Abstract
Introduction
Recent clinical trials show Lactobacillus rhamnosus GG (LGG) administration in critical illness has the potential to reduce nosocomial infections and improve clinical outcome. However, the mechanism(s) of LGG-mediated benefit following illness and injury remain elusive.
Objective
The aim of this study was to determine the effect of LGG treatment on survival and lung injury in a mouse model of Pseudomonas aeruginosa–induced pneumonia. As increased T regulatory (Treg) cell numbers have been shown to improve outcome in experimental pneumonia, we examined the potential role of Treg cells in probiotic-mediated benefit.
Methods
FVB/N mice were subjected to intratracheal injection of either P. aeruginosa or saline and received LGG or vehicle immediately before procedure. T regulatory cell responses in the lung were evaluated by polymerase chain reaction, Western blotting, and flow cytometry.
Results
Mice treated with LGG had significantly improved 7-day survival (P < 0.01) compared with saline-treated control pneumonia mice (55% LGG vs. 14% control). The survival advantage was associated with reduced bacterial counts in bronchoalveolar lavage and with decreased markers of the systemic inflammatory response and improved lung pathology in the probiotic group. Probiotic treatment influenced immune response in the lungs of mice with pneumonia as demonstrated by increased levels of Treg cell marker Foxp3.
Conclusions
These data demonstrate that early administration of LGG improves outcome following P. aeruginosa–induced pneumonia. An effect of LGG on Treg cells may play a role in this protection.
Keywords: Lactobacillus rhamnosus GG, Treg cells, survival, lung injury
INTRODUCTION
Despite advances in antimicrobial therapy and supportive care in critical illness, infection and pneumonia remain a common cause of mortality in the United States with more than 50,000 patients dying of the disease every year (1). Furthermore, in a recent multinational intensive care unit (ICU) study of 14,414 patients in 1,265 participating ICUs from 75 countries, 51% were considered infected on the day of survey, and 71% were receiving antibiotics (2). Sixty-four percent of the infections in this study were of respiratory origin, and the ICU mortality rate of infected patients was more than twice that of noninfected patients (25% vs. 11%, P < 0.001), as was the hospital mortality rate (33% infected vs. 15% noninfected, P < 0.001). These data reveal that infection remains a major issue in critical care worldwide, leading to significant mortality. Furthermore, antibiotic use occurs in a majority (almost three quarters) of all ICU patients. This antibiotic use can lead to significant adverse effects, such as overgrowth of indigenous microflora, as well as pathogenic bacterial strains, which can be resistant to the standard antimicrobial agents (3).
Specific to ventilator-associated pneumonia (VAP), up to 30% of patients undergoing mechanical ventilation acquire VAP, which causes increased morbidity, mortality, and prolonged ICU and hospital lengths of stay (4). The pathogenesis of VAP is complex and involves colonization of the aerodigestive tract with pathogenic bacteria (4). Preventive strategies, such as selective digestion decontamination protocols, have shown some promise in preventing respiratory tract infections in the ICU patients; however, clinical use has been limited and may be complicated by subsequent bacterial overgrowth of pathogenic bacteria (5).
To inhibit or prevent the gastrointestinal colonization and overgrowth by pathogenic bacteria, the use of probiotic bacteria has been recently studied. Results from recent randomized controlled trials suggest a clinical benefit to the use of probiotics in the ICU (6, 7). These trials have shown significant reduction of overall infections, particularly VAP (8). By definition, probiotics are living nonpathogenic bacteria that colonize the intestine and provide a benefit to the host (9). Lactobacillus species are one of the most frequently used probiotic strains in the treatment of gastrointestinal disorders (10) or as therapy for clinical conditions such as antibiotic-associated diarrhea (11), VAP (7, 12), sepsis, and postoperative infections (13). More specifically, a recent randomized controlled trial of Lactobacillus rhamnosus GG (LGG) demonstrated a significant reduction of VAP by 50% in the LGG-treated group via intention-to-treat analysis (7). Although probiotics appear to show promise in various clinical conditions, the mechanisms responsible for their clinical benefit are not well understood (14). We have recently shown that LGG administration improves several important outcomes in polymicrobial sepsis induced by cecal ligation and puncture in mice (15). Based on the results from our previous work and other in vivo and in vitro studies, probiotics have been shown to attenuate inflammation (16, 17), reduce the overgrowth of pathogenic bacteria, decrease bacterial translocation (18), and reduce apoptosis in intestinal epithelial cells (19). Finally, probiotics have been hypothesized to affect T regulatory (Treg) cell induction (20, 21). In light of this potential effect of probiotics on Treg cells, very recent data propose an immunoregulatory role for Treg cells in containing the excessive inflammatory infiltrate from the lung immune response to infection. One potential hypothesis is that excess inflammation following bacterial infection can lead to substantial tissue damage through the actions of degradative enzymes and other soluble mediators released from the infiltrating immune cells (22). Such tissue damage can worsen lung injury and compromise the integrity of the epithelial cell barrier of the lung aiding in bacterial dissemination to the bloodstream (22). Supporting this hypothesis, increased Treg cell numbers via adoptive transfer techniques have been shown to improve outcome from experimental Pneumococcal pneumonia in rodents (22).
Thus, we hypothesize that LGG will reduce lung injury and bronchoalveolar lavage (BAL) bacterial count and ultimately improve survival in a Pseudomonas pneumonia model. Furthermore, we hypothesize that probiotic administration will attenuate the proinflammatory response to the bacterial exposure, and this will be associated with increased Treg cell content in the lung.
MATERIALS AND METHODS
Probiotic treatment and pneumonia model
The animal protocol used in these studies was approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus. An initial power analysis on the effect of the probiotic treatments on survival was performed utilizing our data studying the same probiotic interventions in a CLP model, which we recently reported (15). From our previous experience with this pneumonia model, we expected a similar mortality to our CLP model (~90%). In our previous experiments with these probiotic treatments, we achieved a mortality reduction of 35% to 40% (15). Thus, using this as an initial starting point, we used an SD of 0.4 and determined that the sample size required for an α of 0.05 and a power of 80% was 17 to 22 animals per group to detect a mortality difference of 35% to 40%. Our pneumonia model utilized 6- to 8-week-old FVB/N mice that were orally gavaged with 200 µL of either LGG (1 × 109 colony-forming units [CFUs]/mL) or sterile water (vehicle) immediately before procedure. Pneumonia was induced via direct intratracheal instillation of Pseudomonas aeruginosa. Under isoflurane anesthesia, mice received a midline cervical incision, and P. aeruginosa (ATTC 27853) was introduced into the trachea via a 29-gauge syringe. Forty microliters of 4 × 108 CFUs of bacteria diluted in sterile saline was used. Mice were then held vertically for 5 s to enhance the delivery into the lungs. Sham mice were treated identically except that they received an intratracheal injection of saline. All mice received antibiotic therapy (gentamicin 0.2 mg/mL, subcutaneously) after the surgery to mimic clinical setting. Animals were killed at either 12 or 24 h (for acute studies) or followed 7 days for survival. For acute studies, mice received a single dose of LGG. For survival studies, mice were treated with LGG daily for 7 days and received antibiotic treatment at 0, 12, and 24 h. Note that, for standardization throughout the article, control animals not receiving probiotics are referred to as control pneumonia animals or pneumonia alone.
LGG culture
Lactobacillus rhamnosus GG (ATCC, Manassas, Va) was incubated in de Man, Rogosa, and Sharpe broth (BD, Sparks, Md) for 24 h at 37°C and 5% CO2. A600 was measured to determine the number of CFUs per 1 mL. Lactobacillus rhamnosus GG species were pelleted from the broth (10,000 revolutions/min [rpm]; 10 min) and resuspended in distilled water.
Bacterial cultures
Bronchoalveolar lavage fluid was obtained following tracheal instillation with 1 mL of sterile 0.9% saline at 12 h, serially diluted (1:10; 1:100; 1:1,000) in sterile 0.9% saline, and cultured on Trypticase soy agar plates with 5% sheep blood (BD) for 24 h at 37°C/5% CO2. Colony-forming units were then enumerated for each animal.
Pulmonary pathology and neutrophil infiltration into the lungs
Lung tissue was collected from each animal at 12 h and fixed overnight in 10% formalin, paraffin-embedded, and sectioned at 4 to 6 µm. Serial sections were stained with hematoxylin-eosin and evaluated for severity of pneumonia by blinded evaluator using a grading scale from 0 (no abnormality) to 4 (severe pneumonia) as described previously (23).
Neutrophil infiltration into the lungs was evaluated by staining for myeloperoxidase (MPO). After deparaffinization and rehydration, sections were blocked with 1.5% rabbit serum (Vector Laboratories, Burlingame, Calif) in phosphate-buffered saline for 30 min, then incubated with goat polyclonal MPO (1:50; R&D Systems, Minneapolis, Minn) antibody for 1 h, washed with phosphate-buffered saline, and incubated with rabbit anti–goat biotinylated secondary antibody (Vector Laboratories) for 30 min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as substrate. Sections were counterstained with hematoxylin, dehydrated, and cover-slipped. Myeloperoxidase-positive cells were quantified in 10 random high-power fields per section. All counting was performed by a blinded evaluator.
Serum interleukins 6 and 10 analysis
Enzyme-linked immunosorbent assay (R&D Systems) was used to determine the concentrations of interleukin 6 (IL-6) and IL-10 in serum 12-h time point) according to the manufacturer’s instructions. Serum was collected after centrifuging blood for 10 min at 5,000 rpm and stored at −80°C until the assay was performed.
RNA preparation, reverse transcription, and real-time polymerase chain reaction
Total RNA was isolated from lung tissue (snap frozen in liquid N2, collected at 12 h) using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, Calif) as described in the manufacturer’s protocol. RNA concentrations were quantified at 260 nm, and the purity and integrity were determined using a NanoDrop. Reverse transcription and real-time polymerase chain reaction (PCR) assays were performed to quantify steady-state messenger RNA levels of Foxp3, IL-6, and IL-10. Complementary DNA was synthesized from 0.2 µg of total RNA. Predeveloped TaqMan primers and probes (Applied Biosystems, Foster City, Calif) were used for detection. Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7300 Real-Time PCR System (Applied Biosystems). Real-time PCR quantification was performed with TaqMan GAPDH controls and relative mRNA expression calculated using the 2−ΔΔCT method (24).
Western blot
For Western blots, lung tissue was harvested and frozen immediately in liquid nitrogen. Samples were homogenized with a handheld homogenizer in a 5× volume of ice-cold homogenization buffer (Tris HCl, 50 mm; pH, 7.4; NaCl, 100 mm; EDTA, 10 mm; Triton X-100, 0.5%) with added protease inhibitors (Roche Diagnostics, Mannheim, Germany). The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected. Total protein concentration was quantified using the Bradford protein assay. Twenty micrograms of each sample was added to a 1× treatment buffer (0.125 M Tris, pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol, and 10% β-mercaptoethanol), boiled for 3 min, and then loaded into a NuPAGE 4% to 12% bis-tris Gel (Invitrogen, Carlsbad, Calif). Proteins were electrophoretically separated with a minigel system and transferred to polyvinylidine difluoride membranes (Millipore, Billerica, Mass), using the wet transfer system (BioRad, Hercules, Calif). Membranes were blocked in 5% nonfat milk in phosphate-buffered saline (PBS)–Tween for 1 h at room temperature. Primary antibodies against Foxp3 (1:000) (catalog ABE75; EMD Millipore) or β-actin (1:10,000) (Sigma-Aldrich, St Louis, Mo) were added to antibody buffer (blocking solution) and incubated overnight at 4°C. After washing three times with PBS-Tween for 30 min, secondary antibodies, peroxidase-conjugated goat anti–mouse or goat anti–rabbit immunoglobulin G (Pierce, Rockford, Ill), were applied at a 1:3,000 dilution for 1.5 h. Blots were washed three times with PBS-Tween for 30 min, incubated in commercial enhanced chemiluminescence reagents (Pierce), and exposed using a UVP chemiluminescent darkroom system (UVP, Upland, Calif).
Flow cytometry
For flow cytometry, lungs were perfused with a 2 mM EDTA saline solution and harvested. Tissue was minced, digested in collagenase D (Sigma-Aldrich) for 1 h, and ground through 100-µm cell strainer for staining. After red blood cell lysis, cells were stained with eF-450–labeled CD4 (catalogRM4-5; eBiosciences, San Diego, Calif) and PerCP-labeled major histocompatibility complex class II (catalog M5/114.15.2; Biolegend, San Diego, Calif) for 30 min at room temperature in the dark, in staining buffer containing the anti-Fc receptor blocking antibody 2.4G2. Cells were then fixed, permeabilized, and stained with PE-labeled Foxp3 using the Foxp3 staining kit (catalog 00-5523-00; eBiosciences) as per manufacturer’s instructions. Cells negative for major histocompatibility complex class II but positive for Foxp3 and CD4 were identified as Treg cells. A FACSCanto II (BD) was used for flow cytometry acquisition. FlowJo software (TreeStar, Ashland, Ore) was used for data analysis.
Statistical analysis
Comparisons were performed with t test analysis (unpaired, two-tailed). Survival studies were analyzed via the log-rank test. To analyze the bacterial culture results, two-tailed NPar, Mann-Whitney U test was used. No measurements or animals were lost to observation or missing in the analysis. Data were analyzed using Prism 4.0 (GraphPad Software, San Diego, Calif) and reported as means ± SE. P ≤ 0.05 was considered to be statistically significant.
RESULTS
LGG improves survival in P. aeruginosa–induced pneumonia
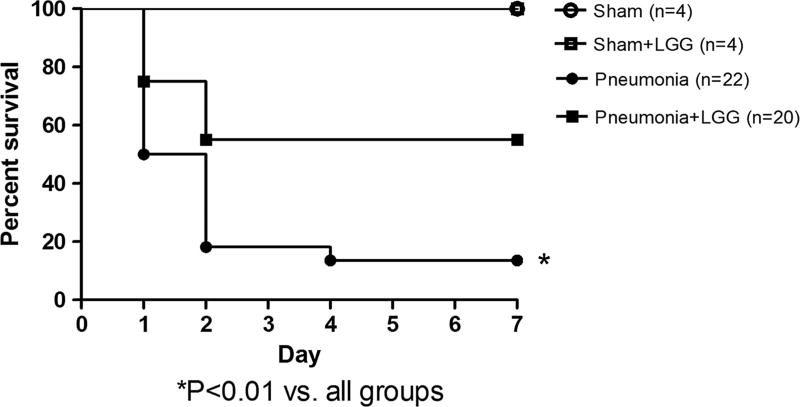
To determine if treatment with LGG affected mortality in P. aeruginosa–induced pneumonia, separate cohorts of mice (shams n = 4 per group, pneumonia [control] n = 22, pneumonia + LGG n = 20) were intratracheally injected with P. aeruginosa and followed 7 days for survival and treated with/without LGG (Fig. 1). Mice treated with LGG had significantly improved 7-day survival (P < 0.01) compared with control mice with pneumonia (55% vs. 14%). All sham animals survived.
Fig. 1.
Effect of LGG treatment on mortality in P. aeruginosa pneumonia. Six- to 8-week-old FVB/N mice were subjected to P. aeruginosa–induced pneumonia with or without probiotic (LGG) treatment that was initiated immediately before surgery. Sham animals received intratracheal injections of saline. All mice were given antibiotics at 0, 12, and 24 h and probiotic treatment every 12 h and followed for survival for 7 days. Mice treated with LGG had significantly decreased mortality (P < 0.01) compared with control mice with pneumonia (pneumonia alone) (55% vs. 14%). Shams n = 4 per group, pneumonia n = 22, pneumonia + LGG n = 20. All sham mice survived.
LGG treatment decreases bacterial counts in BAL fluid
To examine the effect of LGG treatment on bacterial counts in BAL fluid, BAL fluid was cultured, and bacteria numbers counted. The data are expressed as CFUs per 1 mL.
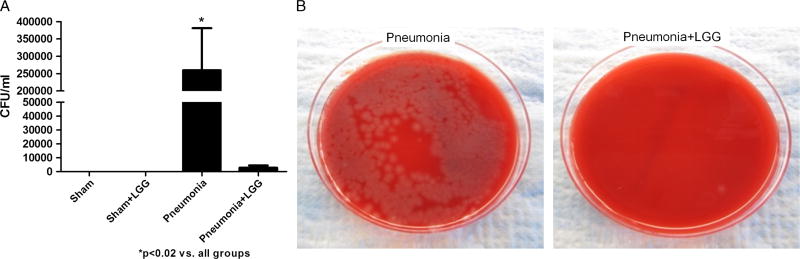
Bacterial counts in BAL obtained from mice with pneumonia were significantly increased in comparison to shams (P < 0.02). Mice receiving LGG treatment before the surgery had significantly lower number of bacteria in BAL fluid versus control animals with pneumonia (P < 0.02) (Fig. 2, A and B).
Fig. 2.
Effect of LGG treatment on BAL cultures in P. aeruginosa pneumonia at 12 h. A, Mice with pneumonia had significantly increased bacterial count in BAL compared with shams (P < 0.02), whereas mice treated with LGG had significantly reduced bacterial load in the BAL (P < 0.02). Shams n = 3 to 4 per group, pneumonia, pneumonia + LGG n = 6 to 7 per group. Data are expressed as the mean ± SE. B, Bacterial growth on representative TSA plates with sheep blood (5%) for pneumonia and pneumonia + LGG groups is shown.
LGG improves pulmonary pathology and normalizes the number of infiltrating neutrophils in lungs of mice with pneumonia
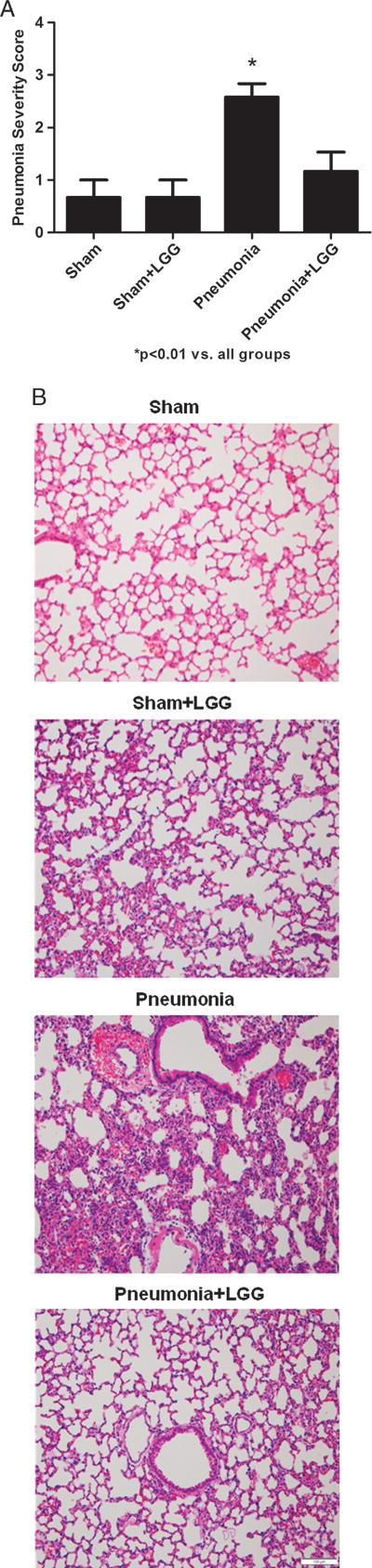
To determine whether the improved survival may be related to reduced lung injury, the effect of probiotic treatment on lung pathology was assessed. Pseudomonas aeruginosa infection caused marked histological injury 12 h after the intratracheal injection in control animals, which was significantly improved in animals treated with LGG (Fig. 3, A and B).
Fig. 3. Effect of LGG treatment on pulmonary pathology at 12 h.
A, The severity of pneumonia (from 0: no abnormality to 4: severe pneumonia) was significantly reduced in the lungs of mice treated with LGG (P < 0.02). B, Representative hematoxylin-eosin–stained sections of lung are shown. Original magnification ×100. Shams n = 4 per group, pneumonia, pneumonia + LGG n = 8 to 10 per group.
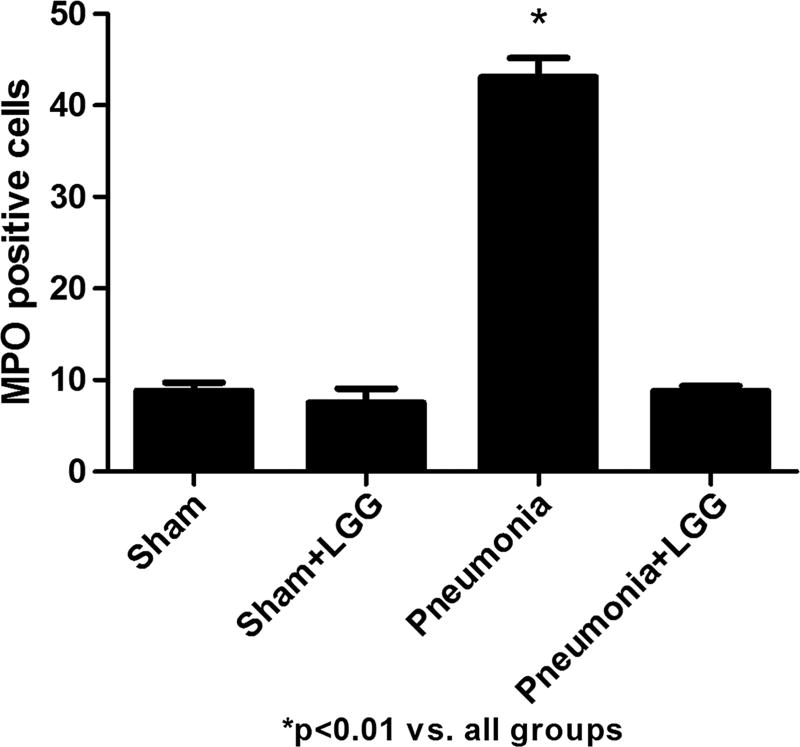
Pseudomonas aeruginosa–mediated lung injury was associated with a significantly higher number of infiltrating neutrophils, represented by the number of MPO-positive cells in the control pneumonia animals when compared with shams (P < 0.01). Treatment with LGG normalized (P < 0.01) the number of MPO-positive cells in the lungs to that observed in sham mice (Fig. 4).
Fig. 4. Effect of LGG treatment on neutrophil infiltration at 12 h.
Numbers of MPO-positive cells were enumerated in the lung to evaluate the neutrophil infiltration. Mice with pneumonia had significantly higher number of MPO-positive cells in comparison to shams (P < 0.01). Lactobacillus rhamnosus GG treatment significantly decreased the neutrophil infiltration into the lung (P < 0.01). Shams n = 4 per group, pneumonia, pneumonia + LGG n = 8 to 10 per group.
LGG attenuates systemic inflammatory response during pneumonia
Attenuation of the proinflammatory response has been hypothesized to be a potential protective mechanism following probiotic administration (16, 17). To determine the effect of LGG on inflammatory markers in P. aeruginosa–induced pneumonia model, serum levels of the proinflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 were measured by enzyme-linked immunosorbent assay.
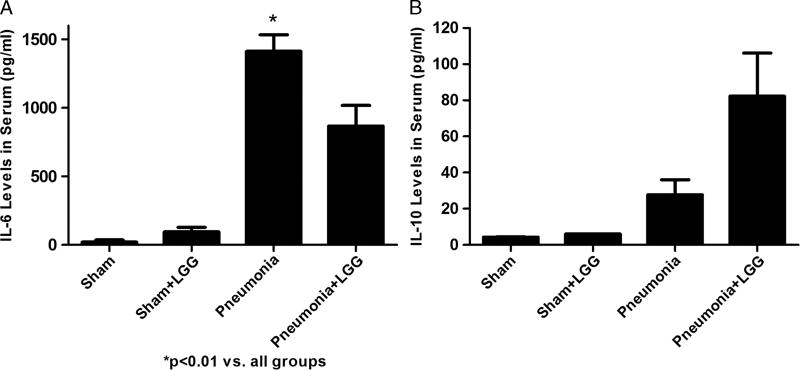
Levels of IL-6 significantly increased in control mice with pneumonia compared with shams (P < 0.05), whereas treatment with LGG led to significantly reduced (P < 0.05) systemic IL-6 levels compared with untreated pneumonia mice (Fig. 5A).
Fig. 5. Effect of LGG treatment on the systemic inflammatory response at 12 h.
Enzyme-linked immunosorbent assay was used to determine the concentrations of IL-6 and IL-10 in serum. A, Interleukin 6 was significantly elevated in the serum of mice with pneumonia compared with shams (P < 0.01). Treatment with LGG in pneumonia mice led to significantly reduced systemic IL-6 levels (P < 0.05) compared with control mice with pneumonia. B, Serum levels of IL-10 showed increasing trend in pneumonia mice treated with LGG compared with untreated pneumonia mice. The change has not reached significance. Shams n = 4 to 5 per group; pneumonia, pneumonia + LGG n = 6 to 12 per group. Data are expressed as the mean ± SE.
Serum levels of IL-10 showed increasing trend in pneumonia mice treated with LGG compared with untreated pneumonia mice. This change did not reach statistical significance (Fig. 5B).
LGG treatment effects mRNA expression of cytokines in lungs of mice with pneumonia
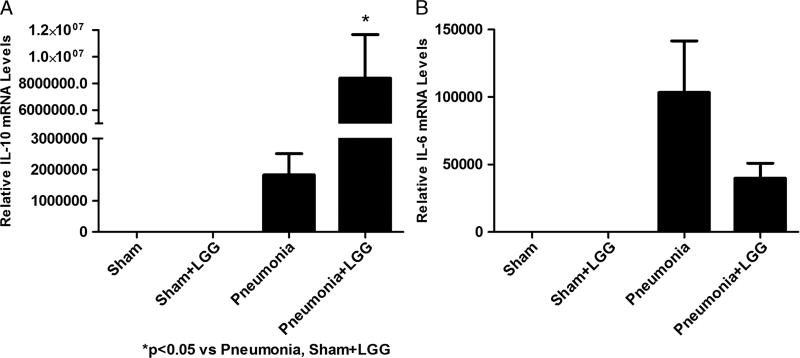
The induction of anti-inflammatory cytokines is thought to be one of protective mechanisms following probiotic administration (16). We found the mRNA levels of IL-10 significantly increased in the lungs of animals infected with P. aeruginosa and treated with LGG compared with control pneumonia mice (P < 0.05) (Fig. 6A).
Fig. 6. Effect of LGG treatment on IL-10 and IL-6 expression in the lungs at 12 h.
Reverse transcription and real-time PCR assays were performed to quantify steady-state mRNA levels of IL-6 and IL-10. A, Lactobacillus rhamnosus GG treatment significantly increased the gene expression of IL-10 in the lungs of pneumonia mice compared with all other experimental groups (P < 0.05). B, mRNA levels of proinflammatory cytokine IL-6 were elevated in control pneumonia mice and decreased in the lungs of mice treated with LGG, although this change has not reached significance. Shams n = 3 to 4 per group; pneumonia, pneumonia + LGG n = 5 to 7 per group. Data are expressed as the mean ± SE.
mRNA levels of proinflammatory cytokine IL-6 were elevated in pneumonia mice and showed a trend toward reduced levels in the lungs of mice treated with LGG, although this change did not achieve statistical significance (Fig. 6B).
LGG modulates the Treg cell response in lungs of mice with pneumonia
To examine whether LGG regulates Treg cells in the lungs of mice infected with P. aeruginosa, mRNA and protein levels of Foxp3 were analyzed. Flow cytometry was used to further confirm these results.
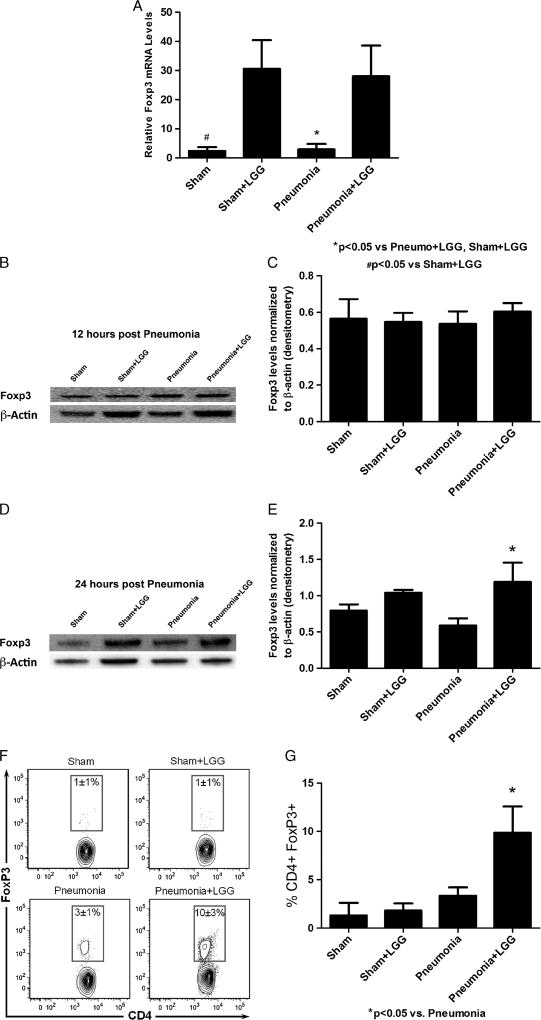
Interestingly, treatment with LGG significantly increased gene expression of Foxp3, the marker of Treg cells, in mice following pneumonia versus control pneumonia mice at the 12-h time point (P < 0.05) (Fig. 7A). Protein levels of Foxp3 were not changed at the 12-h time point (Fig. 7, B and C) but were significantly elevated in lungs of LGG-treated pneumonia mice compared with control pneumonia mice at 24 h after intratracheal injection of P. aeruginosa (Fig. 7, D and E). The changes in lung tissue were also confirmed by flow cytometry analysis (Fig. 7, F and G) as cells positive for Foxp3 and CD4 significantly increased in animals treated with LGG compared with lungs of control pneumonia animals.
Fig. 7. Effect of LGG treatment on T cells in the lungs at 12 and 24 h.
A, Reverse transcription and real-time PCR assays were performed to quantify steady-state mRNA levels of Foxp3 at 12 h. Gene expression of Foxp3 was significantly increased in pneumonia mice receiving LGG treatment compared with control pneumonia animals (P < 0.05). Shams n = 3 to 4 per group; pneumonia, pneumonia + LGG n = 5 to 7 per group. Data are expressed as the mean ± SE. B and C, Protein levels of Foxp3 analyzed by Western blot have not changed at the 12-h time point. D and E, Twenty-four hours after intratracheal injection of P. aeruginosa, Foxp3 levels were significantly elevated in lungs of LGG-treated pneumonia mice compared with control pneumonia mice (shams, n = 3 per group; pneumonia, n = 8; pneumonia + LGG, n = 5). F and G, CD4+ Foxp3+ cells were significantly increased in lungs of LGG-treated pneumonia mice compared with control pneumonia mice (P < 0.05). Representative zebra plots showing outliers, using log10 scales for each group, are shown (shams, n = 2 per group; pneumonia, n = 7; pneumonia + LGG, n = 8).
DISCUSSION
This study demonstrates that administration of the probiotic bacteria LGG before onset of infection leads to significantly improved survival and decreased systemic inflammatory response in mice exposed to intratracheal instillation of P. aeruginosa (P. aeruginosa pneumonia). This improvement was associated with reduced bacterial counts in BAL in LGG-treated mice. Lungs of mice treated with LGG demonstrated reduced histological lung injury and lower number of infiltrating neutrophils compared with control pneumonia mice. Finally, administration of LGG was associated with increased Treg cell content in the lungs.
Critical illness and its treatment create a hostile environment in the gut, which alters the microbiota and can lead to overgrowth of pathogens (25). Oral administration of probiotics may modify the gastrointestinal environment and create one that favors the growth of less pathogenic species. It is unknown whether probiotic treatment and potential modification of the upper aerodigestive microflora could cause reduction of nosocomial infections. Recently published data from randomized clinical trials demonstrate that the use of probiotics in critically ill patients is a promising preventive strategy against VAP (7, 14), sepsis, and postoperative infections (15). It is likely that currently used probiotic strains at their recommended doses may require early (preinfection, preoperatively, or at ICU admission) and continued administration to have positive effects in critically ill and trauma patients (26–29). In our pneumonia model, the animals were given LGG at the onset of P. aeruginosa infection to better imitate the desired clinical setting of early treatment in mechanically ventilated ICU patients at risk for VAP to prevent progressive infection and sepsis. This study demonstrates that oral administration of LGG significantly improves the survival of mice infected with P. aeruginosa and reduces bacterial growth in BAL.
Traditionally, Treg cells were felt to lead to immunosuppression, which could potentially impair immune defense. This effect could be hypothesized to worsen outcome from an insult such as a bacterial infection (22). However, specific to pneumonia, previous work has shown that Foxp3 deficiency causes increased lung inflammation in Foxp3mut mice (30). Another study has shown an essential role of T cells in clearing acute Streptococcus pneumoniae infection when using Rag1−/− mice with innate immunity but no lymphocytes (31). Furthermore, very recent data indicate that increased Treg cell numbers via adoptive transfer techniques can improve the outcome from experimental pneumonia in rodents (22). In regard to this finding, there are at least two types of Treg cells performing similar functions. The first type is defined by expression of CD4 and CD25 developing in the thymus under the control of Foxp3 transcription factor (32). Second, inducible Treg cells have been identified that are generated in the periphery after activation of naive T cells in the presence of inflammation. Inducible Treg cells are also able to produce cytokines such as IL-10 that regulate inflammation (33). In this report, we show that mRNA levels of Foxp3 in the lungs of mice following LGG treatment are significantly increased 12 h after the surgery. Protein levels follow this mRNA increase and are elevated at 24 h following the onset of pneumonia. This may suggest an involvement of Treg cells in the protective effect of LGG against P. aeruginosa infection. Indeed, it is currently unknown if induction of Treg cells from naive T cells or naturally occurring Treg cells or both is responsible for this response. Interestingly, the mRNA levels of IL-10 were also elevated in the lungs of pneumonia mice receiving LGG treatment. It is possible LGG influences the activation of inducible Treg cells, which leads to increased levels of anti-inflammatory cytokine IL-10 in the lungs of P. aeruginosa–infected mice. In support of our findings, a recent clinical study has demonstrated that probiotic administration is associated with Foxp3 Treg cell induction and IL-10 secretion in human peripheral blood after probiotic administration (21). Further clinical trials have shown a significant increase in Foxp3 mRNA expression in ileal biopsies following probiotic administration (34).
New findings on the key role of Treg cells in protection against pneumococcal pneumonia (22), in conjunction with our initial findings in pseudomonal pneumonia may begin to alter our perception of host immune responses to bacterial infection, emphasizing the importance of balancing proinflammatory responses in the lung with controlled anti-inflammatory and immunomodulatory activity by cells such as Treg cells.
A key limitation of our work at this point is that we cannot make a direct mechanistic link between improved outcomes following LGG administration and Treg cell induction. In future studies, we plan to examine the specific role of Treg cells in LGG-mediated protection against pneumonia.
In conclusion, infections (and specifically respiratory infections) continue to be a major cause of mortality and morbidity in ICUs worldwide (2). Furthermore, with the majority of ICU patients receiving antibiotics, derangement of the normal microbiome and potential overgrowth of drug-resistant pathogenic bacterial strains continue to be a significant issue. The potential for the restoration of “healthy” microbiome flora via administration of probiotics to restore appropriate balance to the gut microbiome and the immune response to infection could have significant therapeutic effects. Our data show that early administration of LGG before tracheal instillation of P. aeruginosa improves survival, reduces bacteremia and BAL bacterial content, and reduces lung injury. From a clinical perspective, probiotic treatment significantly reduced the acquisition of “clinical” pneumonia following exposure to an otherwise lethal P. aeruginosa exposure. This effect may in part be mediated by an influence on the lung immune response to infection, including upregulation of Treg cells. Future research is needed to further explore the connection between probiotics and Treg cell induction in infection and critical illness.
We believe our data add to a growing body of existing clinical trial and mechanistic data supporting the potential efficacy of probiotics in critical illness. The broad clinical application of probiotics in the ICU and other clinical areas has recently been limited by concerns regarding safety and potential increased risk of infection from probiotic administration. Although we did not specifically evaluate for bacteremia from our administered probiotic strains, we did observe a marked and statistically significant reduction in mortality in our animals treated with live probiotic bacteria. Furthermore, the results of the recent American Health Care Research and Quality report on the safety of probiotic therapy in more than 600 published clinical trials and case reports are quite reassuring with regard to the safety of probiotic administration (35). One recent clinical trial studying probiotics in severe pancreatitis (the PROPATRIA trial) found an unexpected increase in mortality in probiotic-treated patients (36). This trial was unique as it administered multiple stains of probiotic bacteria and prebiotic-like fiber via a postpyloric feeding tube (placed in the small bowel). This postpyloric method of administration was associated with an increase in small bowel necrosis, which was subsequently associated with death in a number of patients receiving the prebiotic fiber/probiotic mixture. It has been hypothesized that the postpyloric administration of this fiber/multiple probiotic strain mixture in pancreatitis patients may carry significant risk and should likely be avoided (37). In any case, careful and appropriate safety monitoring in all future probiotic clinical trials should be conducted, specific patient groups should be excluded or studied with great caution (i.e., prosthetic heart valve patients), and probiotic administration should be confined to widely studied and thus far safe oral and gastric administration. It is likely the time has come to proceed with carefully designed, carefully monitored, multicenter randomized clinical trials of probiotic therapy to attempt to reduce the risk of infection, sepsis, and mortality in critically ill children and adults. In closing, the use of probiotic bacteria in critically ill patients alongside traditional antibiotic therapies has the potential to be an ideal and cost-effective strategy to prevent hospital-acquired infections, such as VAP, and maintain appropriate immune “balance.”
Acknowledgments
This work was funded in part by NIH R01 GM078312 (to P.E.W.).
Footnotes
This article was presented at the 36th Annual Conference on Shock as one of the finalists of the New Investigator Awards Competition, San Diego, California, June 1–4, 2013.
None of the authors have any conflict of interests to declare.
References
- 1.Heron M. Deaths: leading causes for 2008. Natl Vital Stat Rep. 2012;60(6):1–94. [PubMed] [Google Scholar]
- 2.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 3.Rello J, Ausina V, Ricart M, Castella J, Prats G. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest. 1993;104(4):1230–1235. doi: 10.1378/chest.104.4.1230. [DOI] [PubMed] [Google Scholar]
- 4.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care. 2005;50(6):714–721. discussion 714–721. [PubMed] [Google Scholar]
- 5.Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Gensini GF, Gusinu R. Antibiotic prophylaxis to prevent nosocomial infections in patients in intensive care units: evidence that struggle to convince practising clinicians. Intern Emerg Med. 2006;1(2):160–162. doi: 10.1007/BF02936546. [DOI] [PubMed] [Google Scholar]
- 6.Hojsak I, Abdovic S, Szajewska H, Milosevic M, Krznaric Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics. 2010;125(5):e1171–e1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- 7.Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182(8):1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrof EO, Dhaliwal R, Manzanares W, Johnstone J, Cook D, Heyland DK. Probiotics in the critically ill: a systematic review of the randomized trial evidence. Crit Care Med. 2012;40(12):3290–3302. doi: 10.1097/CCM.0b013e318260cc33. [DOI] [PubMed] [Google Scholar]
- 9.Hammerman C, Bin-Nun A, Kaplan M. Germ warfare: probiotics in defense of the premature gut. Clin Perinatol. 2004;31(3):489–500. doi: 10.1016/j.clp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32(2):147–152. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104(5):e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 12.Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008;12(3):R69. doi: 10.1186/cc6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma. 2009;67(4):815–821. doi: 10.1097/TA.0b013e31819d979e. [DOI] [PubMed] [Google Scholar]
- 14.Shanahan F. Probiotics and inflammatory bowel disease: from fads and fantasy to facts and future. Br J Nutr. 2002;88(Suppl 1):S5–S9. doi: 10.1079/BJN2002624. [DOI] [PubMed] [Google Scholar]
- 15.Khailova L, Frank DN, Dominguez JA, Wischmeyer PE. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology. 2013;119:166–177. doi: 10.1097/ALN.0b013e318291c2fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguero G, Villena J, Racedo S, Haro C, Alvarez S. Beneficial immunomodulatory activity of Lactobacillus casei in malnourished mice pneumonia: effect on inflammation and coagulation. Nutrition. 2006;22(7–8):810–819. doi: 10.1016/j.nut.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Ishikawa H, Tateda K, Yaeshima T, Ishibashi N, Yamaguchi K. Oral administration of Bifidobacterium longum prevents gut-derived Pseudomonas aeruginosa sepsis in mice. J Appl Microbiol. 2008;104(3):672–680. doi: 10.1111/j.1365-2672.2007.03593.x. [DOI] [PubMed] [Google Scholar]
- 18.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55(11):1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1118–G1127. doi: 10.1152/ajpgi.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4(8):e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, Quigley EM, Kiely B, Akdis CA, O’Mahony L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61(3):354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 22.Neill DR, Fernandes VE, Wisby L, Haynes AR, Ferreira DM, Laher A, Strickland N, Gordon SB, Denny P, Kadioglu A, et al. T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice. PLoS Pathogens. 2012;8(4):e1002660. doi: 10.1371/journal.ppat.1002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Diacovo MJ, van Rooijen N, Hogue LA, Cannon CL, et al. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res. 2008;150(2):278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–466. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 26.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, Fedorak R, Madsen K. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85(3):816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 27.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. 2002;82(2):103–108. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 28.McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24(2):211–219. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Honeycutt TC, El Khashab M, Wardrop RM, 3rd, McNeal-Trice K, Honeycutt AL, Christy CG, Mistry K, Harris BD, Meliones JN, Kocis KC. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: a randomized placebo-controlled trial. Pediatr Crit Care Med. 2007;8(5):452–458. doi: 10.1097/01.PCC.0000282176.41134.E6. quiz 464. [DOI] [PubMed] [Google Scholar]
- 30.Rivas MN, Koh YT, Chen A, Nguyen A, Lee YH, Lawson G, Chatila TA. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122(5):1933–1947. doi: 10.1172/JCI40591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blair C, Naclerio RM, Yu X, Thompson K, Sperling A. Role of type 1 T helper cells in the resolution of acute Streptococcus pneumoniae sinusitis: a mouse model. J Infect Dis. 2005;192(7):1237–1244. doi: 10.1086/444544. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 33.Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115(6):1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Pronio A, Montesani C, Butteroni C, Vecchione S, Mumolo G, Vestri A, Vitolo D, Boirivant M. Probiotic administration in patients with ileal pouchanal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis. 2008;14(5):662–668. doi: 10.1002/ibd.20369. [DOI] [PubMed] [Google Scholar]
- 35.Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JNV, Suttorp MJ, Johnsen B, Shanman R, Slusser W, Fu N, et al. Safety of probiotics to reduce risk and prevent or treat disease. Evidence report/technology assessment no. 200. 2011 AHRQ publication 11-E007. [PMC free article] [PubMed] [Google Scholar]
- 36.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 37.Morrow LE, Gogineni V, Malesker MA. Synbiotics and probiotics in the critically ill after the PROPATRIA trial. Curr Opin Clin Nutr Metab Care. 2012;15(2):147–150. doi: 10.1097/MCO.0b013e32834fcea8. [DOI] [PubMed] [Google Scholar]