Abstract
Background
Although colorectal cancer (CRC) usually metastasizes to the liver and/or lungs, factors influencing the anatomic pattern of metastases remain poorly understood.
Methods
We assessed the relationship between primary CRC site and pattern of synchronous metastasis among 1,202 individuals diagnosed with incident metastatic CRC between 2010–2014 and identified through the Seattle-Puget Sound Surveillance, Epidemiology, and End Results (SEER) registry. Polytomous logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between primary tumor site and synchronous metastatic pattern.
Results
Compared to patients with proximal colon primaries, patients with rectal primaries were more likely to present with lungs-only or liver and lungs metastases versus liver-only metastases (ORlungs–only vs. liver-only: 2.39, 95% CI: 1.35–4.24, ORliver+lungs vs. liver-only: 2.20, 95% CI: 1.46–3.32).
Conclusion
These findings suggest that patients with rectal primaries are more likely than patients with colon primaries to present with synchronous lung metastases.
Keywords: colorectal neoplasms, colonic neoplasms, rectal neoplasms, neoplasm metastasis, population-based cancer registry, epidemiology
INTRODUCTION
Although prognosis is generally favorable for colorectal cancer (CRC) patients diagnosed with early-stage disease, roughly 21% of cases are diagnosed with stage IV or metastatic disease, for which the five-year survival is only 13%[1]. Within this patient population, some variability in survival exists dependent on the site of metastasis and the number of sites involved [2–4]. However, while it is well-known that CRC typically metastasizes to the liver and/or lungs, little is known as to why the disease metastasizes to different sites in different patients [2, 3, 5, 6]. In this population-based analysis, we assessed the association between primary colorectal tumor site and synchronous anatomic pattern of metastasis to the liver, lungs, or other organs in individuals diagnosed with metastatic CRC.
MATERIALS AND METHODS
We identified patients diagnosed with incident metastatic CRC between 2010–2014 in the 13-county catchment area covered by the Surveillance, Epidemiology and End Results (SEER) cancer registry of the Seattle-Puget Sound region (https://www.fredhutch.org/en/labs/phs/projects/cancer-surveillance-system.html). As of 2010, SEER requires reporting of CRC metastasis at diagnosis (present/absent) for four distinct sites: liver, lungs, brain, and bone.
In this analysis, patients were classified into four groups based on the presentation of synchronous metastatic lesions at diagnosis: metastases in the liver only (not in the lungs), in the lungs only (not in the liver), in both the liver and lungs, and only in other sites (not in the liver or lungs).
Primary tumors reported in the cecum through the splenic flexure (ICD-O-3 codes C180, C182–C185) were defined as proximal colon cancers, while neoplasms in the descending or sigmoid colon (C186–187) were categorized as distal colon cancers. Cancers arising in the rectosigmoid junction or rectum (C199, C209) were grouped together as rectal cancers. We limited our analysis to patients with complete information on primary tumor site and site of synchronous metastases. Of the 1287 stage IV cases originally sampled, 1202 (93%) had complete data for analysis.
Polytomous logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between primary tumor site and synchronous pattern of distant metastases, comparing patients with other metastatic patterns to individuals with liver-only metastasis. All models were adjusted for age at diagnosis, sex, race, and T- and N-staging [7]. Two-sided tests were considered statistically significant at the 0.05 level. We conducted all analyses in STATA (College Station, Texas). This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
RESULTS
Regardless of the primary tumor site, the most common site for metastatic spread was to the liver, with or without lung metastases (Table 1). Compared to patients presenting with liver-only metastasis, individuals with lung metastases were less likely to be white, male, and <60 years of age at diagnosis.
Table 1.
Study population characteristics by metastatic pattern to the liver, lungs, or other site, SEER of the Seattle-Puget Sound region, 2010–2014
| Pattern of Metastasis | ||||
|---|---|---|---|---|
|
| ||||
| Liver Only [N=700] n (%) |
Lungs Only [N=83] n (%) |
Liver and Lungs [N=208] n (%) |
Other [N=211] n (%) |
|
| Primary Tumor Site | ||||
| Proximal colon | 267 (38) | 23 (28) | 49 (24) | 99 (47) |
| Distal colon | 213 (30) | 18 (22) | 68 (33) | 55 (26) |
| Rectum | 220 (31) | 42 (51) | 91 (44) | 57 (27) |
| T-stage* | ||||
| T1 – T2 | 44 (6) | 3 (4) | 12 (6) | 5 (2) |
| T3 | 273 (39) | 26 (31) | 49 (24) | 61 (29) |
| T4 | 160 (23) | 28 (34) | 47 (23) | 106 (50) |
| TX | 223 (32) | 26 (31) | 100 (48) | 39 (18) |
| N-stage* | ||||
| N0 | 141 (20) | 20 (24) | 33 (16) | 42 (20) |
| N1 | 242 (35) | 28 (34) | 72 (35) | 60 (28) |
| N2 | 192 (27) | 18 (22) | 43 (21) | 79 (37) |
| NX | 125 (18) | 17 (20) | 60 (29) | 30 (14) |
| M-stage* | ||||
| M1a | 525 (75) | 46 (55) | 3 (1) | 78 (37) |
| M1b | 170 (24) | 36 (43) | 204 (98) | 126 (60) |
| M1NOS | 5 (<1) | 1 (1) | 1 (<1) | 7 (3) |
| Age, in years | ||||
| <50 | 116 (17) | 14 (17) | 29 (14) | 35 (17) |
| 50 – 59 | 174 (25) | 14 (17) | 62 (30) | 50 (24) |
| 60 – 69 | 249 (36) | 29 (35) | 85 (41) | 64 (30) |
| ≥70 | 161 (23) | 26 (31) | 32 (15) | 62 (29) |
| Mean in years (SD) | 61 (10.9) | 62 (10.8) | 60 (10.2) | 62 (11.3) |
| Sex | ||||
| Male | 439 (63) | 48 (58) | 128 (62) | 107 (51) |
| Female | 261 (37) | 35 (42) | 80 (38) | 104 (49) |
| Race | ||||
| White | 577 (82) | 61 (73) | 173 (83) | 183 (87) |
| Non-white | 120 (17) | 20 (24) | 33 (16) | 27 (13) |
| Unknown | 3 (<1) | 2 (2) | 2 (1) | 3 (<1) |
| Ethnicity | ||||
| Hispanic | 27 (4) | 0 (0) | 6 (3) | 5 (2) |
| Non-Hispanic | 671 (96) | 83 (100) | 202 (97) | 206 (98) |
| Unknown * [7] | 2 (<1) | 0 (0) | 0 (0) | 0 (0) |
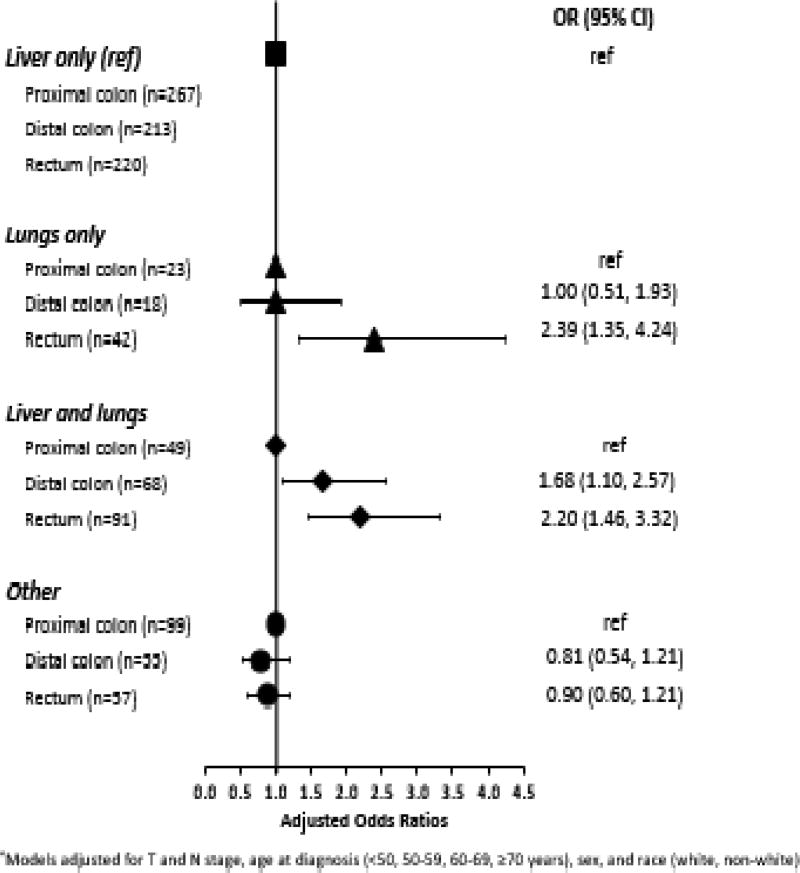
Analyses indicated an association between rectal primary tumors and the presence of synchronous metastatic lesions in the lungs (Figure 1). Specifically, compared to patients with proximal colon primary tumors, we found that patients with rectal primaries were more likely to have metastases in the lungs- only or in both liver and lungs (ORlungs–only vs. liver-only: 2.39, 95% CI: 1.35–4.24; ORliver and lungs vs. liver-only: 2.20, 95% CI: 1.46–3.32). Similarly, there also appeared to be a difference in the metastatic spread of proximal and distal colon primaries. Patients with distal colon primaries had a higher odds of synchronous metastatic spread to both liver and lungs (OR liver and lungs vs. liver-only: 1.68, 95% CI: 1.10–2.57).
Figure 1.
Adjusted ORs for synchronous metastasis pattern in comparison to patients with liver only metastasis, SEER of the Seattle-Puget Sound region, 2010–2014*
DISCUSSION
Prior investigation into other primary cancer sites (i.e., breast, lung) supports the paradigm that primary tumor characteristics, including tumor site and molecular markers, can inform the pattern of metastatic spread [8–10]. Consistent with the findings presented here, previous epidemiologic research also indicates a relationship between primary CRC tumor site and the synchronous pattern of metastasis to the liver and/or lungs, with three past studies specifically noting that patients with rectal primaries were, compared to colon cancer patients, more likely to present with lung metastases and less likely to present with liver metastases at diagnosis.[2, 3, 11] However, these past findings have been limited by small sample sizes [5, 6, 12], lack of population-based data [3, 5, 6, 11, 12], and/or by grouping together proximal and distal colon primaries [2].
The underlying mechanisms driving patterns of CRC metastasis are also somewhat unclear. With regard to hematogenous pathways, clinical evidence indicates that venous drainage of the colon into the portal system likely influences the pattern of metastatic spread first to the liver and then to the lungs among those with a primary tumor in the proximal or distal colon [11, 13]. Conversely, the spread of metastatic rectal cancer to the lungs, either in isolation or as the first of several distant sites, may be attributable to venous drainage which bypasses the portal system and instead enters systemic circulation via the inferior and middle rectal veins [11, 13].
Our findings should be interpreted in light of study limitations, which included the absence of finer metastatic site detail and small numbers for less common sites of synchronous metastasis (e.g., bone, brain) [2, 3]. Additionally, non-differential misclassification of primary and metastatic site is possible. Finally, we lacked data on somatic mutations and microsatellite instability status of the primary neoplasm, which have been previously indicated to impact patterns of CRC metastasis [5, 6, 12].
In summary, our findings suggest a relationship between rectal location of a primary CRC and presentation of synchronous metastasis in the lungs for incident stage IV CRC cases. Results from this population-based analysis may have implications for future clinical practice. At present, it is unclear whether regular observation of patients with rectal primaries for the development of lung metastases would be advantageous, as such metastases are often asymptomatic and resection of synchronous lung metastases may not confer a survival benefit [14, 15]. However, characterizing the factors underpinning anatomic patterns of CRC metastatic spread could ultimately facilitate more targeted surveillance for individuals who do not initially present with distant metastases, which may contribute to better survival.
Highlights.
Tumors arising in the rectum were more likely to spread to the lungs than liver.
Distal colon tumors were less likely to spread to one versus two visceral organs.
Population-based data findings are consistent with previous clinical results.
Observed metastatic patterns may relate to differences in venous drainage.
Acknowledgments
This research was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by Contract No. HHSN261201300012I/NCI Control No. N01 PC-2013-00012 from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington.
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [T32 ES015459 to J.R.R.] and by the National Cancer Institute at the National Institutes of Health [K05 152715 to P.A.N., R25 CA94880 to S.H., K07 CA172298 to A.I.P.]. None of the supporting funding sources were involved in the study design, data collection, data analysis, interpretation of findings, or writing/submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contribution.
JRR conceived the research question, designed and performed the statistical analysis, interpreted findings, wrote and edited the manuscript, and was responsible for all submission processes. AIP conceived the project, requested and obtained necessary data, and supervised the statistical analysis, the interpretation of findings, and the writing of the manuscript.
PAN, SH, and SAC contributed to the design of the analysis and the interpretation of findings. All authors participated in manuscript revision and all approved its final version.
Conflicts of interest: none.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6(36):38658–66. doi: 10.18632/oncotarget.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25(3):651–7. doi: 10.1093/annonc/mdt591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordholm-Carstensen A. Pulmonary nodules and metastases in colorectal cancer. Dan Med J. 2016;63(1):B5190. [PubMed] [Google Scholar]
- 5.Cejas P, Lopez-Gomez M, Aguayo C, Madero R, de Castro Carpeno J, Belda-Iniesta C, Barriuso J, Moreno Garcia V, Larrauri J, Lopez R, Casado E, Gonzalez-Baron M, Feliu J. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS One. 2009;4(12):e8199. doi: 10.1371/journal.pone.0008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira AA, Rego JF, Morris V, Overman MJ, Eng C, Garrett CR, Boutin AT, Ferrarotto R, Lee M, Jiang ZQ, Hoff PM, Vauthey JN, Vilar E, Maru D, Kopetz S. Association between KRAS mutation and lung metastasis in advanced colorectal cancer. Br J Cancer. 2015;112(3):424–8. doi: 10.1038/bjc.2014.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 8.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–8. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 9.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 10.Stenbygaard LE, Sorensen JB, Larsen H, Dombernowsky P. Metastatic pattern in non-resectable non-small cell lung cancer. Acta Oncol. 1999;38(8):993–8. doi: 10.1080/028418699432248. [DOI] [PubMed] [Google Scholar]
- 11.Tan KK, Lopes Gde L, Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg. 2009;13(4):642–8. doi: 10.1007/s11605-008-0757-7. [DOI] [PubMed] [Google Scholar]
- 12.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–32. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, Giuliano R, Archie V, Calvin D, Mensha L, Shivers S, Cox C, Werner JA, Kitagawa Y, Kitajima M. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25(2):221–32. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- 14.Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, Takkenberg JJ, Treasure T. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg. 2016;103(10):1259–68. doi: 10.1002/bjs.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence-based approach. Eur J Cardiothorac Surg. 2016;50(5):792–798. doi: 10.1093/ejcts/ezw140. [DOI] [PubMed] [Google Scholar]