Abstract
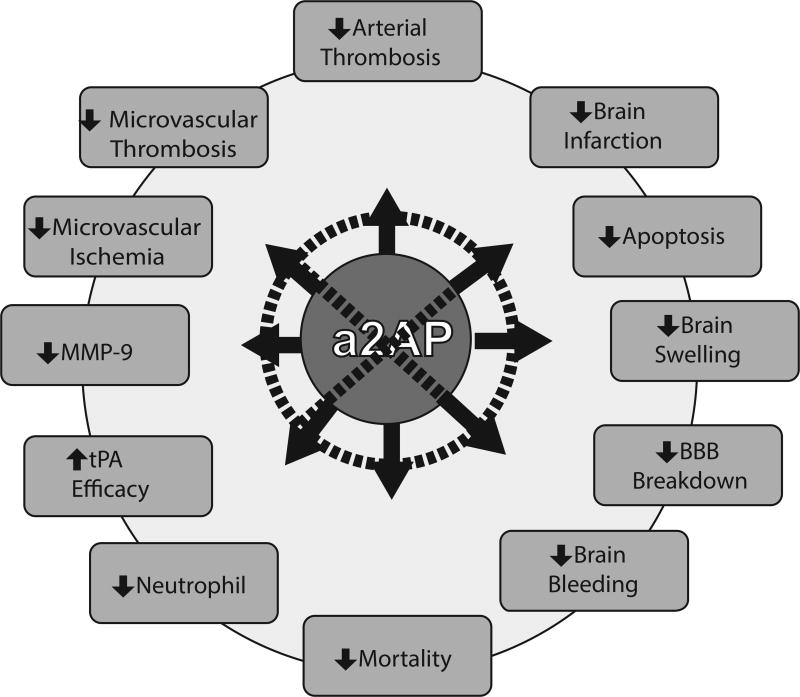
Thrombotic vascular occlusion is the leading cause of ischemic stroke. High blood levels of α2-antiplasmin, an ultrafast, covalent inhibitor of plasmin, have been linked in humans to increased risk of ischemic stroke and failure of tissue plasminogen activator therapy. Consistent with these observations, α2-antiplasmin neutralizes the therapeutic benefit of tissue plasminogen activator therapy in experimental stroke. In addition, α2-antiplasmin has deleterious, dose-related effects on ischemic brain injury in the absence of therapy. Experimental therapeutic inactivation of α2-antiplasmin markedly reduces microvascular thrombosis, ischemic brain injury, brain swelling, brain hemorrhage and death after thromboembolic stroke. These data provide new insights into the critical importance of α2-antiplasmin in the pathogenesis of ischemic brain injury and suggest that transiently inactivating α2-antiplasmin may have therapeutic value in ischemic stroke.
Keywords: Cerebral infarction, thromboembolism, fibrinolysis, blood brain barrier, survival
α2-antiplasmin is a Potent Plasmin Inhibitor
Since its original description, 1–3 α2-antiplasmin (a2AP) or α2-plasmin inhibitor (serpinf2) has been known as the primary, fast inhibitor of plasmin. It is a member of the serine protease inhibitor family, whose members typically act as highly specific, suicide inhibitors. a2AP is such a potent inhibitor that the half-life of plasmin in vivo is thought to be less than 100 msec. 4 a2AP rapidly inactivates plasmin, forming covalent plasmin-a2AP (protease serpin) complexes, whose presence in the blood is a marker of plasmin generation in clinical states associated with plasminogen activation.5 Aoki and colleagues showed that activated factor XIII crosslinks a2AP to fibrin during fibrin formation.6 Crosslinking of a2AP to the fibrin significantly enhances the resistance of fibrin to degradation by plasmin.6,7 The initial reactions between a2AP and plasmin appear to occur through kringle 1 and/or 3 of plasmin and one or more lysine residues in the C-terminus of a2AP.8 When the kringles of plasmin are engaged with lysine residues of fibrin, or lysine analogs such as epsilon-aminocaproic acid, plasmin inactivation by a2AP is delayed.9
Molecular Forms of a2AP
The primary site of a2AP synthesis in humans is the liver but low levels of transcripts for a2AP have been found in other tissues by gene profiling experiments. a2AP has been detected in neurons in the normal human brain and, for unknown reasons, its expression appears to be increased in Aβ plaques in Alzheimer’s disease.10
The mean plasma level of a2AP is 1 micromolar (~70 micrograms/ml) and the plasma half-life is 2.6 days.11 The rate of synthesis or catabolism was found to be 1.4 mg/kg/day.11 During therapeutic fibrinolysis with plasminogen activators, a2AP disappears quickly (within 1 h) from the blood due to formation of plasmin-a2AP complexes. 12 Levels of a2AP levels rebound quickly (within 24 h) after streptokinase therapy.13 Higher levels are associated with myocardial infarction.14 Levels of a2AP increased with increasing total cholesterol, triglyceride, and plasminogen levels. Increased levels of a2AP are associated with an increased risk of stroke in univariate analysis. 15 Atrial fibrillation, which is a risk factor for ischemic stroke, is associated with increased levels of plasmin-antiplasmin complex. 16 Circulating levels of a2AP may be significantly reduced in patients with severe cirrhosis and severely diminished in patients with acute liver failure.17,18 In contrast, a2AP levels may be mildly elevated post-partum or post-surgery, or in patients with thrombosis, peripheral arterial disease, or coronary artery disease.17,19
A2AP is synthesized as a glycoprotein with a relative molecular mass of 70,000 Da. A partial X-ray crystal structure of a2AP shows unique structural elements.20 In the blood, a2AP is secreted with an N-terminal methionine (Met-a2AP) and converted to another form, Asn-a2AP, when the amino terminal twelve residues are cleaved by a2AP cleaving enzyme, a soluble form of fibroblast activating protein .21 Crosslinking of a2AP to fibrin protects against fibrinolysis. Since Met-a2AP is crosslinked to fibrin at a slower rate by activated factor XIII, high levels of Met-a2AP may enhance fibrinolysis.21 This observation has prompted consideration of inhibitors of fibroblast activating protein as potential therapeutics.21 A tryptophan polymorphism in the N-terminus of a2AP (Arg6Trp) is associated with delayed N-terminal cleavage and the crosslinking of a2AP which may affect fibrinolysis.22 In the blood a2AP is also modified at the C-terminus which may slow its initial inhibition of plasmin.23 While these modifications of a2AP may eventually be shown to affect fibrinolysis in vivo, 8 recent studies failed to find a linkage between amino and carboxyl terminal modification of a2AP and, the risks of myocardial infarction.24
Regulation of Fibrinolysis
Role of a2AP in fibrinolysis
Early studies using specific monoclonal antibody inhibitors showed that a2AP was a potent regulator of fibrinolysis. Inhibition of a2AP induced spontaneous dissolution of human plasma clots even without addition of exogenous plasminogen activators. 25,26 The combination of a2AP-inhibition and plasminogen activators was synergistic, yet for the same degree of fibrinolysis, it did not enhance the degradation of fibrinogen.25 In vivo, specific inhibition of fibrin-bound a2AP alone was sufficient to increase thrombus dissolution in experimental venous thrombosis. 27 In vivo studies of experimental pulmonary embolism established that a2AP was a significant inhibitor of thrombus dissolution initiated by r-tPA.28,29
Insights from a2AP Deficiency
Since a2AP is a less efficient inhibitor of fibrin-bound plasmin than of plasmin in solution, studies have suggested that the primary role of a2AP is not to regulate plasmin-mediated fibrinolysis, but to inhibit plasmin in the circulation in order to prevent degradation of clotting factors such as fibrinogen, factor V and factor VIII.30 Similarly, illnesses associated with systemic activation of the fibrinolytic system may also cause depletion of a2AP and coagulation factors. Coagulation factor depletion has been associated with increased bleeding risk, particularly in patients undergoing invasive procedures. However, it is clear from studies of humans and mice, that primary genetic a2AP deficiency alone does not affect hemostatic parameters, coagulation or create a systemic fibrinolytic state (see a2AP-deficiency below).
Secondary or acquired a2AP deficiency
Pharmacologic activation of the fibrinolytic system by plasminogen activators, plasmin and microplasmin causes secondary or acquired a2AP-deficiency and α2-macroglobulin is also depleted. 23,31 In fibrinolytic states, a2AP depletion induced by high plasmin levels leads to deficiencies of coagulation proteins such as fibrinogen and factor V, causing impaired coagulation with prolongation of prothrombin time (PT), activated partial thromboplastin time (aPTT), and reptilase time tests. 31,32 Patients are at risk for bleeding complications, which are typically related to puncture wounds from cardiac catheterizations, arterial lines, etc. 31
Patients with severe liver disease may have reduced synthesis of a2AP. Secondary depletion of a2AP may occur also with disseminated intravascular coagulation or systemic fibrinolysis such as in acute promyelocytic leukemia.33 In these settings, patients usually have laboratory findings of hyperfibrinolysis or disseminated intravascular coagulation, which includes low platelet counts as well as decreased levels of fibrinogen, antithrombin and plasminogen. There is typically evidence of elevated fibrinogen degradation products.33 Occasionally, these conditions are associated with serious bleeding, though treatment with antifibrinolytic agents such as epsilon-aminocaproic acid has been shown to stop hemorrhage.33
Primary or genetic a2AP deficiency
Congenital a2AP deficiency provides insights into the safety and tolerability of transient a2AP inactivation. Only a small number of individuals have been identified who are genetically deficient in a2AP and the true prevalence of complete a2AP deficiency is unknown.34 Clinical assays for a2AP levels are still not widely available and most patients have been identified only during comprehensive hematologic evaluations for a cause of ‘delayed re-bleeding’ episodes after surgery or trauma. 35 Studies of humans with genetic a2AP deficiency have shown that their blood coagulation is normal, and they do not have evidence of a systemic lytic state or disseminated intravascular coagulation.34 However, after trauma or surgery they are at risk of delayed oozing from the site of injury because the process of blood clot dissolution or fibrinolysis is enhanced. Fourteen cases of homozygous a2AP deficiency have been reported worldwide according to the most comprehensive review.34 In sharp contrast to coagulation factor deficiencies, none of these individuals were reported to have brain bleeding. Reports of bleeding in these individuals most commonly include hemarthroses and hematomas, some of which occur in muscle or bone. The ages of patients ranged up to 35 years of age at detection. More recently a patient was first identified at age 63, which indicates that severe a2AP deficiency can be associated with survival into older age. 36 The etiology of bleeding in 11 of 11 a2AP-deficient patients was thought to be related to premature dissolution of formed thrombi following trauma or surgery, but ‘spontaneous hemorrhage’ was also reported in 4 of 11 patients.34,37 Patients with a2AP deficiency suffering hemorrhage are treated with fibrinolytic inhibitors (tranexamic acid or epsilon-aminocaproic acid) or fresh frozen plasma. With antifibrinolytic therapy (epsilon-aminocaproic acid), patients with complete a2AP deficiency can be taken successfully through severe hemostatic challenges including major operations (open heart surgery, orthopedic procedures).34
Mice with genetic deficiency of a2AP (a2AP−/−) induced by gene targeting have normal fertility, viability and coagulation.38 There was no increased bleeding or re-bleeding after surgical procedures, including tail bleeding.38 In a similar fashion, selective a2AP inactivation in vivo by monoclonal antibodies has not been associated with bleeding or consumption of fibrinogen or plasminogen, in pre-clinical studies (see below). In considering the potential safety of transient, therapeutic a2AP inhibition, it is interesting to compare the effects of genetic deficiencies of pro-thrombin or factor X, which are targets of multiple current therapies. Genetic deficiency of pro-thrombin is lethal—no mice are born and no humans have been identified. Similarly, humans with complete deficiency of factor X do not appear to be viable and knockout of murine factor X is also lethal. In contrast, genetic deficiency of a2AP is tolerated in humans and in mice. Since the activity of both factor Xa and thrombin can be inhibited therapeutically with acceptable safety in patients, transient therapeutic a2AP inactivation may also prove to be safe.
When treated with anticoagulants, antiplatelet agents or r-tPA, a2AP-deficient mice do not have increased bleeding.29,39 In early studies of venous thromboembolism in ferrets, a2AP-inactivation did not cause bleeding.28 a2AP-inactivation significantly reduced brain bleeding in ischemic stroke by comparison to controls (no treatment) and r-tPA; a2AP-inactivation had marked effects on reducing mortality by comparison to controls or r-tPA (see below).40,41
a2AP in Ischemic Stroke
Epidemiologic and clinical linkage of a2AP to ischemic stroke
The Atherosclerosis Risk in Communities Study found that elevated a2AP levels were associated with an increased risk of subsequent stroke in univariate analysis. 9 In patients treated with r-tPA for ischemic stroke, higher a2AP blood levels were associated with a decreased rate of successful reperfusion.42 a2AP-plasmin complexes are elevated in patients with atrial fibrillation and increased risk of ischemic stroke.16
Endogenous Activation of the fibrinolytic System in Ischemic stroke
There is significant activation of the fibrinolytic system after stroke in humans. Levels of a2AP-plasmin complex are higher, signifying increased endogenous plasminogen activation, dissolution of fibrin and inhibition of the process by a2AP.43–47 Levels of D-dimer and a2AP-plasmin complexes are increased in stroke patients versus controls, particularly in older patients.48
Effects of a2AP on Experimental Stroke
Experimental Studies of the fibrinolytic system in experimental stroke
The NINDS study demonstrated that r-tPA treatment reduced disability when given to stroke patients within three hours of the onset of ischemia.49 However, in a series of surprising experiments, Tsirka and colleagues found that r-tPA had deleterious effects on the ischemic brain; r-tPA enhanced neurotoxicity and increased brain infarction following mechanical arterial occlusion.50 Tsirka and colleagues induced brain ischemia by mechanical arterial occlusion and not by thrombosis. Their goal was to determine the effects of endogenous tPA and r-tPA on the ischemic brain, separate from their effects on thrombus dissolution and reperfusion. In studies of gene-deleted mice, Nagai and colleagues expanded these findings by examining the effect of endogenous tPA and other key components of the fibrinolytic system. 51 In an open skull model of cerebral ischemia induced by surgical ligation of the middle cerebral artery (MCA), deletion of tPA or of a2AP (Table 1) significantly reduced ischemic brain injury.51 Nagai et al. also found that deficiency of PAI-1 or of plasminogen significantly increased stroke size, whereas deficiency of urinary-type PA mice had no effect.51 In subsequent studies, using the same model, Nagai et al demonstrated that depletion of a2AP by administration of microplasmin, plasmin or polyclonal antibodies significantly reduced ischemic brain injury.52 In related studies, Nagai and colleagues showed that in ischemia induced by MCA ligation, pharmacologic treatment with all plasminogen activators tested (r-tPA, streptokinase and staphylokinase) caused some degree of neurotoxic effects that enhanced ischemic brain damage.53
Table 1.
Effects of a2AP on Experimental Ischemic Stroke
| Effect and Comparison | |||||
|---|---|---|---|---|---|
| Outcome | Normal a2AP Blood Levels | High a2AP Blood Levels | |||
| a2AP-I vs. r-tPA41 |
a2AP-I (or deficiency) vs. controlb,41,51,52 |
a2AP-I + r-tPA vs. r-tPA 40 |
vs. normal a2AP levels41 |
Effect on r-tPA treatment40 |
|
| Ischemic brain injury | ⇩⇩b | ⇩⇩ | ⇩⇩ | ⇧⇧ | ⇧⇧ |
| Brain Swelling | ⇩⇩ | ⇩⇩ | ⇩⇩ | ⇧⇧ | ⇧⇧ |
| Brain Hemorrhage | ⇩⇩ | ⇩⇩ | ⇩⇩ | ⬄ | ⬄⇩ |
| Microvascular thrombosis | ⇩⇩ | ⇩⇩ | - | ⇧⇧ | - |
| MMP-9 expression | ⇩⇩ | ⇩⇩ | - | ⇧⇧ | - |
| Blood brain barrier breakdown | ⇩⇩ | - | ⇩⇩ | - | - |
| MCA thrombus dissolution | ⇩⇧c | ⇧⇧ | ⇧⇧ | ⇩⇩ | ⇩⇩ |
| Survival | ⇧⇧ | ⇧⇧ | ⇧⇧ | - | - |
| Apoptosis | - | - | ⇩⇩ | - | |
Control, normal a2AP levels.
⇧⇧ markedly greater, ⇧ greater, ⬄ no significant difference, ⇩ less than or ⇩⇩ markedly less in comparison
Dose-related
Experimental models with translational relevance to human stroke
The deleterious effects of endogenous tPA and r-tPA in non-thrombotic, arterial occlusion studies led some to question the relevance of this non-thrombotic experimental model for human ischemic stroke, a disease largely caused by thrombotic occlusion. 54 Since almost all patients with ischemic stroke have evidence of an occlusive arterial thrombus, 55,56 the Stroke Academic Industry Roundtable and other expert groups consider animal models of experimental thromboembolic stroke to have translational relevance to human disease, 57 particularly when evaluating the fibrinolytic system. In some models of thrombotic ischemic stroke, autologous clots are placed by catheter in the proximal MCA to induce hemispheric ischemia. Thrombotic obstruction of the proximal MCA is confirmed by greater than 80% reductions in relative hemispheric blood flow and by post-mortem inspection of the base of the brain. Thromboembolic stroke has been evaluated in a number of different animals including mice. The mouse fibrinolytic system is comparable to that of humans; mouse a2AP inhibits mouse plasmin with similar kinetics and a2AP is the dominant regulator of fibrinolysis.38,58,59 In these models, r-tPA has been shown to reduce stroke size in mice when given within 15 minutes of thromboembolism60 or 20 minutes of clot inititation,61 although its effectiveness diminishes significantly when given later.62
Narrow therapeutic time window for r-tPA
When mice are treated after 15 min. after thromboembolism-induced ischemia, the standard r-tPA dose in mice (10 mg/kg) significantly increased dissolution of the MCA thrombus and markedly reduces brain infarction. However, treatment of mice one hour after stroke onset significantly increased dissolution of the MCA thrombus but did not decrease stroke size by comparison to controls. Treatment with r-tPA 2.5 hours after the onset of ischemic dissolved the MCA thrombus, but it markedly increased stroke infarction vs. controls. 40,41 Treatment with r-tPA also significantly increased hemorrhage by comparison to controls.
a2AP contributes to the therapeutic failure of r-tPA
In the fibrinolytic pathway, a2AP is downstream of tPA and high blood levels have been associated with r-tPA failure to restore reperfusion in stroke patients.42 To examine this, blood levels of a2AP were experimentally increased (~doubled) by intravenous infusion prior to cerebral thromboembolism (Table 1). With r-tPA treatment, mice with high a2AP blood levels had 2.2-fold larger brain infarcts than mice with normal a2AP levels. During r-tPA treatment, high a2AP blood levels increased brain swelling (3.7-fold) and decreased dissolution of the MCA thrombus.
a2AP inactivation improves the effectiveness and therapeutic window of r-tPA
To confirm the effects of a2AP on r-tPA and stroke outcomes, mice were treated with the combination of r-tPA and a monoclonal antibody that inactivated a2AP (a2AP-I). This combination markedly enhanced the dissolution of the MCA thrombus vs. r-tPA alone (Table 1). Combination therapy also diminished brain infarction, swelling and hemorrhage. Combination therapy with a2AP-I and r-tPA extended the therapeutic time window for reduction in ischemic brain injury and improved survival by comparison to r-tPA therapy alone.
Effects of endogenous a2AP on ischemic stroke
The existence of a dose-response relationship between a2AP levels and ischemic brain injury was examined in mice with increased a2AP levels (following supplementation), normal a2AP levels and a2AP deficiency. Brain infarction was increased by more than 50% in mice with high a2AP vs. normal a2AP levels. Brain infarction was reduced by more than two-fold in a2AP-deficient (a2AP−/−) vs. a2AP+/+ mice and, it was reduced about four-fold vs. mice with high a2AP levels. These data indicated a strong dose response relationship whereby high a2AP levels were deleterious and low a2AP levels were protective in ischemic stroke. The relative importance of circulating vs. thrombus bound a2AP was assessed in a2AP−/− mice. There was faster MCA thrombus dissolution and brain reperfusion in a2AP−/− mice with a2AP-deficient thrombi, than in a2AP−/− mice with normal levels of a2AP in the thrombi. In these experiments, brain infarction was comparably reduced in both groups, suggesting that the presence or absence of a2AP in the MCA thrombus affected the ischemic brain injury less than circulating a2AP. Thus circulating levels of a2AP appeared to be the most important determinant of outcomes, potentially through its effects on microvascular thrombosis (see below).
Comparative therapeutic time windows for r-tPA and a2AP-I
When blood flow is reduced due to arterial thrombosis, severely ischemic brain tissue begins to infarct. Brain tissue in the penumbra, the area between severe ischemia and normal blood flow, which may be rescued by therapy, remains marginally viable for a period of time. The duration of viability of the penumbra is much shorter in mice than in humans. The time-related effects of a2AP-I and r-tPA therapies alone were compared (Table 1, Fig. 1) in experimental ischemic stroke‥ Treatment 1 hour after stroke onset with r-tPA did not reduce brain injury. In contrast, treatment of ischemic stroke with a2AP-I 1 h after stroke onset significantly reduced brain infarction by comparison to control (un-treated) and r-tPA-treated mice. In addition, treatment of ischemic stroke with a2AP-I 2.5 hours after ischemia also significantly reduced infarction vs. control and vs. r-tPA. Treatment with r-tPA significant increased brain hemorrhage vs. control or a2AP-I. However, a2AP-I treatment did not increase brain bleeding vs. controls.
Figure 1.
Effects of a2AP-inactivation on outcomes in experimental ischemic stroke. See text for details. *Reed GL, Houng AK, Singh S, unpublished data.
Comparative effects of r-tPA and a2AP-I on longer term outcomes and survival
Since r-tPA treatment had a short therapeutic time window, mice were treated 30 min. following MCA thromboembolism. Treatment with a2AP-I decreased brain infarction by 4–5-fold by comparison to control or to r-tPA therapy. Treatment with a2AP-I also markedly reduced brain swelling (>10-fold). Importantly, a2AP-I treatment significantly reduced brain hemorrhage when compared with control or r-tPA treatment.
Proximal MCA thromboembolism was associated with large brain infarctions. There was significant mortality in mice treated with r-tPA and in control mice. In comparison to these two groups, mice treated with low dose a2AP-I showed a significant improvement in survival. All mice treated with high doses a2AP-I survived, which was a significant improvement over low dose a2AP-I, r-tPA therapy or controls. These experiments showed that a2AP inactivation treatment after stroke onset conferred a significant survival advantage.
Effects of a2AP on the Ischemic Brain
Microvascular thrombosis
Downstream from a thrombotic occlusion, ischemia may trigger the formation of microvascular thrombi, which further reduce blood flow and thereby enhance blood brain barrier breakdown and worsen brain tissue injury.63–66 Microvascular thrombosis may worsen ischemia by causing vascular obstruction.67 In experimental stroke, r-tPA treatment significantly enhanced microvascular thrombus formation vs. control (untreated mice); this is consistent with the pro-thrombotic effects of r-tPA therapy in humans.41,68 High blood levels of a2AP also increased microvascular thrombosis. However, a2AP-I treatment or a2AP deficiency significantly decreased microvascular thrombosis, by comparison to control or to r-tPA treated mice.69 Consistent with this observation, plasminogen deficiency enhances, and high plasminogen levels reduce microvascular thrombosis in experimental ischemic stroke.70
The extent of microvascular thrombosis was more strongly associated with the sequelae of severe ischemic brain injury than the magnitude of dissolution of the middle cerebral thrombus. While r-tPA therapy more efficiently dissolved the MCA thrombus than a2AP-I, the a2AP-I induced a greater reduction in microvascular thrombosis, which was associated with larger decrease in ischemic brain injury.
Matrix metalloproteinase-9 expression
Fibrin stimulates neutrophil adherence; 71 in turn neutrophils adhering to fibrin(ogen) promote thrombin generation and fibrin deposition.72 In human stroke, matrix metalloproteinase-9 (MMP-9) expression is associated with neutrophil infiltration in infarcted and hemorrhagic areas of the brain.73 However, MMP-9 is also expressed by endothelial cells and to a lesser extent by astrocytes and neurons in ischemic brain.74 MMP-9 is a potential mediator of breakdown of the blood brain barrier, brain swelling and hemorrhage. There was a dose-response relationship between a2AP and acute MMP-9 expression. High a2AP levels or r-tPA treatment, significantly increased MMP-9 levels. Deficiency of a2AP deficiency or a2AP-I markedly diminished MMP-9 expression. The relationship of a2AP to expression of MMP-3 and other MMPs, which are linked to breakdown of the blood brain barrier, is unknown. 75
Blood brain barrier breakdown
MMP-9 has been implicated as a cause of breakdown of the blood brain barrier, hemorrhage, and brain edema in ischemic stroke. 75–77 Mice treated with r-tPA alone showed leakage of albumin outside vascular spaces, indicated by collagen IV immunostaining, consistent with blood brain barrier breakdown; this was reduced in mice treated with the combination of r-tPA and a2AP-I. By comparison to normal mice, or r-tPA-treated mice there was also significantly less breakdown of the BBB in mice treated with an a2AP-I alone (Reed, unpublished data).
Apoptosis
In comparative studies, combination treatment with r-tPA and a2AP-I was significantly more effective than r-tPA alone at reducing brain apoptosis as indicated by reductions in cleaved caspase-3 and TUNEL staining.
Therapeutic Potential of a2AP Inactivation
The therapeutic potential of a2AP inactivation in human ischemic stroke has been suggested by epidemiologic, clinical and experimental studies. While there is as yet no approved therapy for modulating a2AP activity, several approaches hold promise.
Monoclonal antibodies
Because of their exquisite specificity and affinity monoclonal antibody therapy has emerged as a safe and effective treatment for cancer and inflammatory diseases, though none yet have been developed for stroke. Potent and specific inhibitors of human a2AP have been described that markedly accelerate the dissolution of human clots in vitro. 25,59,78–80 Some antibodies act through an elegant mechanism to convert a2AP from an inhibitor of plasmin to a plasmin substrate, which leads to permanent inactivation of the a2AP molecule. 59 Inhibition of a2AP in vivo significantly enhances dissolution of experimental venous as well as pulmonary and cerebral arterial thrombi.27,28,40,41,59,81 Recent studies in different laboratories have shown that monoclonal antibody inhibition of a2AP significantly reduces ischemic stroke injury.40,41,52
Microplasmin
Microplasmin is a small fragment of plasmin that lacks the kringle domains, which modulate interactions with potential regulatory molecules and substrates. As a result, microplasmin is a relatively indiscriminant protease that is preferentially inactivated by α2-macroglobulin and by a2AP. Infusion of microplasmin or plasmin, significantly depletes a2AP and reduces experimental brain infarction induced by surgical ligation of the cerebral artery.52 Recombinant microplasmin also significantly reduced brain infarction and disability in a rat photochemical, thrombotic model of ischemic stroke.82 Intravenous microplasmin was investigated in a Phase 1, placebo-controlled, double-blind, ascending-dose study in 60 healthy volunteers.83 Volunteers were given an intravenous bolus of microplasmin of 0.1 to 2 mg/kg over 15 min. Additional volunteers also received a bolus followed by an infusion of 1–4 mg/kg of microplasmin over 60 min. Subjects were monitored for 21 days after the infusion. There was a dose-dependent prolongation of the PT and activated PTT with a reduction in fibrinogen levels. The PT and aPTT levels normalized within 9 hours. There was no change in plasminogen levels.
Notable adverse events included: allergic-type (urticarial) events that were considered to be related to the study medication and were associated with transient decreases in total serum hemolytic complement; all resolved without treatment. Procedure-site reactions were reported in 6 subjects given microplasmin including pain at infusion site in leading to cessation of infusion (2), pain during (2) and after (1) infusion that didn’t lead to cessation, bruising at the intravenous site 11 days post-infusion, prolonged bleeding after infusion (1) and a hematoma (1). No clinically significant changes were seen in the vital signs, urinalysis, hematology tests or clinical chemistry.
Microplasmin was subsequently tested in a Phase 2, randomized, placebo-controlled trial of patients with stroke who were not eligible for r-tPA and had ischemia of 3–12 h.84 Three different bolus- infusion doses were tested. Microplasmin decreased a2AP levels up to 80% and reduced fibrinogen levels. The PT and aPTT prolonged and, D-dimer levels increased. Microplasmin decreased circulating MMP-2 but had no effect on other biomarkers of stroke or brain imaging tests. There were no allergic reactions, but one of thirty treated patients had a symptomatic intracerebral hemorrhage. Brain hemorrhage can be a complication of stroke itself and there were insufficient numbers of patients to conclude whether there was any statistical benefit or risk from therapy. 84 The company (ThromboGenics) producing microplasmin is not currently developing it for ischemic stroke.
Plasmin
While Nagai et al. had used infusions of plasmin to deplete systemic a2AP, Marder et al. conversely viewed a2AP as a molecular mechanism to control the systemic effects of catheter-infused plasmin. 52,85 After promising studies shown experimental recanalization of thrombotically occluded middle cerebral arteries in rabbits, catheter delivery of plasmin into a thrombus was studied in a Phase 1 safety trial of 83 patients. 86,87 Seven dose escalation cohorts of 25–175 mg of plasmin were given by 5 h catheter infusion.87 There was major bleeding in 5% of patients and minor bleeding in 16%; the bleeding risk did not appear to be related to dose. At the highest doses, plasmin infusions lowered fibrinogen (20%), a2AP (39%) and α2-macroglobulin (25%). Dissolution of the thrombus by ≥ 50% occurred in 79% of the patients receiving the highest doses vs. 50% receiving lower doses. Adverse events occurred in 57 subjects (69%) including hypertension (17%), γ-glutamyltransferase elevation (7%), constipation (7%), nausea (6%), and hypotension (6%), of which one event (hypertension and sinus tachycardia) was considered to be plasmin-related. 87 Serious adverse events occurred in 23% of patients: “postprocedural hematoma and hematoma, artery or graft complication or occlusion, pseudoaneurysm, peripheral ischemia, GI hemorrhage, MI, arterial rupture, hypertension, abdominal pain, wound infection, sepsis, compartment syndrome, joint effusion, convulsion, ischemic stroke, multiorgan failure, metastatic neoplasm, and psychotic disorder.” 87 However, some of these events were not attributed to plasmin, due to the timing of the event and the lack of a relationship between the dose of plasmin and the frequency of serious adverse events. Plasmin is now in a Phase 1/2a trial for treatment of ischemic stroke and a Phase 2 trial for peripheral arterial ischemia sponsored by Grifols.85
A2AP cleaving enzyme inhibition
Inhibitors of a2AP cleaving enzyme or a soluble form of fibroblast activating protein may be of value in accelerating fibrinolysis by delaying the production of Asn-a2AP which is more rapidly crosslinked to fibrin. 21 While there appears to have been no published clinical investigations of a fibroblast activation protein-targeted therapy for thrombotic disease, there are ongoing clinical trials to investigate the clinical utility of modifying its activity on carcinoma-associated fibroblasts as a novel chemotherapeutic strategy.88
Summary and Prospects
Evidence suggests that a2AP plays a critically important role in ischemic stroke. Epidemiologic and clinical studies indicate that a2AP levels are linked to the risk of developing stroke and to the therapeutic success of r-tPA. Experimental studies confirm that a2AP levels affect brain injury and outcomes after thromboembolic stroke, as well as stroke induced by surgical ligation without reperfusion. Therapeutic inactivation of a2AP by monoclonal antibodies or microplasmin-plasmin significantly reduces brain infarction (Fig. 1). Experimental a2AP-I had a longer therapeutic window for reducing ischemic brain injury than r-tPA. By comparison to r-tPA, a2AP inactivation markedly diminishes brain swelling, brain hemorrhage and mortality. Inactivation of a2AP markedly reduces microvascular thrombosis, MMP-9 expression and breakdown of the blood brain barrier. Taken together, these studies demonstrate enormous potential for a2AP-targeted therapies in ischemic stroke. Early clinical studies are planned or underway to test whether modulating a2AP by microplasmin, plasmin or monoclonal antibodies are effective in reducing thrombosis, morbidity and disability in patients with ischemic stroke.
Acknowledgments
Supported in part by National Institute of Neurological Diseases and Stroke (NS089707) and the American Heart Association (GRBT3870005).
References
- 1.Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976;69(1):209–216. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- 2.Moroi M, Aoki N. Isolation characterization of alpha2-plasmin inhibitor from human plasma A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976;251(19):5956–5965. [PubMed] [Google Scholar]
- 3.Mullertz S, Clemmensen I. The primary inhibitor of plasmin in human plasma. Biochem J. 1976;159(3):545–553. doi: 10.1042/bj1590545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edy J, Collen D. The interaction in human plasma of antiplasmin, the fast-reacting plasmin inhibitor, with plasmin, thrombin, trypsin and chymotrypsin. Biochim Biophys Acta. 1977;484(2):423–432. doi: 10.1016/0005-2744(77)90098-5. [DOI] [PubMed] [Google Scholar]
- 5.Aoki N, Moroi M, Matsuda M, Tachiya K. The behavior of alpha2-plasmin inhibitor in fibrinolytic states. J Clin Invest. 1977;60(2):361–369. doi: 10.1172/JCI108784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakata Y, Aoki N. Significance of cross-linking of alpha 2-plasmin inhibitor to fibrin in inhibition of fibrinolysis and in hemostasis. J Clin Invest. 1982;69(3):536–542. doi: 10.1172/JCI110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed GL, Matsueda GR, Haber E. Platelet factor XIII increases the fibrinolytic resistance of platelet-rich clots by accelerating the crosslinking of alpha 2-antiplasmin to fibrin. Thromb Haemost. 1992;68(3):315–320. [PubMed] [Google Scholar]
- 8.Abdul S, Leebeek FW, Rijken DC, Uitte de Willige S. Natural heterogeneity of alpha2-antiplasmin: functional and clinical consequences. Blood. 2016;127(5):538–545. doi: 10.1182/blood-2015-09-670117. [DOI] [PubMed] [Google Scholar]
- 9.Wiman B, Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem. 1978;84(2):573–578. doi: 10.1111/j.1432-1033.1978.tb12200.x. [DOI] [PubMed] [Google Scholar]
- 10.Barker R, Kehoe PG, Love S. Activators and inhibitors of the plasminogen system in Alzheimer’s disease. J Cell Mol Med. 2012;16(4):865–876. doi: 10.1111/j.1582-4934.2011.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collen D, Wiman B. Turnover of antiplasmin, the fast-acting plasmin inhibitor of plasma. Blood. 1979;53(2):313–324. [PubMed] [Google Scholar]
- 12.Collen D, Bounameaux H, De Cock F, Lijnen HR, Verstraete M. Analysis of coagulation and fibrinolysis during intravenous infusion of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction. Circulation. 1986;73(3):511–517. doi: 10.1161/01.cir.73.3.511. [DOI] [PubMed] [Google Scholar]
- 13.Kluft C, Los P, Jie AF, et al. The mutual relationship between the two molecular forms of the major fibrinolysis inhibitor alpha-2-antiplasmin in blood. Blood. 1986;67(3):616–622. [PubMed] [Google Scholar]
- 14.Meltzer ME, Doggen CJ, de Groot PG, Rosendaal FR, Lisman T. Plasma levels of fibrinolytic proteins and the risk of myocardial infarction in men. Blood. 2010;116(4):529–536. doi: 10.1182/blood-2010-01-263103. [DOI] [PubMed] [Google Scholar]
- 15.Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2010;29(5):497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg WM, Macy E, Cornell ES, et al. Plasmin-alpha2-antiplasmin complex in patients with atrial fibrillation. Stroke Prevention in Atrial Fibrillation Investigators. Thromb Haemost. 1999;82(1):100–103. [PubMed] [Google Scholar]
- 17.Cucuianu M, Knauer O, Roman S. Alpha 2-antiplasmin, plasminogen activator inhibitor (PAI) and dilute blood clot lysis time in selected disease states. Thromb Haemost. 1991;66(5):586–591. [PubMed] [Google Scholar]
- 18.Lisman T, Bakhtiari K, Adelmeijer J, et al. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J Thromb Haemost. 2012;10(7):1312–1319. doi: 10.1111/j.1538-7836.2012.04770.x. [DOI] [PubMed] [Google Scholar]
- 19.Teger-Nilsson AC, Gyzander E, Myrwold H, et al. Determination of fast-acting plasmin inhibitor (alpha2-antiplasmin) in plasma from patients with tendency to thrombosis and increased fibrinolysis. Haemostasis. 1978;7(2–3):155–157. doi: 10.1159/000214255. [DOI] [PubMed] [Google Scholar]
- 20.Law RH, Sofian T, Kan WT, et al. X-ray crystal structure of the fibrinolysis inhibitor alpha2-antiplasmin. Blood. 2008;111(4):2049–2052. doi: 10.1182/blood-2007-09-114215. [DOI] [PubMed] [Google Scholar]
- 21.Lee KN, Jackson KW, Christiansen VJ, Dolence EK, McKee PA. Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor alpha2-antiplasmin. J Thromb Haemost. 2011;9(5):987–996. doi: 10.1111/j.1538-7836.2011.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christiansen VJ, Jackson KW, Lee KN, McKee PA. The effect of a single nucleotide polymorphism on human alpha 2-antiplasmin activity. Blood. 2007;109(12):5286–5292. doi: 10.1182/blood-2007-01-065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leebeek FW, Kluft C, Knot EA, et al. Plasmin inhibitors in the prevention of systemic effects during thrombolytic therapy: specific role of the plasminogen-binding form of alpha 2-antiplasmin. J Am Coll Cardiol. 1990;15(6):1212–1220. doi: 10.1016/s0735-1097(10)80003-8. [DOI] [PubMed] [Google Scholar]
- 24.Uitte de Willige S, Miedzak M, Carter AM, et al. Proteolytic and genetic variation of the alpha-2-antiplasmin C-terminus in myocardial infarction. Blood. 2011;117(24):6694–6701. doi: 10.1182/blood-2010-11-320325. [DOI] [PubMed] [Google Scholar]
- 25.Reed GL, 3rd, Matsueda GR, Haber E. Synergistic fibrinolysis: combined effects of plasminogen activators and an antibody that inhibits alpha 2-antiplasmin. Proc Natl Acad Sci U S A. 1990;87(3):1114–1118. doi: 10.1073/pnas.87.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed GL, 3rd, Singer DE, Picard EH, DeSanctis RW. Stroke following coronary-artery bypass surgery A case-control estimate of the risk from carotid bruits. N Engl J Med. 1988;319(19):1246–1250. doi: 10.1056/NEJM198811103191903. [DOI] [PubMed] [Google Scholar]
- 27.Reed GL, 3rd, Matsueda GR, Haber E. Inhibition of clot-bound alpha 2-antiplasmin enhances in vivo thrombolysis. Circulation. 1990;82(1):164–168. doi: 10.1161/01.cir.82.1.164. [DOI] [PubMed] [Google Scholar]
- 28.Butte AN, Houng AK, Jang IK, Reed GL. Alpha 2-antiplasmin causes thrombi to resist fibrinolysis induced by tissue plasminogen activator in experimental pulmonary embolism. Circulation. 1997;95(7):1886–1891. doi: 10.1161/01.cir.95.7.1886. [DOI] [PubMed] [Google Scholar]
- 29.Matsuno H, Okada K, Ueshima S, Matsuo O, Kozawa O. Alpha2-antiplasmin plays a significant role in acute pulmonary embolism. J Thromb Haemost. 2003;1(8):1734–1739. doi: 10.1046/j.1538-7836.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- 30.Weitz JI, Leslie B, Hirsh J, Klement P. Alpha 2-antiplasmin supplementation inhibits tissue plasminogen activator-induced fibrinogenolysis and bleeding with little effect on thrombolysis. J Clin Invest. 1993;91(4):1343–1350. doi: 10.1172/JCI116335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho CH, Wang SP. Serial thrombolysis-related changes after thrombolytic therapy with TPA in patients with acute myocardial infarction. Thromb Res. 1990;58(3):331–341. doi: 10.1016/0049-3848(90)90102-i. [DOI] [PubMed] [Google Scholar]
- 32.Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial--phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11(1):1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- 33.Williams EC. Plasma alpha 2-antiplasmin activity. Role in the evaluation and management of fibrinolytic states and other bleeding disorders. Arch Intern Med. 1989;149(8):1769–1772. doi: 10.1001/archinte.149.8.1769. [DOI] [PubMed] [Google Scholar]
- 34.Favier R, Aoki N, de Moerloose P. Congenital alpha(2)-plasmin inhibitor deficiencies: a review. Br J Haematol. 2001;114(1):4–10. doi: 10.1046/j.1365-2141.2001.02845.x. [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka A, Kamitsuji H, Takase T, et al. Congenital deficiency of alpha 2-plasmin inhibitor in three sisters. Haemostasis. 1982;11(3):176–184. doi: 10.1159/000214659. [DOI] [PubMed] [Google Scholar]
- 36.Harish VC, Zhang L, Huff JD, Lawson H, Owen J. Isolated antiplasmin deficiency presenting as a spontaneous bleeding disorder in a 63-year-old man. Blood Coagul Fibrinolysis. 2006;17(8):673–675. doi: 10.1097/MBC.0b013e3280108e1a. [DOI] [PubMed] [Google Scholar]
- 37.Aoki N. The past, present and future of plasmin inhibitor. Thromb Res. 2005;116(6):455–464. doi: 10.1016/j.thromres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Lijnen HR, Okada K, Matsuo O, Collen D, Dewerchin M. Alpha2-antiplasmin gene deficiency in mice is associated with enhanced fibrinolytic potential without overt bleeding. Blood. 1999;93(7):2274–2281. [PubMed] [Google Scholar]
- 39.Matsuno H, Kozawa O, Okada K, et al. Inhibitors of fibrinolytic components play different roles in the formation and removal of arterial thrombus in mice. J Cardiovasc Pharmacol. 2002;39(2):278–286. doi: 10.1097/00005344-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Houng AK, Wang D, Reed GL. Reversing the deleterious effects of alpha2-antiplasmin on tissue plasminogen activator therapy improves outcomes in experimental ischemic stroke. Experimental neurology. 2014;255C:56–62. doi: 10.1016/j.expneurol.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed GL, Houng AK, Wang D. Microvascular Thrombosis, Fibrinolysis, Ischemic Injury, and Death After Cerebral Thromboembolism Are Affected by Levels of Circulating alpha2-Antiplasmin. Arterioscler Thromb Vasc Biol. 2014;34(12):2586–2593. doi: 10.1161/ATVBAHA.114.304530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marti-Fabregas J, Borrell M, Cocho D, et al. Hemostatic markers of recanalization in patients with ischemic stroke treated with rt-PA. Neurology. 2005;65(3):366–370. doi: 10.1212/01.wnl.0000171704.50395.ba. [DOI] [PubMed] [Google Scholar]
- 43.Isenegger J, Meier N, Lammle B, et al. D-dimers predict stroke subtype when assessed early. Cerebrovasc Dis. 29(1):82–86. doi: 10.1159/000256652. [DOI] [PubMed] [Google Scholar]
- 44.Lane DA, Wolff S, Ireland H, Gawel M, Foadi M. Activation of coagulation and fibrinolytic systems following stroke. Br J Haematol. 1983;53(4):655–658. doi: 10.1111/j.1365-2141.1983.tb07316.x. [DOI] [PubMed] [Google Scholar]
- 45.Uchiyama S, Yamazaki M, Hara Y, Iwata M. Alterations of platelet, coagulation, and fibrinolysis markers in patients with acute ischemic stroke. Seminars in thrombosis and hemostasis. 1997;23(6):535–541. doi: 10.1055/s-2007-996132. [DOI] [PubMed] [Google Scholar]
- 46.Kataoka S, Hirose G, Hori A, Shirakawa T, Saigan T. Activation of thrombosis and fibrinolysis following brain infarction. J Neuro Sci. 2000;181(1–2):82–88. doi: 10.1016/s0022-510x(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 47.Olah L, Misz M, Kappelmayer J, et al. Natural coagulation inhibitor proteins in young patients with cerebral ischemia. Cerebrovasc Dis. 2001;12(4):291–297. doi: 10.1159/000047723. [DOI] [PubMed] [Google Scholar]
- 48.Ono N, Koyama T, Suehiro A, et al. Clinical significance of new coagulation and fibrinolytic markers in ischemic stroke patients. Stroke. 1991;22(11):1369–1373. doi: 10.1161/01.str.22.11.1369. [DOI] [PubMed] [Google Scholar]
- 49.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 50.Wang YF, Tsirka SE, Strickland S, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4(2):228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 51.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99(18):2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 52.Nagai N, De Mol M, Van Hoef B, Verstreken M, Collen D. Depletion of circulating alpha(2)-antiplasmin by intravenous plasmin or immunoneutralization reduces focal cerebral ischemic injury in the absence of arterial recanalization. Blood. 2001;97(10):3086–3092. doi: 10.1182/blood.v97.10.3086. [DOI] [PubMed] [Google Scholar]
- 53.Nagai N, Vanlinthout I, Collen D. Comparative effects of tissue plasminogen activator, streptokinase, and staphylokinase on cerebral ischemic infarction and pulmonary clot lysis in hamster models. Circulation. 1999;100(25):2541–2546. doi: 10.1161/01.cir.100.25.2541. [DOI] [PubMed] [Google Scholar]
- 54.Tabrizi P, Wang L, Seeds N, et al. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler Thromb Vasc Biol. 1999;19(11):2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- 55.Albers GW. Antithrombotic agents in cerebral ischemia. Am J Cardiol. 1995;75(6(S1)):34B–38B. doi: 10.1016/0002-9149(95)80008-g. [DOI] [PubMed] [Google Scholar]
- 56.Fieschi C, Argentino C, Lenzi GL, et al. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. J Neuro Sci. 1989;91(3):311–321. doi: 10.1016/0022-510x(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 57.(Stroke Therapy Academic Industry Roundtable) S. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 58.Lijnen HR, van Hoef B, Beelen V, Collen D. Characterization of the murine plasma fibrinolytic system. Eur J Biochem. 1994;224(3):863–871. doi: 10.1111/j.1432-1033.1994.00863.x. [DOI] [PubMed] [Google Scholar]
- 59.Sazonova IY, Thomas BM, Gladysheva IP, Houng AK, Reed GL. Fibrinolysis is amplified by converting alpha-antiplasmin from a plasmin inhibitor to a substrate. J Thromb Haemost. 2007;5(10):2087–2094. doi: 10.1111/j.1538-7836.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 60.Kilic E, Hermann DM, Hossmann KA. Recombinant tissue-plasminogen activator-induced thrombolysis after cerebral thromboembolism in mice. Acta Neuropathol. 2000;99(3):219–222. doi: 10.1007/pl00007430. [DOI] [PubMed] [Google Scholar]
- 61.Orset C, Macrez R, Young AR, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38(10):2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- 62.Hara T, Mies G, Hossmann KA. Effect of thrombolysis on the dynamics of infarct evolution after clot embolism of middle cerebral artery in mice. J Cereb Blood Flow Metab. 2000;20(10):1483–1491. doi: 10.1097/00004647-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112(9):3555–3562. doi: 10.1182/blood-2008-04-144758. [DOI] [PubMed] [Google Scholar]
- 64.Momi S, Tantucci M, Van Roy M, et al. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs. Blood. 2013;121(25):5088–5097. doi: 10.1182/blood-2012-11-464545. [DOI] [PubMed] [Google Scholar]
- 65.Heye N, Paetzold C, Cervos-Navarro J. The role of microthrombi and microcirculatory factors in localization and evolution of focal cerebral ischemia. Neurosurg Rev. 1991;14(1):7–16. doi: 10.1007/BF00338186. [DOI] [PubMed] [Google Scholar]
- 66.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25(9):1847–1853. doi: 10.1161/01.str.25.9.1847. discussion 1853-1844. [DOI] [PubMed] [Google Scholar]
- 67.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23(8):879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmeister HM, Szabo S, Kastner C, et al. Thrombolytic therapy in acute myocardial infarction: comparison of procoagulant effects of streptokinase and alteplase regimens with focus on the kallikrein system and plasmin. Circulation. 1998;98(23):2527–2533. doi: 10.1161/01.cir.98.23.2527. [DOI] [PubMed] [Google Scholar]
- 69.Gross S, Janssen SW, de Vries B, et al. Collaborative study for the validation of alternative in vitro potency assays for human tetanus immunoglobulin. Pharmeur Bio Sci Notes. 2009;2009(1):11–25. [PubMed] [Google Scholar]
- 70.Singh S, Houng AK, Wang D, Reed GL. Physiologic Variations in Blood Plasminogen Levels Affect Outcomes after Acute Cerebral Thromboembolism in Mice: A Pathophysiologic Role for Microvascular Thrombosis. J Thromb Haemost. 2016 doi: 10.1111/jth.13390. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper JA, Lo SK, Malik AB. Fibrin is a determinant of neutrophil sequestration in the lung. Circ Res. 1988;63(4):735–741. doi: 10.1161/01.res.63.4.735. [DOI] [PubMed] [Google Scholar]
- 72.Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2001;21(12):2093–2098. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 73.Rosell A, Cuadrado E, Ortega-Aznar A, et al. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39(4):1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 74.Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 75.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8(1):82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33(11):2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 77.Asahi M, Asahi K, Jung JC, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20(12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Reed GL, 3rd, Matsueda GR, Haber E. Acceleration of plasma clot lysis by an antibody to alpha 2-antiplasmin. Trans Assoc Am Physicians. 1988;101:250–256. [PubMed] [Google Scholar]
- 79.Mimuro J, Koike Y, Sumi Y, Aoki N. Monoclonal antibodies to discrete regions in alpha 2-plasmin inhibitor. Blood. 1987;69(2):446–453. [PubMed] [Google Scholar]
- 80.Reed GL. Functional characterization of monoclonal antibody inhibitors of alpha 2-antiplasmin that accelerate fibrinolysis in different animal plasmas. Hybridoma. 1997;16(3):281–286. doi: 10.1089/hyb.1997.16.281. [DOI] [PubMed] [Google Scholar]
- 81.Reed GL, Houng AK. The contribution of activated factor XIII to fibrinolytic resistance in experimental pulmonary embolism. Circulation. 1999;99(2):299–304. doi: 10.1161/01.cir.99.2.299. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki Y, Chen F, Ni Y, et al. Microplasmin reduces ischemic brain damage and improves neurological function in a rat stroke model monitored with MRI. Stroke. 2004;35(10):2402–2406. doi: 10.1161/01.STR.0000140628.00927.1a. [DOI] [PubMed] [Google Scholar]
- 83.Pakola S, Cahillane G, Stassen JM, Lijnen HR, Verhamme P. Neutralization of alpha(2)-antiplasmin by microplasmin: a randomized, double-blind, placebo-controlled, ascending-dose study in healthy male volunteers. Clin Ther. 2009;31(8):1688–1706. doi: 10.1016/j.clinthera.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Thijs VN, Peeters A, Vosko M, et al. Randomized, placebo-controlled, dose-ranging clinical trial of intravenous microplasmin in patients with acute ischemic stroke. Stroke. 2009;40(12):3789–3795. doi: 10.1161/STROKEAHA.109.560201. [DOI] [PubMed] [Google Scholar]
- 85.Marder VJ. Historical perspective and future direction of thrombolysis research: the re-discovery of plasmin. J Thromb Haemost. 2011;9(Suppl 1):364–373. doi: 10.1111/j.1538-7836.2011.04370.x. [DOI] [PubMed] [Google Scholar]
- 86.Marder VJ, Jahan R, Gruber T, Goyal A, Arora V. Thrombolysis with plasmin: implications for stroke treatment. Stroke. 2010;41(10 Suppl):S45–49. doi: 10.1161/STROKEAHA.110.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marder VJ, Comerota AJ, Shlansky-Goldberg RD, et al. Safety of catheter-delivered plasmin in patients with acute lower extremity arterial or bypass graft occlusion: phase I results. J Thromb Haemost. 2012;10(6):985–991. doi: 10.1111/j.1538-7836.2012.04728.x. [DOI] [PubMed] [Google Scholar]
- 88.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11(2):257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]