dimer levels, coagulation testing, and pertinent radiologic studies e.g., Doppler ultrasounds of the extremities and CT scan of the chest. Components of the hypercoagulable studies included: PT, PTT, antithrombin III activity, Factor V Leiden mutation, fibrinogen, lupus anticoagulant, Protein C and S activities, prothrombin mutation, c.*97G>A, (G20210A) in F2, thrombin time, and homocysteine. The D-dimer assay used at the NIH is the STA Liatest (Diagnostica Stago, Parsippany, NJ), which has been validated for screening in the general population with a threshold of 0.5 mcg/ml [Aguilar et al., 2002; Kulstad et al., 2004; Rathbun et al., 2004].
RESULTS
Of 57 individuals with Proteus syndrome, six of ten deceased individuals had DVT and bilateral PE as the confirmed cause of death. Review of the 47 living patients, ages two to 66 years, showed six of them had non-fatal thromboembolism. All occurred postoperatively, and the DVT was in an affected limb. One patient had recurrent thromboembolic events. The clinical scenarios in these six patients were as follows: 1) A female, age 14 years with left leg DVT (left popliteal vein with extension to the greater saphenous vein, which was still present at this writing). Her examination showed asymmetric overgrowth of her legs, left more than right, and superficial varicosities. 2) A female, age 40 years with right leg DVT, whose examination showed asymmetric overgrowth of her legs (right more than left). 3) A male, age 18 years, who had four separate episodes of DVTs in the right leg, and after the first event in the upper right leg, he had bilateral PE, which improved after anticoagulation therapy for 6 months. However, 8 months later, he had a DVT in the overgrown right leg. 4) A male, age 16 years with right leg overgrowth, who had right saphenous vein DVT and bilateral PE. 5) A male, age 21 years with left leg overgrowth and prominent varicosities in both legs, who had left popliteal vein DVT; ten years later at 31 years, he was diagnosed with PE and has been treated with warfarin without recurrence. 6) A male, age 16 years with left leg asymmetric overgrowth and left buttock thrombophlebitis, who had left upper thigh femoral vein DVT.
Of the 47 patients, 23 had a NIH hematology consult and 21/47 (44%) had an evaluation for clinical laboratory testing associated with thrombosis. Eleven of the 21 (52%) patients had heterogeneous abnormalities on the hypercoagulable panel, including two with Factor V Leiden heterozygous variants, one with mild Factor 12 deficiency, one with mildly low Anti-thrombin III (ATIII) activities, and Protein C and S deficiencies, one with partial Anti-thrombin III deficiency, one with low Protein C and Protein S activities, one with borderline Lupus anticoagulant and normal aPTT, three with positive Lupus anticoagulant, and one with prolonged PT, which suggested a vitamin K deficiency. One patient with the mildly low ATIII, and protein C and S deficiencies had a DVT. A second patient with low protein C and S activities had three superficial venous clots in the lower extremity. There were two of three patients who had positive lupus anticoagulant; two of these had DVTs, one with thrombophlebitis and the other with a PE. Among the 11 patients with abnormalities on the hypercoagulable panel, four had thromboembolic event, which was not significantly different (p = 0.0902).
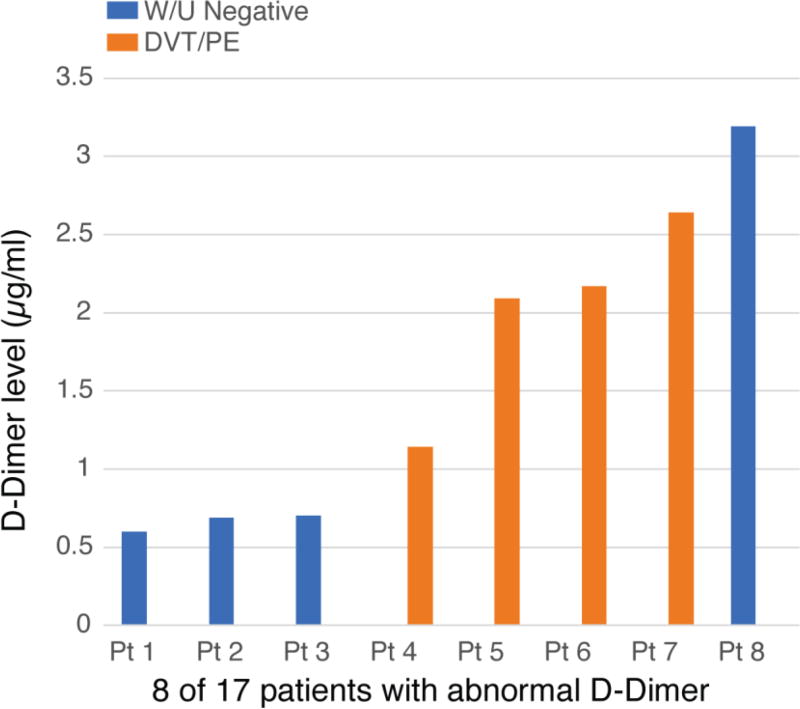
Of the 17 patients who had D-dimer levels, eight had an abnormal level [Figure 3]. Three with elevated levels (0.69, 0.6, 0.7 µg/ml) had no evidence of DVT/PE, on the basis of a normal Doppler ultrasound of the legs and CT scan of the chest (ages 6, 42, and 32 years, respectively). Five individuals had levels >1 mcg/ml (1.14, 2.09, 2.17, 2.64, 3.19 mcg/ml), four had positive findings of DVT and/or PE, and one had recurrent superficial thrombi. Six individuals with median age 16.5 years were diagnosed with DVT (two also had PE) after surgery (of the spine in one and of affected limb in four); one was recurrent. Two of these individuals did not have D-dimer levels [Table 1]. Seven patients had other venous abnormalities. These venous abnormalities were: enlarged portal venous malformation, phlebitis and extensive varicosities, phleboliths, underdevelopment of the deep venous system in the leg, and superficial venous thromboses.
Figure 3.
Abnormal D-dimer levels in 8 of 17 patients with Proteus syndrome, four of which had deep venous thromboembolism and/or pulmonary embolism.
Table I.
Clinical findings in the patients with Proteus syndrome having abnormal D-Dimer levels and/or thrombosis
| Patient | Age (yrs) |
Gender | Affected limb |
DVT in affected limb |
Pulmonary Embolism |
Post- surgery |
D- dimer level (µg/ ml) |
L.A. | Protein C/S Levels |
Antithrombin III activity |
Prothrombin 20210 Mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | F | R Leg | − | − | − | 0.60 | + | nl/nl | nl | − |
| 2 | 6 | M | L Leg | − | − | − | 0.69 | − | nl/nl | nl | − |
| 3 | 32 | M | R Leg | − | − | − | 0.7 | N.D. | N.D. | N.D. | N.D. |
| 4 | 18 | M | R Leg | + | + | + | 1.14 | N.D. | N.D. | N.D. | + |
| 5 | 14 | F | L Leg | + | − | + | 2.09 | − | Low/low | Mildly low | − |
| 6 | 6 | F | R Leg | − | + | + | 2.17 | − | Low/nl | nl | − |
| 7 | 17 | M | R Leg | + | + | + | 2.64 | N.D. | N.D. | N.D. | N.D. |
| 8 | 54 | M | Bil Legs L>R | −* | − | − | 3.19 | − | Low/nl | − | − |
| 9 | 16 | M | L Leg | + | − | + | N.D. | + | N.D. | N.D. | N.D. |
| 10 | 21 | M | L Leg | + | + | + | N.D. | N.D. | N.D. | N.D. | N.D. |
−, none; +, present;
, recurrent superficial thrombi;
abnl, abnormal; Bil, bilateral; DVT, Deep venous thrombosis; L, left; L.A. Lupus Anticoagulant; lat, laterality; N.D., Not done; nl, normal; R, right; yrs, years
DISCUSSION
This retrospective cohort study confirms the conclusions of earlier case reports of an increased risk for DVT and/or PE in individuals with Proteus syndrome [Skovby et al., 1993; Eberhard et al., 1994; Slavotinek et al., 2000; Staub et al., 2006]. These reports included six individuals with Proteus syndrome, who developed pulmonary or portal thromboembolism, two postoperatively, all with ages <27 years, and all but one fatal. It has been suggested that the vascular malformations are the primary risk factor for thromboembolism in patients with Proteus syndrome [Slavotinek et al., 2000], but other risk factors have not been investigated. In this review of the clinical, radiological, and laboratory evaluations of a large cohort of patients with Proteus syndrome, we found that six of ten deaths (60%) were the result of a DVT and bilateral PE at a young age (median age of 17 years), and amongst living patients, that six of 47 (16%) had DVTs, with three of the six having co-occurrence of PE, and another 7 of 47 (15%) had evidence of thrombophlebitis and other venous malformations. We also know that among the ten deceased patients that DVT and PE occurred in several patients, some perioperative, and some associated with acute illnesses. This younger age and frequency of superficial venous thromboses, DVT, and PE in patients with Proteus syndrome is striking. Amongst the 47 currently living patients, the recognized DVT/PE events occurred postoperatively in an affected (overgrown) limb. In addition, venous varicosities were visible on examination in half of these patients. We found that the occurrence of thrombosis in patients with Proteus syndrome was not primarily attributable to an increase in any one of the well-known prothrombotic conditions such as factor V Leiden, prothrombin mutation, c.*97G>A, (G20210A) in F2, deficiency of antithrombin III, protein C, or protein S, that typically increase the rate of thrombin production and fibrin clot generation. While we recognize that a study of this size is limited in power, the high frequency of DVT and PE in Proteus syndrome argues against these being major factors, because they are relatively uncommon. Therefore, our findings point to other potential mechanisms for thromboembolism in Proteus syndrome. We speculate that the biology of Proteus syndrome may have adverse effects on hemostasis by other mechanisms, such as endothelial abnormalities or platelet dysfunction, beyond the well-recognized effects of stasis associated with vascular malformations. Indeed, this strikingly high rate of DVT and PE in patients with Proteus syndrome lead us to speculate that this disorder may have a level of risk that is out of proportion to the vascular malformations and may be attributable to specific vascular and/or platelet dysfunction caused by the AKT1 p.E17K mutation.
The D-dimer is a widely used and sensitive screening test for venous thrombosis [Wells et al., 2003; Lees et al., 2011; Molugu et al., 2013]. D-dimer is a circulating fibrin degradation product that forms when a thrombus is degraded by thrombin, coagulation factor XIIIa and fibrinolysin [Molugu et al., 2013; Wells, 2004; Wedlund and Voslar, 2014]. The D-dimer levels reflect endogenous fibrinolysis, which may be used as a marker of DVT [Lees et al., 2011]. In this study, of the eight of 17 individuals with Proteus syndrome who had D-dimer levels, four of eight (50%) with levels >1 mcg/dl had thrombotic events. In contrast, none of three patients with a level between 0.5 and 1.0 had a DVT or PE detectable by imaging. If we assume that the individuals with a D-dimer level of <0.5 mcg/ml did not have a DVT or PE, this yields a sensitivity of 100% and a PPV of 37% (4/11). This compares favorably with the performance of the D-dimer test as a DVT/PE screen in the general population with suspected DVT, which has a PPV of ~20–30% and a sensitivity of ~96% (we assume here that all of the patients reported here with a D-dimer <0.5 mcg/ml did not have DVT or PE) [Nelson et al., 2009; Pulivarthi and Gurram, 2014]. We conclude that the D-dimer test is useful in patients with Proteus syndrome and should be used liberally. What is less clear is whether the screening threshold used in this context should be the same as it is for the general population (which is 0.5 mcg/ml for the STA Liatest D-dimer assay).
Adjustments to the D-dimer threshold for screening based on age have been proposed, tested and validated in prospective clinical trials that utilized the STA Liatest D-dimer assay among others, and found to decrease the proportion of patients tested who required further diagnostic evaluation without loss of sensitivity (Age-Adjusted D-Dimer Cutoff Levels to Rule Out Pulmonary Embolism - The ADJUST-PE Study) [Righini et al., 2014]. A strategy of adjusting the threshold for D-dimer screening for DVT in patients with Proteus syndrome, based on the observed increase in D-dimer levels in those without DVT should be considered. Notably, most patients with Proteus syndrome with DVT in our cohort have had their events at early ages, well before adjustments to the D-dimer threshold for DVT are made (typically age 50). Patients who are positive for DVT or PE on imaging should be undergo acute anticoagulation according to the standard American College of Chest Physicians guidelines (Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report) [Kearon et al., 2016].
Thromboembolism has also been reported in patients with PROS, which subsumes the previous phenotypic descriptors of CLOVES syndrome KTS [Alomari et al. 2010; Keppler-Noreuil et al., 2014; Mirzaa et al., 2013], and other phenotypes. Oishi and Ezaki [2010] described three preadolescent patients with the clinical diagnosis of KTS who had large upper extremity venous malformations and developed DVT; two had documented PE, one of which was fatal. The specific pathogenesis for thromboembolism in PROS is also unknown, although the presence of vascular malformations as a risk factor is assumed, as for Proteus syndrome, and these disorders are both caused by somatic mutations in the PI3K-AKT signaling pathway. The p110α catalytic subunit of PI3K is essential for endothelial cell migration and angiogenesis, and sustained endothelial activation of AKT1 has been shown to induce the formation of structurally and functionally abnormal blood vessels [Graupera et al., 2008; Phung et al., 2006]. In addition, RAS activation in endothelial cells resulted in abnormal vascular morphogenesis, which is regulated by PI3K signaling [Bajaj et al., 2010]. There are also many reports of other patients with congenital vascular malformations not associated with an underlying syndrome who have increased prevalence of thromboembolism [Enjolras et al, 1997; Mason et al, 2001; Mazoyer et al, 2002; Merli, 2005; Dompmartin et al, 2008; Oduber et al, 2009]. Of course, it is possible that many of these reports of patients with presumed non-syndromic vascular malformations may in fact have an undiagnosed or subclinical form of Proteus syndroem or PROS. It is therefore possible that the risk of DVT and PE is substantially attributable to dysfunction of the AKT/PI3K pathway.
In general, the three primary clinical factors associated with thrombosis risk include those comprising Virchow’s triad: 1) vessel wall damage due to inflammation or trauma; 2) changes in blood flow or volume due to immobility, ischemia, or other conditions; and 3) hypercoagulable factors present in the blood, including inherited and acquired coagulation disorders [Merli et al., 2005]. Many patients with large venous malformations have had serologic evidence of coagulopathy (decreased fibrinogen, elevated D-dimer, elevated prothrombin times and normal to low platelet counts [Oduber et al., 2009]. In several studies, the severity of the coagulopathy correlated with the extent of the malformation [Enjolras et al., 1997; Mason et al., 2001; Mazoyer et al., 2002]. Of course, the Virchow’s triad model of pathogenesis was proposed long before the molecular delineation of the causes of vascular malformations. It is now recognized that mosaicism is not as rare as previously supposed [Biesecker and Spinner, 2013] and that many vascular malformations are caused by mosaic mutations of the AKT/PI3K pathway. We hypothesize that the outsize risk of DVT/PE in patients with Proteus syndrome, the elevated risk in patients with PROS, coupled with a speculation that many apparently non-syndromic vascular malformations are also due to mosaic mutations in this pathway, all point to AKT/PI3K pathway being a fourth major underlying factor of susceptibility to DVT and PE.
We conclude that patients with Proteus syndrome have a striking predisposition to DVT and PE and that liberal use of the D-dimer test as a screen for these complications is useful, as it can identify patients, including young children, who can benefit from anticoagulation. While most DVT and PE in patients with Proteus syndrome developed perioperatively, others did not, and clinicians must maintain a high index of suspicion, especially since DVT and PE are rare in children who do not have Proteus syndrome. We suggest that D-dimer is a useful screen for the presence of a DVT in patients with Proteus syndrome when the level is above 0.5 mcg/dl (for the STA Liatest D-dimer assay). To better understand the diagnosis, management, etiology, genetic mechanisms, and natural history of thromboembolism in Proteus syndrome and related disorders, including PROS, we plan to undertake several efforts. First, we will establish a registry to collect data on D-dimer screening and subsequent evaluations for all patients with Proteus syndrome or PROS. We also plan to conduct a prospective evaluation of clinical, radiological and laboratory studies, and contribute to development of therapeutic approaches for these disorders. Using these approaches, we are optimistic that we can lower the morbidity and mortality of Proteus syndrome and PROS.
Acknowledgments
The authors are grateful to the patients and their families who participated in this research study. This research was supported by the Intramural Research Program of the National Human Genome Research Institute, Grant HG200388-03.
References
- Aguilar C, Martinez A, Martinez A, Del Rio C, Vazquez M, Rodriguez FJ. Diagnostic value of d-dimer in patients with a moderate pretest probability of deep venous thrombosis. Br J Haematol. 2002;118:275–257. doi: 10.1046/j.1365-2141.2002.03614.x. [DOI] [PubMed] [Google Scholar]
- Alomari AI, Burrows PE, Lee EY, Hedequist DJ, Mulliken JB, Fishman SJ. CLOVES syndrome with thoracic and central phlebectasia: Increased risk of pulmonary embolism. J Thorac Cardiovasc Surg. 2010;140:459–63. doi: 10.1016/j.jtcvs.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Bajaj A, Zheng Q, Adam A, Vincent P, Pumiglia K. Activation of endothelial ras signaling bypasses senescence and causes abnormal vascular morphogenesis. Cancer Res. 2010;70:3803–3812. doi: 10.1158/0008-5472.CAN-09-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker LG, Happle R, Mulliken JB, Weksberg R, Graham JM, Viljoen DL, Cohen MM., Jr Proteus syndrome: diagnostic criteria, differential diagnosis and patient evaluation. Am J Med Genet. 1999;84:389–395. doi: 10.1002/(sici)1096-8628(19990611)84:5<389::aid-ajmg1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151–1157. doi: 10.1038/sj.ejhg.5201638. [DOI] [PubMed] [Google Scholar]
- Biesecker LG, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14:307–320. doi: 10.1038/nrg3424. [DOI] [PubMed] [Google Scholar]
- Dompmartin A, Acher A, Thibon P, Toubach S, Hermans C, Deneys V, Pocock B, Lequerrec A, Labbé D, Barrellier MT, Vanwijck R, Vikkula M, Boon LM. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144:873–877. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma RA, Oduber CEU, Gerdees VEA, van Delden OM, van Eck-Smit BLF, Meijers JCM, van Beers EJ, Douma BJ, van der Horst CM, Bresser P. Chronic pulmonary embolism in Klippel-Trenaunay syndrome. J Am Acad Dermatol. 2012;66:71–77. doi: 10.1016/j.jaad.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Eberhard DA. Two-year-old boy with Proteus Syndrome and fatal pulmonary thromboembolism. Pediatr Pathol. 1994;14:771–779. doi: 10.3109/15513819409037674. [DOI] [PubMed] [Google Scholar]
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36:219–225. doi: 10.1016/s0190-9622(97)70284-6. [DOI] [PubMed] [Google Scholar]
- Gianlupi A, Harper RW, Dwyre DM, Marelich GP. Recurrent pulmonary embolism associated with Klippel-Trenaunay-Weber syndrome. Chest. 1999;115:1199–1201. doi: 10.1378/chest.115.4.1199. [DOI] [PubMed] [Google Scholar]
- Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- Huiras EE, Barnes CJ, Eichenfield LF, Pelech AN, Drolet BA. Pulmonary thromboembolism associated with Klippel-Trenaunay syndrome. Pediatrics. 2005;116:e596–e600. doi: 10.1542/peds.2004-1607. [DOI] [PubMed] [Google Scholar]
- Karalezli A, Sevgili S, Ernam Turgut D, Hasanoglu A, Hasanoglu HC. Pulmonary embolism in a patient with Klippel-Trenaunay-Weber syndrome. Tuberk Toraks. 2006;54:281–287. [PubMed] [Google Scholar]
- Kearon C, Aki EA, Ornelas J, Blavais A, Jimenez D, Bounameaux H, King CS, Morris TA, Sood N, Stevens SM, Vintch JR, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, Parker VE, Blumhorst C, Darling T, Tosi LL, Huson SM, Whitehouse RW, Jakkula E, Grant I, Balasubramanian M, Chandler KE, Fraser JL, Gucev Z, Crow YJ, Brennan LM, Clark R, Sellars EA, Pena LD, Krishnamurty V, Shuen A, Braverman N, Cunningham ML, Sutton VR, Tasic V, Graham JM, Jr, Geer J, Jr, Henderson A, Semple RK, Biesecker LG. Clinical Delineation and Natural History of the PIK3CA-Related Overgrowth Spectrum. Am J Med Genet A. 2014;164A:1713–1733. doi: 10.1002/ajmg.a.36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulstad EB, Kulstad CE, Lovell EO. A rapid quantitative turbimetric d-dimer assay has high sensitivity for detection of pulmonary embolism in the ED. Am J Emerg Med. 2004;22:111–114. doi: 10.1016/j.ajem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lees D, Griffiths P, Paxton C, Wahbi Z. Can D-dimer assay, together with clinical probability predict computed tomography pulmonary angiogram (CTPA) outcomes for pulmonary embolism (PE)? Eur Respir J. 2011;38(Suppl 55):582. [Google Scholar]
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, Blumhorst C, Brockmann K, Calder P, Cherman N, Deardorff MA, Everman DB, Golas G, Greenstein RM, Kato BM, Keppler-Noreuil KM, Kuznetsov SA, Miyamoto RT, Newman K, Ng D, O'Brien K, Rothenberg S, Schwartzentruber DJ, Singhal V, Tirabosco R, Upton J, Wientroub S, Zackai EH, Hoag K, Whitewood-Neal T, Robey PG, Schwartzberg PL, Darling TN, Tosi LL, Mullikin JC, Biesecker LG. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhurst MJ, Parker VE, Payne F, Sapp JC, Rudge S, Harris J, Witkowski AM, Zhang Q, Groeneveld MP, Scott CE, Daly A, Huson SM, Tosi LL, Cunningham ML, Darling TN, Geer J, Gucev Z, Sutton VR, Tziotzios C, Dixon AK, Helliwell T, O'Rahilly S, Savage DB, Wakelam MJ, Barroso I, Biesecker LG, Semple RK. Mosaic overgrowth with Fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44:928–933. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KP, Neufeld EJ, Karian VE, Zurakowski D, Koka BV, Burrows PE. Coagulation abnormalities in pediatric and adult patients after sclerotherapy or embolization of vascular anomalies. Am J Roentgenol. 2001;177:1359–1363. doi: 10.2214/ajr.177.6.1771359. [DOI] [PubMed] [Google Scholar]
- Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L. Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol. 2002;24:243–251. doi: 10.1046/j.1365-2257.2002.00447.x. [DOI] [PubMed] [Google Scholar]
- Mazoyer E, Enjolras O, Bisdorff A, Perdu J, Wassef M, Drouet L. Coagulation disorders in patients with venous malformations of the limbs and trunk: a case series of 118 patients. Arch Dermatol. 2008;144:861–867. doi: 10.1001/archderm.144.7.861. [DOI] [PubMed] [Google Scholar]
- Merli G. Diagnostic assessment of deep vein thrombosis and pulmonary embolism. Am J Med. 2005;118(Suppl 8A):3S–12s. doi: 10.1016/j.amjmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Mirzaa G, Conway R, Graham JM, Jr, Dobyns WB. PIK3CA-Related Segmental Overgrowth. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 2013b. Aug 15, pp. 1993–2016. 2013 Available from: https://www.ncbi.nlm.nih.gov/books/NBK153722/ [Google Scholar]
- Molugu C, Fisher G, Hirons B, Hughes D, Raftery S. P151 V-DimERS study – value of D-Dimers in estimating risk of significant pulmonary embolism and deep vein thrombosis. Thorax. 2013;68(Suppl 3):A144–A144. [Google Scholar]
- Muluk SC, Ginns LC, Semigran MJ, Kaufman JA, Gertler JP. Klippel-Trenaunay syndrome with multiple pulmonary emboli: an unusual cause of progressive pulmonary dysfunction. J Vasc Surg. 1995;21:686–690. doi: 10.1016/s0741-5214(95)70199-0. [DOI] [PubMed] [Google Scholar]
- Nielsen H. Pathophysiology of venous thromboembolism. Semin Thromb Hemost. 1991;17(suppl 3):250–253. [PubMed] [Google Scholar]
- Nelson CM, Wright GS, Silbaugh TR, Cota LJ. Improving D-dimer positive predictive value for outpatients with suspected deep vein thrombosis. Perm J. 2009;13:4–7. doi: 10.7812/tpp/08-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduber CE, Gerdes VE, van der Horst CM, Bresser P. Vascular malformations as underlying cause of chronic thromboembolism and pulmonary hypertension. J Plast Reconstr Aesthet Surg. 2009;62:684–689. doi: 10.1016/j.bjps.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Oger E. Incidence of venous thromboembolism: a community-based study in Western France; EPI-GETBP study group, Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost. 2000;83:657–660. [PubMed] [Google Scholar]
- Oishi SN, Ezaki MB. Venous Thrombosis and Pulmonary Embolus in Pediatric Patient with Large Upper Extremity Venous Malformations. J Hand Surg. 2010;35A:1330–1333. doi: 10.1016/j.jhsa.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahna-Earley RA, Shiojima I, Nagy JA, Lin ML, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6:491–499. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun SW, Whitsett TL, Raskob GE. Negative D-dimer result to exclude recurrent deep venous thrombosis: a management trial. Ann Int Med. 2004;141:839–846. doi: 10.7326/0003-4819-141-11-200412070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righini M, Van Es J, Den Exter PL, Roy PM, Vershchuren F, Ghuysen A, Rutschmann OT, Sanchez O, Jaffrelot M, Trinh-Duc A, Le Gall C, Moustafa F, Principe A, Van Houten AA, Ten Wolde M, Douma RA, Hazelaar G, Erkencs PM, Van Kralingen KW, Grootenboers MJ, Durian MF, Cheung YW, Meyer G, Bounameaux H, Huisman MV, Kamphuisen PW, Le Gal G. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- Silverstein MD, Heit JAA, Mohr CN, Petterson TM, O’Fallon WM, Melton LJ., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- Skovby F, Graham JM, Jr, Sonne-Holm S, Cohen MM., Jr Compromise of the spinal canal in Proteus syndrome. Am J Med Genet. 1993;47:656–659. doi: 10.1002/ajmg.1320470516. [DOI] [PubMed] [Google Scholar]
- Skourtis G, Lazoura O, Panoussis P, Livieratos L. Klippel-Trenaunay syndrome: an unusual cause of pulmonary embolism. Int Angiol. 2006;25:322–326. [PubMed] [Google Scholar]
- Slavotinek AM, Vacha SJ, Peters KF, Biesecker LG. Sudden death caused by pulmonary thromboembolism in Proteus syndrome. Clin Genet. 2000;58:386–389. doi: 10.1034/j.1399-0004.2000.580509.x. [DOI] [PubMed] [Google Scholar]
- Ulrich S, Fischler M, Walder B, Pfammatter T, Speich R. Klippel-Trenaunay syndrome with small vessel pulmonary arterial hypertension. Thorax. 2005;60:971–973. doi: 10.1136/thx.2004.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlund A, Voslar A. Is the D-dimer test a viable option for the detection of PE? J Nucl Med. 2014;55(Suppl 1):2708. [Google Scholar]
- Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- Wells P. Overview and comparison of D-dimer assay kits for DVT and PE. Clin Adv Hematol Oncol. 2004;160:178. [PubMed] [Google Scholar]