Abstract
Although both human epidemiologic and animal model studies have suggested that caffeine/coffee protects against Alzheimer’s disease, direct human evidence for this premise has been lacking. In the present case-control study, two separate cohorts consisting of 124 total individuals (65–88 years old) were cognitively assessed and a blood sample taken for caffeine/biomarker analysis. Subjects were then monitored for cognitive status over the ensuing 2–4 year period to determine the extent to which initial plasma caffeine/biomarkers levels would be predictive of changes in cognitive status. Plasma caffeine levels at study onset were substantially lower (−51%) in mild cognitive impairment (MCI) subjects who later progressed to dementia (MCI→DEM) compared to levels in stable MCI subjects (MCI→MCI). Moreover, none of the MCI→DEM subjects had initial blood caffeine levels that were above a critical level of 1200 ng/ml, while half of stable MCI→MCI subjects had blood caffeine levels higher than that critical level. Thus, plasma caffeine levels greater than 1200 ng/ml (≈6 µM) in MCI subjects were associated with no conversion to dementia during the ensuing 2–4 year follow-up period. Among the 11 cytokines measured in plasma, three of them (GCSF, IL-10, and IL-6) were decreased in MCI→DEM subjects, but not in stable MCI→MCI subjects with high plasma caffeine levels. Coffee would appear to be the major or perhaps only source of caffeine for such stable MCI patients. This case-control study provides the first direct evidence that caffeine/coffee intake is associated with a reduced risk of dementia or delayed onset, particularly for those who already have MCI.
Keywords: Alzheimer’s disease, caffeine, coffee, dementia, immune response, mild cognitive impairment, plasma cytokines
INTRODUCTION
There is a critical need to identify prophylactics that reduce risk, or delay onset, of Alzheimer’s disease (AD), particularly from the standpoint of lifestyle choices. In this context, an increasing body of scientific evidence supports the premise that caffeine/coffee intake can reduce the risk of AD and/or delay the disease’s onset [see Journal of Alzheimer’s Disease, Special Issue, Volume 20, Supplement 1, 2010]. That evidence began with epidemiologic human studies and has been further supported by highly controlled studies in AD transgenic mice. As well, these later mouse studies have provided clear insight into the disease-modifying mechanisms whereby caffeine/coffee appear to provide protection against AD. What has been lacking to solidify caffeine/coffee as perhaps the first dietary component to be prophylactic against AD are controlled clinical studies. In that context, the present case-control study provides the first direct evidence that caffeine/coffee may indeed reduce risk and/or delay onset of dementia, notably in those that already have the prelude to AD, mild cognitive impairment (MCI).
Epidemiologic studies have largely supported caffeine/coffee as protective against cognitive impairment and AD. Early prospective studies reported significantly less cognitive decline over a 4–10 year period in aged men drinking 3 cups of coffee per day [1] and in aged women whose daily caffeine intake was equivalent to 3+ cups of coffee [2]. Two recent epidemiologic studies evaluated mid-life coffee intake and risk of later AD, with one study reporting a robust 65% decreased risk of AD in individuals who drank 3–5 cups of coffee daily during their 40 s–50 s [3], while the other study found no association [4]. Parenthetically, the former study involved a typical in-clinic assessment of AD, whereas the later study utilized a telephone interview questionnaire. Perhaps most compelling among the epidemiologic studies is Maia and de Mendonca [5], wherein AD subjects were found to have consumed much less caffeine (calculated from questionnaires) during the 20 years preceding diagnosis of AD compared with age-matched subjects without AD. Though insightful, these epidemiologic studies cannot provide direct evidence for a prophylactic effect of caffeine/coffee against AD because they are largely based on recall and cannot unequivocally isolate caffeine/coffee intake from other factors that affect cognition over a lifetime (e.g., they are not controlled).
Fortunately, the creation of AD transgenic mice has allowed highly controlled studies to be performed that can delve into AD pathogenesis and therapeutic development. These AD mouse models produce the same abnormal human protein (amyloid-β; Aβ) that is produced and aggregates in the brains of humans destined for AD [6, 7]. During this brain Aβ pathogenesis, which many researchers believe to be critical in precipitating AD [8], AD transgenic mice become memory-impaired and are, thus, considered appropriate (though incomplete) models for the disease. We have utilized “young adult” AD mice in demonstrating that long-term administration of a physiologic level of caffeine in drinking water protects them from otherwise inevitable memory impairment in older age [9], as well as reverses already-present memory impairment in “aged” AD mice [10]. Caffeine likely induced these protective and treatment effects through its unique ability to suppress both enzymes required for Aβ production (β- and γ-secretase), resulting in much lower brain Aβ aggregation/deposition [9, 10]. Moreover, there are other complementary mechanisms of caffeine action that we have identified that could contribute to the cognitive benefits of caffeine against AD. Specifically, long-term caffeine treatment in AD transgenic mice: 1) decreases brain levels of pro-inflammatory cytokines such as TNF-α and IFN-γ [11], and 2) induces beneficial effects on signal transduction factors important for neuronal plasticity and survival [12]. Thus, our studies in AD mice indicate that caffeine is likely to be a multi-mechanistic, disease-modifying therapeutic against development of AD. The extent to which adenosine receptor antagonism by caffeine is involved in the aforementioned mechanisms has yet to be determined.
Aside from caffeine, coffee is rich in many other components (e.g., antioxidants, anti-inflammatory compounds) that may also complement caffeine’s actions to reduce risk of AD [13–17]. In this regard, we most recently reported that AD mice treated twice-weekly with caffeinated coffee (but not those treated with decaffeinated coffee) showed enhanced memory [18]. Since treatment was given every 72 hours, the cognitive-enhancing ability of caffeinated coffee involved a mechanism that out-lives the presence of coffee’s components (including caffeine) in plasma. In that same study, we showed that a single oral administration of caffeinated coffee induced dramatic elevations in three plasma cytokines (granulocyte-colony stimulating factor (GCSF), IL-10, and IL-6) several hours thereafter, with all remaining cytokines unaffected. This plasma cytokine profile was not seen following administration of either decaffeinated coffee or caffeine, indicating that some as-yet unidentified component of coffee synergizes with caffeine to greatly enhance plasma levels of three beneficial cytokines [18]. This cytokine response, particularly for GCSF, appears to trigger long-term beneficial mechanisms against AD (e.g., recruitment of bone marrow cells to remove brain Aβ, enhanced synaptogenesis, increased neurogenesis) that out-live coffee’s various plasma components. Thus, coffee would seem to provide protective effects against AD that are not possible with caffeine or decaffeinated coffee alone. Consistent with this premise are epidemiologic studies showing that caffeinated coffee intake (but not caffeinated tea or overall caffeine intake) was associated with better cognitive function in aged humans [19], while mid-life caffeinated coffee (but not caffeinated tea intake) was associated with later reduced risk of AD [3].
Although AD starts in the brain several decades prior to AD diagnosis, performing prospective (longitudinal) human studies over decades to test therapeutics for their “protective” potential against AD would be most challenging. Patients with mild cognitive impairment (MCI) already have considerable AD neuropathology accompanied by a mild loss of short-term memory [20]. Inasmuch as 12–15% of MCI patients will go on to develop dementia per year in populations seeking evaluation for memory disorders [21, 22], they are a good population to test the ability of candidate prophylactics to protect against AD or conversion to AD over a relatively short study period. The present case-control study links epidemiologic evidence suggesting caffeine/coffee as prophylactic against AD to our recent findings from AD mice in reporting that: 1) MCI patients with high blood caffeine levels at the beginning of a 2–4 year assessment period had a 100% chance of avoiding conversion to dementia over that period, and 2) caffeine/coffee may have provided this protection, in part, by preventing a selective immune decline that we found to occur in MCI patients several years prior to dementia conversion. Although our findings are associative and require verification via controlled clinical trials with caffeine/coffee administrated over several years to MCI patients, the present study establishes a linkage between higher caffeine/coffee intake in MCI patients and prevention or delaying of progression to dementia.
MATERIALS AND METHODS
Study population
Subjects were previously recruited through the Florida Alzheimer’s Disease Research Center (FADRC) as part of a multisite study of persons aged 65 years and over from the Miami and Tampa areas. The present case-control study involved a total of 124 randomly-selected subjects between 65 and 88 years of age at study onset, with the Miami cohort comprised of 81 subjects and the Tampa cohort comprised of 43 subjects. The original FADRC study protocol, to longitudinally monitor cognitive status and blood biomarkers, was approved by both the University of Miami and University of South Florida Institutional Review Boards. Prior to the start of the study, all participants gave their written informed consent.
General protocol
At the initial visit (between February 2006–July 2007), all subjects were neurologically assessed through the following evaluations: 1) full clinical history, obtained from the participant and corroborated by a reliable informant; 2) neurological evaluation; 3) psychiatric evaluation, including administration of the Geriatric Depression Scale [23] and the Neuropsychiatric Inventory [24]; 4) Clinical Dementia Rating scale (CDR) [25]; 5) Mini-Mental State Evaluation (MMSE) [26]; and 6) a neuropsychological test battery, as outlined by the National Alzheimer’s Coordinating Center for National Alzheimer’s Disease Research and Clinical Centers (NACC) protocol [27], which includes standard measures of memory, language, visuospatial and executive function. Also included were additional tests, including the Three-Trial Fuld Object Memory Evaluation [28] and Hopkins Verbal Learning Test-Revised [29]. In addition, MRI scans were acquired using a proprietary 3-D (volumetric) protocol on a Siemens Symphony, 1.5 Tesla machine (Iselin, NJ).
Based on the above neurologic evaluation at the initial visit, subjects were diagnosed as either aged normal, MCI (both amnestic and non-amnestic), or dementia (DEM). At that same visit, a fasting blood sample was taken via venous puncture during the morning hours. Plasma was immediately separated by centrifugation, frozen, and stored at −80°C until assay. Over the ensuing 2–4 year period, subjects came in on a yearly basis for re-assessment of cognitive status. Five groups resulted from the follow-up analysis:
| N→N | Initially normal and remained so during 2–4 year follow-up |
| N→MCI | Initially normal, but converted to MCI during 2–4 year follow-up |
| MCI→MCI | Initially MCI and remained so during 2–4 year follow-up |
| MCI→DEM | Initially MCI, but converted to dementia during 2–4 year follow-up |
| DEM | Initially dementia and remained dementia during follow-up |
Diagnostic procedures
The physician initially assigned a cognitive diagnosis of N, MCI, or dementia, based on the subject’s entire clinical history using a reliable informant, including his/her functional status (which was derived from the history itself and from the CDR rating and a functional activity questionnaire), as well as the MMSE score and sub-scores. All neuropsychological tests were administered in the subjects’ native language (English or Spanish), and age and education adjusted normative data applicable to both language groups were used to assess the cut points for impairment in each test, based on a large co-normed normative database used in previous studies [30]. Memory was assessed, with the 3-trial Fuld Object Memory Evaluation [28] and Delayed Visual Reproduction of the Wechsler Memory Scale-R [31]. Tests of non-memory function included category fluency (language) [32], letter fluency (language) [33], Block Design-WAIS-III (visuospatial) [31], Trails B (Executive) [34], and Similarities-WAIS-R (Executive) [31]. Neuropsychological classification was achieved employing methods developed by Loewenstein et al. [30]. The thresholds used were: (a) a test score of 1.5 SD or greater below expected normative values on any single test for MCI syndromes and (b) 2.0 SD or greater below expected normative values in one memory and one non-memory test for dementia (corresponding to NINCDS-ADRDA criteria).
Consensus diagnoses
The final consensus cognitive diagnosis was made using a computational algorithm developed and validated in the Florida ADRC which combined the AlgDx assigned each NACC diagnosis by combining the physician diagnoses with the neuropsychological evaluation. Subjects diagnosed with aMCI or non-amnestic MCI (naMCI) in the FADRC-CC were judged to have met Petersen’s criteria for MCI [21], as well as criteria for a diagnosis of Cognitive Impairment without Dementia [35]. Subjects judged to meet criteria for dementia were impaired on neuropsychological testing and judged by the clinician to have sufficient memory, or other cognitive and functional impairment, to meet criteria for dementia by DSM-IV criteria [36].
Progression over time
Progression from a normal diagnosis to aMCI or naMCI required a diagnosis of MCI by clinical evaluation made by the patient’s physician, with confirmation of cognitive deficits by the neuropsychologist who used a threshold of 1.5 SD or below expected levels of performance on one or more memory measures, with or without non-memory impairment (aMCI) or one or more non-memory measures (naMCI). While the follow-up diagnosis by the physician and the neuropsychologist were made independently, they were not blind to the previous or baseline diagnoses, as is the case with most longitudinal studies. These clinicians were directed to adhere to as strictly as possible to guidelines or rules in making the consensus diagnosis at baseline and all of their yearly follow-up evaluations. Subjects were considered to have progressed to dementia if in their physician’s judgment, social and occupational function was sufficiently impaired to fulfill DSM-IV criteria for a dementia syndrome [3] and the patient had deficits on memory testing equal to or greater than 1.5 SD below expected levels. For several patients diagnosed with dementia upon initial year 01 follow-up, later clinical follow-up was not performed.
Plasma analysis
Caffeine
Plasma caffeine concentrations were measured via compete ELISA Kits from Neogen (WI, USA), following manufacturer’s protocol. In brief, the enzyme conjugate solution was prepared by diluting the 180× enzyme conjugate stock 1 to 180 in the EIA buffer provided. Caffeine was then diluted with EIA buffer at two-fold dilutions from 200 ng/ml to 0.39 ng/ml. Then 20 µl standard of each dilution was added into the coated plate. Plasma samples were then diluted with EIA buffer, with 20 µl of this dilution added into the coated plate. Both standard and samples were run in duplicate in the plate. Positive and negative controls of 20 µl were loaded to each plate. Then 180 µl of diluted drug-enzyme conjugate was added into each well and mixed by gently shaking the plate. Plates were covered with plastic film and incubated at room temperature for 45 min. During the incubation, a 10× wash buffer was diluted to 1× with DI water and mixed thoroughly. Once incubation was completed, the liquid was dumped from the wells. Plates were then taped on a clean lint-free towel to remove any remaining liquid in the wells. Then each well was washed with 300 µl of diluted wash buffer 3 times. After completing the last wash step, the bottom of the wells was wiped with a lint-free towel to remove any liquid on the outside of the wells. Then 150 µl of the K-Blue Substrate was added to each well with a multi-channel pipette. The plate was then mixed by gently shaking, followed by incubation at room temperature for 5 to 20 min. To stop the enzyme reaction, 50 µl of red stop solution was added to each well and gently mixed. The absorbance was then measured with a plate reader (Synergy HT, Biotek, VT) at a wavelength of 650 nm. The absorbance was converted into concentration using Gen5 software.
Aβ1-40 and Aβ1-42
Plasma Aβ1-40 and Aβ1-42 levels were detected by using ELISA kits (Invotrogen, Camarilla, CA). Standard and samples were mixed with detection antibody and loaded on the antibody pre-coated plate as the designated wells after three hours of incubation at room temperature. HRP-conjugated antibody was added after wash, and substrates were added for colorimetric reaction, which was then stopped with sulfuric acid. Optical density was obtained and concentrations were calculated according a standard curve.
Cytokines/chemokines/growth factors
For both human plasma samples, as well as plasma samples from a prior mouse study [18] presented in the Discussion, a total of eight cytokines and chemokines were measured with Lumenix multiplex assay (GCSF, IL-10, IL-6, TNF-α, IL-1α, IL-17, IFN-γ, and IP-10). An addition four cytokines/growth factors (ENA-78, PDGF BB, NGF, and MCP-1) were analyzed from human plasma samples. Expression profiles and levels were detected using the Bio-Rad Bio-Plex, with reagents being ordered from Millipore as customer kits (Millipore, CA). All samples and standards were prepared using company protocols. Plasma samples were prepared for analysis by diluting 1 volume of the serum sample with 3 volumes of the Bio-Plex mouse or human sample diluent. Detailed procedures were performed by following the protocol provided by the manufacture. Finally, the plates were read. Each cytokine level was calculated based on its own standard curve.
Immunoglobulin isotyping assay
Plasma levels of IgG1, IgG2, IgG3, and IgG4 were determined with Beadlyte Human IgG subclass isotyping kits (Millipore, CA) by using Luminex detection assay and following the protocol provided by manufacturer. Briefly, each plasma sample was diluted with dilution buffer in a 96 well sample-preparing plate. Isotyping beads were then added into each well, mixed and incubated for 1 hour at room temperature on a plate shaker. Then samples were transferred into a pre-wet membrane plate and washed under a controlled vacuum system. Detection antibody was then added into each well, followed by incubation at room temperature for 30 minutes. Plates were then washed with controlled vacuum and submitted to Luminex-100 after suspension in wash buffer. The concentration of each IgG subtype was calculated according to the standard curve.
Statistical analysis
Statistical analysis of subject profiles and plasma biomarkers between clinical groups were initially performed using ANOVA, which was then followed by Tukey HSD tests or additional ANOVAs for planned pair-by-pair comparisons. Very infrequently, outlier analysis (Grubb’s test) of a group’s data for a given biomarker indicated removal of a single subject’s data from statistical analysis involving that particular marker, which was then done. All clinical data are presented as mean ± SEM, with significance group differences designated at the p < 0.05 or higher level.
RESULTS
Table 1 shows the subject profiles for both Miami and Tampa cohorts combined (n = 125), as well as for each cohort separately. For age at study onset, a three-group comparison (N, MCI, and DEM) for the Miami and Tampa cohorts separately revealed no overall differences via ANOVA for either cohort [F(2,78) = 2.40; p = 0.10 and F(2,40) = 1.72; p = 0.19, respectively]. For both cohorts combined, there was a significant overall difference in age for the three-group comparison [F(2,121) = 3.83; p < 0.03)], with DEM subjects being significantly older than normals at study initiation (p < 0.05). If age at study onset is compared in terms of the five-groups resulting from the 2–4 year follow-up (e.g., N→N, N→MCI, MCI→MCI, MCI→DEM, DEM), an overall group difference in age was present for both cohorts combined [F(4,119) = 3.56; p < 0.004], with post hoc analysis showing that only the N→N versus MCI→DEM groups differed significantly in age (p < 0.05). For each cohort separately, no age differences were present among the five groups. It is important to underscore that, for the important pair-by-pair comparisons of [N→N versus N→MCI] and [MCI→MCI versus MCI→DEM], there were no differences in age irrespective of combined or separate cohort analysis. For all subjects in this study, the average follow-up period after initial cognitive assessment was around 2½–3 years (Table 1).
Table 1.
Subject profiles for Miami + Tampa cohorts combined, as well as for each cohort separately
| Miami + Tampa Cohorts combined
|
Miami cohort
|
Tampa cohort
|
||||||
|---|---|---|---|---|---|---|---|---|
| Subjects and gender | (M/F) | Age | Follow-up (yrs) | Subjects | Age | Subjects | Age | |
| N | 69 | 20/49 | 73.4±0.7 | 2.75±0.08 | 45 | 73.3±0.8 | 24 | 73.5±1.2 |
| MCI | 32 | 17/15 | 76.5±1.1 | 2.51±0.11 | 24 | 76.7±1.4 | 8 | 75.9±2.0 |
| DEM | 23 | 12/11 | 77.1 ± 1.3* | 2.35 ± 0.14 | 12 | 77.5 ± 2.1 | 11 | 76.7 ± 1.4 |
| N→N | 60 | 18/42 | 72.9 ± 0.7 | 2.73 ± 0.09 | 38 | 72.7 ± 0.9 | 22 | 73.1 ± 1.2 |
| N→MCI | 9 | 2/7 | 76.6 ± 1.3 | 2.88 ± 0.20 | 7 | 76.3 ± 1.4 | 2 | 78.0 ± 4.0 |
| MCI→MCI | 21 | 15/6 | 75.0 ± 1.5 | 2.62 ± 0.15 | 15 | 74.7 ± 1.9 | 6 | 75.7 ± 2.7 |
| MCI→DEM | 11 | 2/9 | 79.4 ± 1.4* | 2.33 ± 0.14 | 9 | 80.0 ± 1.6 | 2 | 76.5 ± 2.5 |
| DEM | 23 | 12/11 | 77.1 ± 1.3 | 2.35 ± 0.14 | 12 | 77.5 ± 2.1 | 11 | 76.7 ± 1.4 |
p < 0.05 versus N or [N→N].
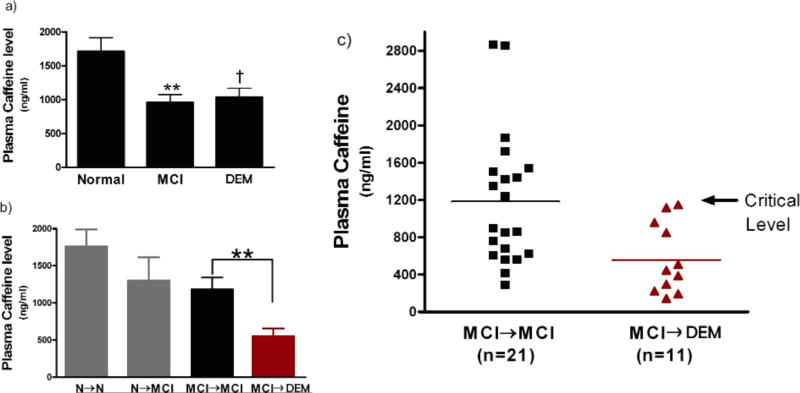
Plasma caffeine levels were analyzed simultaneously by utilizing the same kits for both Miami and Tampa cohorts, thus allowing combination of data from the two cohorts. Plasma caffeine levels did not co-vary with age since the correlation between age and caffeine levels was not significant (r =−0.09; p > 0.34) for the combined cohorts. Moreover, there were no statistically significant caffeine versus age correlations among normal, MCI, or DEM sub-groups. Analysis of plasma caffeine levels from the initial visit in relation to initial diagnosis (Fig. 1a) revealed significantly lower caffeine levels in MCI subjects relative to normals [F(1,99) = 5.52; p < 0.03]. Lower caffeine levels were also present in DEM subjects compared to normals, but not to statistical significance [F(1,90) = 3.42; p < 0.07)] (Fig. 1a). There were no statistically significant differences between MCI and DEM subjects with regards to plasma caffeine levels.
Fig. 1.
Plasma caffeine levels at the beginning of a 2–4 year cognitive assessment period in subjects from two combined cohorts (Miami and Tampa). a) Caffeine levels in subjects grouped by their initial cognitive status as Normal (N), mild cognitive impairment (MCI), or dementia (DEM). Lower caffeine levels were present in MCI and DEM subjects at study initiation. **p < 0.02 versus N; †p = 0.07 versus N. b) Caffeine levels in subjects grouped by their eventual cognitive status during follow-up as stable Normal (N→N), Normal converting to MCI (N→MCI), stable MCI (MCI→MCI), or MCI converting to DEM (MCI→DEM). Blood plasma caffeine levels at study initiation were substantially lower in MCI patients who eventually progressed to DEM compared to those that remained stable MCI. **p < 0.02. c) Plotting of caffeine levels in individual MCI subjects who progressed to DEM and those that remained stable MCI (group means indicated by horizontal lines). None of the MCI→DEM subjects had initial caffeine levels above a critical level of 1200 ng/ml, while half of stable MCI subjects had levels higher than that level. Thus, subjects with the initial diagnosis of MCI and who possessed plasma caffeine levels above 1200 ng/ml at that time had a 100% chance of avoiding DEM during the ensuing 2–4 years.
Normal and MCI groups were then further subdivided according to whether subjects remained stable or declined in cognitive status over the 2–4 year follow-up (Fig. 1b). For initially-diagnosed normal subjects, a 26% lower plasma level of caffeine in normals that converted to MCI (N→MCI) compared to stable normals (N→N) was not significance because of considerable variability in caffeine levels among individuals in both of these sub-groups. In contrast, 11 MCI subjects that progressed to DEM (MCI→DEM) had much lower plasma caffeine levels [F(1,30) = 6.77; p < 0.02)] that were 51% below levels at study initiation when compared to MCI subjects that remained MCI (MCI→MCI; Fig. 1b). Plotting of blood caffeine levels for all individuals in the MCI→MCI and MCI→DEM groups revealed that none of the MCI→DEM subjects had initial blood caffeine levels that were above an apparent critical value of 1200 ng/ml (Fig. 1c). By contrast, approximately half of MCI→MCI subjects had blood caffeine levels at least that high. The data from this combined 2-cohort study indicates that blood caffeine levels greater than 1200 ng/ml (≈6 µM plasma caffeine) in MCI patients at the start of the study were associated with a 100% chance of avoiding progression to dementia during the 2–4 year follow-up.
We then focused on the Miami cohort for additional analysis because the Tampa cohort had several subgroups with very few subjects (Table 1). Moreover, with the exception of caffeine levels, plasma levels of all other biomarkers were analyzed separately for the Miami and Tampa cohorts; ensuing statistical analyses indicated the data from both cohorts could not be combined due to the two independent biomarker analyses.
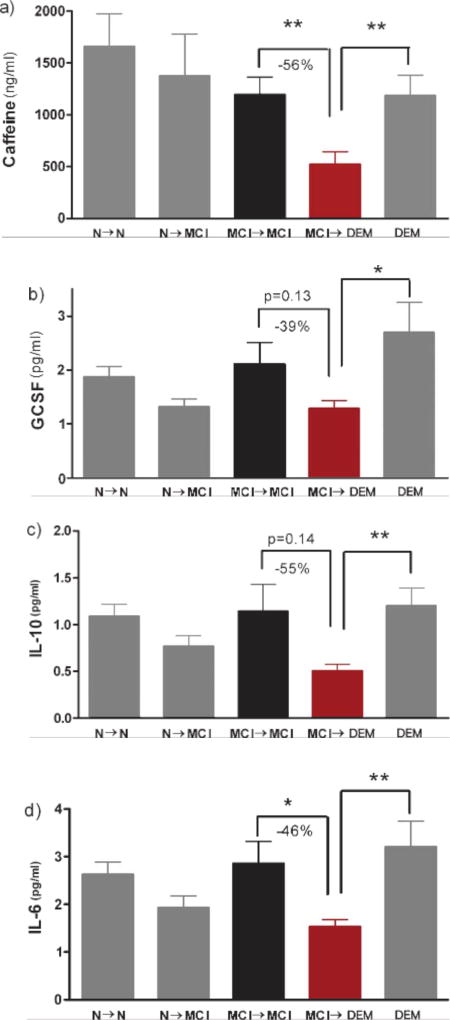
As was the case for both Miami and Tampa cohorts combined (Fig. 1b), MCI subjects in the Miami cohort that progressed to DEM had much lower initial caffeine levels (−56%) compared to MCI subjects that remained stable [F(1,22) = 7.63; p < 0.02] (Fig. 2a). Interestingly, DEM subjects in the Miami cohort had caffeine levels significantly higher than those of MCI subjects that progressed to DEM [F(1,19) = 7.69; p < 0.02] (Fig. 2a). Analysis of all 11 cytokines analyzed from the initial blood sample revealed 3 cytokines that were particularly affected—GCSF, IL-10, and IL-6 (Fig. 2b–d). All three of these cytokines were lower in plasma of MCI patients that were destined for AD conversion (MCI→DEM) in comparison to both non-converting MCI subjects (MCI→MCI) and DEM subjects. For GCSF, IL-10, and IL-6 comparisons involving MCI→DEM versus MCI→MCI, [F(1,22) = 2.38; p = 0.13], [F(1,21) = 2.33; p = 0.14], and [F(1,21) = 4.1; p < 0.05], respectively. For cytokine comparisons involving MCI→DEM versus DEM, [F(1,19) = 5.6; p < 0.05], [F(1,18) = 7.9; p < 0.02], and [F(1,18) = 8.52; p < 0.02], respectively. The 39–55% lower levels of these three cytokines in MCI→DEM subjects compared to MCI→MCI subjects were similar to the 56% lower plasma caffeine levels in the same MCI→DEM subjects. No such differences in plasma caffeine or the same three cytokines were evident for N→N versus N→MCI subjects (Fig. 2). Indeed, there were no differences between these two sub-groups of normal subjects for any of the 11 cytokines, 4 IgGs, and 2 Aβ isoforms analyzed in plasma (data not shown). Moreover, there were no group differences between normal subjects and stable MCI subjects for any biomarker analyzed (Fig. 2). Group differences (suppressions) in plasma caffeine and cytokine levels were largely restricted to the MCI→DEM group.
Fig. 2.
a–d) Plasma caffeine, GCSF, IL-10, and IL-6 levels at the beginning of a 2–4 year cognitive assessment period in subjects from the Miami cohort. All four biomarkers were significantly or near-significantly lower in MCI subjects who later progressed to DEM (MCI→DEM) compared to MCI subjects that remained stable, or compared to subjects initially classified as AD. *p < 0.05; **p < 0.02.
In contrast to the three cytokines shown to be lower in MCI subjects destined for DEM conversion compared to MCI stable subjects (Fig. 2b–d), none of the other 8 plasma cytokines or plasma NGF showed any such profile when the same two MCI sub-groups were compared (Table 2). In MCI→DEM subjects, these 9 cytokines/growth factors varied between reductions to overt elevations compared to MCI→MCI subjects. For all four IgGs and both Aβ isoforms measured in plasma, no differences were observed between the two MCI sub-groups.
Table 2.
A comparison of initial plasma biomarkers in MCI→MCI and MCI→DEM subjects from the Miami cohort
| MCI → MCI | MCI → DEM | Percent change | |
|---|---|---|---|
| (n = 15) | (n = 9) | (%) | |
| Cytokines (pg/ml) | |||
| TNF-α | 0.65±0.07 | 0.73±0.11 | +12 |
| IFN-γ | 5.95±1.28 | 4.62±1.6 | −22 |
| IL-1α | 1.09±0.15 | 0.91±0.14 | −16 |
| IL-17 | 13.1±5.1 | 17.8±15.4 | +35 |
| ENA-78 | 72.1±10.3 | 74.4±11.3 | +3 |
| IP-10 | 71.1±8.3 | 90.8±12.2 | +28 |
| PDGF BB | 5.53±1.42 | 7.21±2.2 | +30 |
| MCP-1 | 2.11±0.34 | 2.42±0.33 | +15 |
| Growth factors | |||
| NGF (pg/ml) | 8.8±2.1 | 2.95±0.5 | −66 |
| IgGs (pg/ml) | |||
| IgG1 | 7147±800 | 6512±519 | −9 |
| IgG2 | 85±20 | 58±17 | −31 |
| IgG3 | 1288±139 | 1700±334 | +32 |
| IgG4 | 718±174 | 592±278 | −17 |
| Amyloid-β (pg/ml) | |||
| Aβ1-40 | 213±18 | 235±20 | +10 |
| Aβ1-42 | 28±2 | 25±4 | −11 |
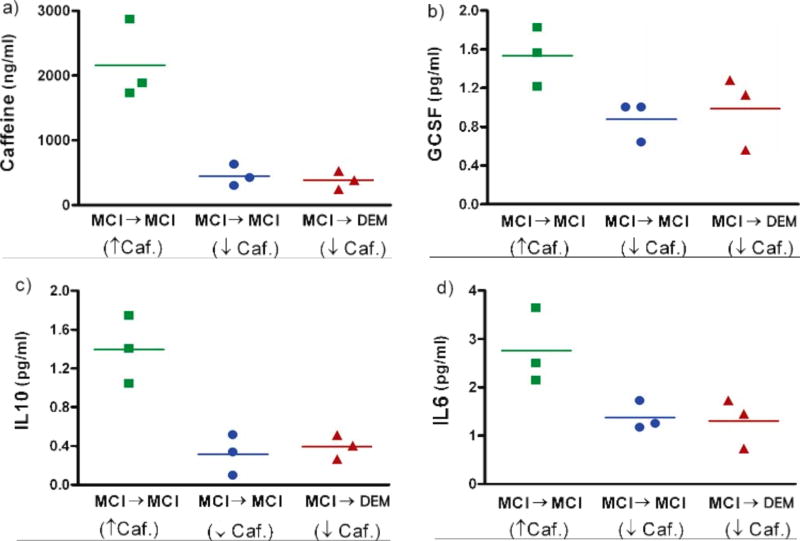
Figure 2 indicates a relationship between blood levels of caffeine and the three cytokines GCSF, IL-10, and IL-6, with low levels of all four being found in MCI patients destined to progress to DEM during the ensuing 2–4 years. To further elucidate the linkage between blood caffeine levels, these three cytokines, and cognitive status, we re-examined caffeine levels for the two MCI subgroups in Fig. 2a. For the MCI→MCI group, we took the three subjects having the highest plasma caffeine levels (↑ caf. group) and the three subjects with the lowest caffeine levels (↓ caf. group). Plasma markers from these two groups of subjects were compared with three subjects from the MCI→DEM group that had plasma caffeine levels very comparable to the (↓ caf.) MCI→MCI group (Fig. 3). When caffeine, GCSF, IL-10, and IL-6 levels were compared between these subjects, it became clear that high blood caffeine levels in MCI→MCI subjects are linked to high blood levels of GCSF, IL-10, and IL-6 in those same subjects. By contrast, low blood caffeine levels in either MCI→MCI or MCI→DEM subjects are linked to lower levels of all three cytokines (Fig. 3).
Fig. 3.
a–d) Plasma caffeine, GCSF, IL-10, and IL-6 levels in the three stable MCI subjects who initially had the highest and lowest plasma caffeine levels. Also plotted are these same four biomarkers in three MCI subjects who eventually progressed to DEM and whose plasma caffeine levels were comparable to stable MCI patients with the lowest caffeine levels. MCI subjects that remained stable and who had the highest caffeine levels at study onset had plasma levels of GCSF, IL-10, and IL-6 that were higher than the other two groups. Horizontal lines are the average for that given triple sampling.
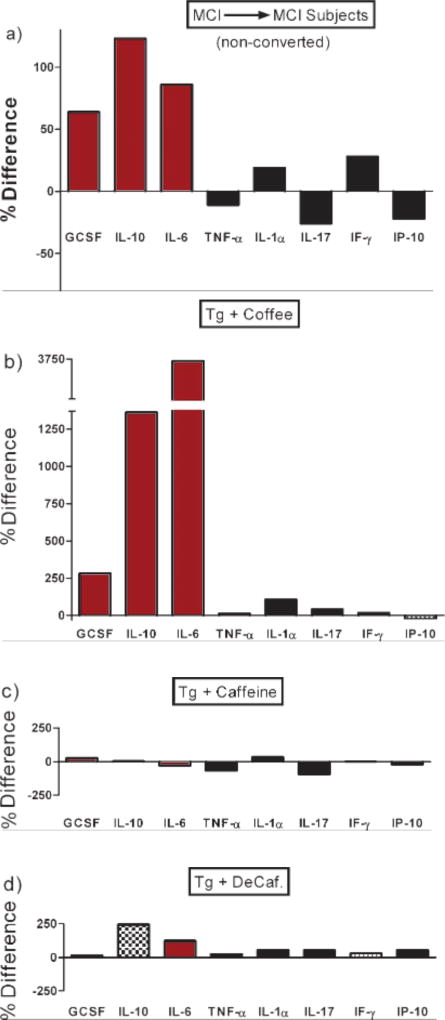
Although the dietary source of caffeine for subjects in this study was not determined or available, the fact that high plasma caffeine levels were selectively associated with high plasma levels of three cytokines (GCSF, IL-10, and IL-6) suggests that coffee was the major or perhaps only source of caffeine for MCI patients that did not convert to DEM (MCI→MCI). Figure 4, which depicts transformed data from our earlier study [18], underscores the reasoning for that premise and is addressed in the Discussion’s Interpretations and Implications sub-section.
Fig. 4.
a) Levels of eight plasma cytokines in MCI subjects that remained stable (MCI→MCI) over the subsequent 2–4 year period, with each cytokine graphed as a percent difference in reference to values from MCI subjects who progressed to DEM (MCI→DEM) during the same time period. Note that three cytokines (GCSF, IL-10, and IL-6) are selectively elevated in stable MCI subjects. b–d) Levels of the same eight plasma cytokines, but in AD transgenic mice that had been given acute treatment three hours earlier with caffeinated coffee (b), caffeine (c), or decaffeinated coffee (d). Cytokine levels for all three treatments are graphed as a percent difference in reference to values from AD mice treated with saline control. The original data (i.e., actual plasma cytokine levels) are presented in Cao et al. [18]. Note that only caffeinated coffee treatment (b) resulted in a plasma cytokine profile in AD mice that was similar to that of stable MCI subjects (a), both having elevated GCSF, IL-10, and IL-6 levels.
DISCUSSION
This study provides an intriguing association between plasma caffeine levels in MCI patients and their ensuing progression (or not) to dementia. High plasma caffeine levels in MCI patients at the beginning of a 2–4 year cognitive assessment period were associated with complete avoidance of progression to dementia over that period. Although several studies have previously associated caffeine/coffee intake with reduced risk of AD [1–3, 5], in the present study we provide more direct evidence of this association by measuring plasma caffeine levels. If caffeine/coffee intake was indeed critical to protection against dementia progression, it likely provides this protection in part by preventing a selective immune decline in MCI patients—an immune decline characterized by decreases in plasma GCSF, IL-10, and IL-6 levels several years prior to dementia conversion. The higher caffeine levels (most likely associated with coffee intake) in many stable MCI subjects were probably important for maintaining plasma levels of these three critical cytokines and preventing dementia conversion. As detailed below under “Interpretations and Implications”, these stable MCI subjects exhibited the exact same plasma cytokine profile (e.g., elevated GCSF, IL-10, and IL-6 levels) as AD transgenic mice given caffeinated coffee, a cytokine profile not provided by decaffeinated coffee or caffeine alone in such mice [18]. Thus, it is likely that stable MCI subjects were getting most or all of their caffeine from caffeinated coffee. Our results clearly warrant controlled clinical trials with caffeine/coffee administration to MCI subjects over a 2–4 year period to definitively elucidate the ability of caffeine/coffee to protect against dementia/AD, as well as the mechanisms involved. It should be noted that the dementia subjects in this study were diagnosed through “cognitive” assessment. As such, the vast majority of these dementia subjects were undoubtedly AD patients (although not all), necessitating use of the term “dementia” rather than “AD” for the results of this study.
Comparison to other studies
Prior epidemiologic-based studies have reported an association between moderate caffeine/coffee intake in mid-life [3] or in older age [1, 2, 5, 19] and reduced risk of cognitive impairment/AD. Highly controlled AD mouse studies have further strengthened the linkage between caffeine/coffee and protection against AD. These studies have demonstrated that long-term oral treatment of AD mice with caffeine [9] or caffeinated coffee [18] prevents cognitive impairment. Utilizing these same AD mouse, we have identified specific “disease-modifying” mechanisms for caffeine alone, and in combination with other components of coffee. Caffeine alone suppresses brain levels of both enzymes (β- and γ-secretase) required for Aβ production [9] via targeting of specific signal transduction mechanisms [10, 12].As well, caffeine has anti-inflammatory actions in AD mouse brains [11]. Most recently, we have uncovered a surprising synergy between caffeine and some as-yet unidentified component of coffee to provide a highly beneficial increase in three key plasma cytokines [18].GCSF is the most notable of these three because of its beneficial cognitive actions in AD mice that involve synaptogenesis, neurogenesis, and recruitment of bone marrow stems cells to phagocytize brain Aβ [6]. Thus, coffee’s caffeine and non-caffeinergic components would appear to exert multiple anti-AD actions.
The aforementioned studies underscore a substantial body of both human epidemiologic and mouse model work that had already linked caffeine/coffee to protection against AD prior to the present study—what has been lacking is direct human evidence for that linkage. The present case-control study addressed this need by directly measuring caffeine levels in blood of aged individuals (65–88) to determine if those levels were predictive of future cognitive status. Thus, certain limitations inherent to standard epidemiologic studies (e.g., recall bias, variable control) were avoided.
Interpretation and implications
When subjects were groups according to their “initial” cognitive status (Normal, MCI, or DEM), there were group differences in plasma caffeine, with MCI subjects having significantly lower levels compared to Normals. When Normal and MCI subjects were further sub-divided into groups that either remained stable or that converted to MCI or DEM, respectively, Normals that converted to MCI had generally lower initial caffeine levels compared to Normals that remained stable. However, this 26% lower caffeine level was not significant due to the large variation in caffeine levels among subjects in both of the initially Normal sub-groups. Factors that could account for this variability in plasma caffeine levels are: marked individual differences in caffeine intake, individual differences in caffeine metabolism, and extent of smoking (which affects caffeine metabolism).Moreover, multiple non-caffeinergic factors could be important in aged Normals for determining whether or not they converted to MCI.
No other plasma biomarkers were initially different for Normals that converted to MCI versus Normals that did not, including the 3 cytokines (GCSF, IL-10, and IL-6) that collectively were different between converting and non-converting MCI subjects. These results suggest that the mild short-term memory impairment of MCI does not involve any advance changes in plasma cytokines, Aβ, or immunoglobulins several years before MCI diagnosis. Moreover, there were no differences between normal subjects and stable MCI subjects for any biomarker analyzed, indicating an inability of “individual” plasma biomarkers to distinguish between Normal and MCI subjects at study initiation. These results are consistent with prior studies, which have largely reported no consistent differences in individual plasma cytokines between aged normal and MCI subjects [37, 38]. Other than the present study, we are aware of no earlier study that investigated whether plasma cytokine levels of normal subjects were predictive of later MCI diagnosis. Although any single cytokine may not have this predictive potential, a combination of plasma cytokines in normal aged individuals may be predictive [38].
In subjects that were initially MCI, however, plasma caffeine levels were predictive for MCI subjects that would remain stable (e.g., not progress to DEM) over the ensuing 2–4 year cognitive assessment period. In MCI subjects, plasma caffeine levels above a critical value of 1200 ng/ml were associated with a 100% chance of avoiding AD conversion over that period. The resultant ≈6 µM plasma caffeine concentration is typically present several hours after intake of 1–2 cups of coffee [39], given that the half-life of plasma caffeine is 3–4 hours and peak plasma caffeine levels of 10–20 µM occur around 1 hour following such oral caffeine ingestion [40]. By contrast, MCI subjects that did progress to DEM had initial caffeine levels that were substantially (−56%) lower compared to stable MCI subjects. Interestingly, both sub-groups of MCI subjects exhibited less variability in plasma caffeine concentration compared to the sub-groups of Normals.
For subjects that were already diagnosed with DEM at the beginning of the study, plasma caffeine levels were comparable to stable MCI subjects and significantly higher than MCI→DEM subjects. Moreover, accompanying levels of GCSF, IL-10, and IL-6 in DEM subjects were also significantly higher than those in MCI→DEM subjects. Although increased plasma cytokine levels in AD were anticipated as part of a heightened inflammatory response following diagnosis of DEM(see next section), the elevated caffeine levels in DEM subjects compared to MCI→DEM subjects were not anticipated. It is important to keep in mind, however, that caffeine levels in DEM subjects were only higher in comparison to the very low levels of MCI→DEM subjects; plasma caffeine levels in DEM subjects were still 40% lower than those of all Normals and near-identical to the low levels present in all MCI subjects.
A number of prior studies have investigated the possibility that plasma cytokine levels could be viable biomarkers for progression from Normal or MCI to AD [37, 41, 42]. Although no single cytokine/growth factor has been identified thus far as predictive of impending MCI or AD, Laske and colleagues have determined that blood levels of several neurotrophic/hematopoietic factors (e.g., GCSF, BDNF, and SCF) are decrease in “early AD” [43–45], resulting in deficient neurotrophic/hematopoietic brain support. We believe this deficient brain support actually begins to occur in late MCI, several years prior to AD conversion, and is important for AD conversion. In this context, the present study provides initial evidence that three key cytokines (GCSF, IL-10, and IL-6) become collectively decreased in MCI patients several years prior to their conversion to DEM. Thus, a selective immune decline would seem to occur during those years. If this immune decline can be verified in larger cohorts of MCI subjects, regular monitoring of these three plasma cytokines beginning at onset of MCI may provide several years warning of impending conversion to DEM. By the time cognitive impairment becomes severe enough to warrant clinical diagnosis of DEM, plasma levels of the 3-cytokines appear to have re-established their higher levels, but this elevated immune response would seem to come too late for cognitive protection. We propose that higher caffeine intake (very likely in association with coffee) maintains the levels of these three critical cytokines in MCI subjects, such that progression to DEM occurs later or perhaps not at all.
Caffeinated coffee was very likely the primary dietary source of caffeine for stable MCI subjects because their blood cytokine profile was very similar to that of AD (AβPPsw+PS1) transgenic mice acutely given caffeinated coffee (see Cao et al. [18]). In that recent study, AD transgenic mice were given a single treatment with caffeinated coffee, decaffeinated coffee, caffeine, or saline. Of all four acute treatments, AD mice only responded to caffeinated coffee with greatly and selectively increased plasma levels of GCSF, IL-10, and IL-6. The methodology of this AD mouse study relevant to the present study is briefly indicated in the Supplementary Data section (available online: http://www.j-alz.com/issues/30/vol30-3.html#supplementarydata02). Figure 4 compares plasma cytokine data from MCI patients of the present study with the same cytokines from the AD mouse study. Only administration of caffeinated coffee provided the same profile of substantially elevated GCSF, IL-10, and IL-6 levels in transgenic mice that is seen in stable MCI→MCI patients. Thus, it is likely that “caffeinated coffee” was the source of caffeine associated with cognitive stability in the present study’sMCI patients.
Although stable MCI→MCI subjects as a group had substantially higher plasma caffeine levels compared to those MCI subjects that progressed to DEM (MCI→DEM), it is important to recognize that half of these stable MCI subjects had caffeine levels below the critical level for protection (e.g., 1200 ng/ml), yet they did not progress to DEM during the 2–4 year follow-up period. Clearly, other dietary/life-style choices, risk factors, and extent of disease progression entered into determining which MCI patients progressed to DEM and which ones did not. Nonetheless, we predict that those stable MCI patients with low caffeine levels (e.g., below 1200 ng/ml) and concomitantly low levels of GCSF, IL-10, and IL-6, will progress to DEM sooner than MCI patients with high caffeine/GCSF/IL-10/IL-6 levels.
Finally, it should be underscored that the lack of differences between the two MCI sub-groups in multiple plasma IgGs, Aβ isoforms, and the 8 unaffected plasma cytokines, indicates that these biomarkers were not independently predictive of impending MCI progression to DEM.
Strengths and limitations
A key strength of our study was the direct measurement of blood caffeine levels, rather than reliance on recall or dietary surveys of caffeine intake, as has been typical of prior retrospective/longitudinal epidemiologic studies. However, our measurement of blood caffeine levels was also the largest study limitation because of a lack of ancillary data collection that would have provided greater insight. First, we did not take multiple blood samples for caffeine analysis during the 2–4 year study period, only at the study’s inception. This is because the study was created retrospectively from available blood (taken at initial clinical exam) and follow-up clinical evaluations available over 2–4 years as part of the FADRC’s ongoing longitudinal assessment of aged individuals for biomarkers and cognitive status. Second, the extent to which caffeine levels at study onset were indicative of daily caffeine intake was not determined. For example, we did not monitor the primary source(s) of caffeine in study participants (although we presented evidence that the primary source was caffeinated coffee for stable MCI subjects). We also did not ask participants when their last caffeine intake was prior to coming in for the initial visit/blood sample. As well, we did not ask subjects about their long-term caffeine/coffee intake habits, although it is likely that subjects with high plasma caffeine levels are habitual/moderate coffee drinkers. Additionally, complete data on ApoE status, education level, ethnicity, dietary habits, and lifestyle choices were not available for all study participants, so none of these can presently be eliminated as contributory to the results observed. Finally, the follow-up time of 2–4 years was relatively short for establishing causality and reverse causation (i.e., subjects with poorer cognitive performance may have reduced caffeine/coffee intake) is possible. Nonetheless, the fact that MCI subjects in Miami and Tampa cohorts independently showed the same relationship between blood caffeine levels and later risk of DEM progression provides a degree of confidence regarding this association.
CONCLUSION
In providing initial direct evidence for caffeine/coffee being protective against dementia/AD, the present case-control study is nonetheless based on association, wherein caffeine/coffee could simply be associated with stable cognitive status in MCI and not contribute to that cognitive stability. It is also important to recognize that the AD pathogenic process begins in the brain 1–2 decades before any evident cognitive impairment; prophylactic intervention should ideally begin that far in advance of AD symptoms. In that context, moderate caffeine/coffee intake is safe for most humans, appears to attack multiple aspects of the disease process, and is convenient for long-term/widespread dietary intake. If controlled clinical trials further support caffeine/coffee as protective against AD diagnosis, compelling evidence will be given for the general public to adopt this strategy to reduce risk of AD.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Aging, NIH 5R01AG020094-03 and NIH 1P50AG025711-03, & USF/Byrd Alzheimer Center and Research Institute funds.
Footnotes
Supplementary data available online: http://dx.doi.org/10.3233/JAD-2012-111781
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1187).
References
- 1.van Gelder B, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur J Clin Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues J, Rouaud O, Barberger-Gateau P, Ancelin M. The neuro-protective effects of caffeine: A prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 3.Eckelinen M, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late life dementia: A population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 4.Laitala V, Kaprio J, Koskenvuo M, Raiha I, Rinne J, Silventoinen K. Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr. 2009;90:640–646. doi: 10.3945/ajcn.2009.27660. [DOI] [PubMed] [Google Scholar]
- 5.Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Ramos J, Song S, Sava V, Catlow B, Mori T, Dickson A, Lin X, Cao C, Arendash G. G-CSF reverses cognitive impairment in Alzheimer’s mice and decreases Aβ levels in both brain and blood. Neuroscience. 2009;163:55–72. doi: 10.1016/j.neuroscience.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao C, Arendash G, Dickson A, Mamcarz M, Lin X, Ethell D. Aβ-specific Th2 cells are sufficient to reverse cognitive impairment, cerebral angiopathy, and pro-inflammatory profiles in Alzheimer’s transgenic mice. Neurobiol Dis. 2009;34:63–70. doi: 10.1016/j.nbd.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selkoe D. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;17:1060–1065. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 9.Arendash G, Schleif W, Rezai-Zadeh K, Jackson E, Zacharia L, Cracchiolo J, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Arendash G, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tan J, Citron B, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain Aβ levels in aged Alzheimer’s mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 11.Cao C, Cirrito J, Lin X, Wang L, Verges D, Dickson A, Mamcarz M, Zhang C, Mori T, Arendash G, Holtzman D, Potter H. Caffeine suppresses β-amyloid levels in plasma and brain of Alzheimer’s transgenic mice. J Alzheimers Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeitlin R, Patel S, Burgess S, Arendash GW, Echeverria V. Caffeine induces beneficial changes in PKA signaling and JNK and ERK activities in the striatum and cortex of Alzheimer’s transgenic mice. Brain Res. 2011;1417:127–136. doi: 10.1016/j.brainres.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Higdon J, Frei B. Coffee and health: A review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 14.Halvorsen B, Carlsen M, Phillips K, Bohn S, Holte K, Jacobs D, Blomhoff R. Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Herder K, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Huha S, Tuomilehto J. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am J Clin Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 16.Pham M, Yosida D, Yin G, Ohnaka K, Takayanagi R, Kono S. The relationship of coffee and green tea consumption with high-sensitivity C-reactive protein in Japanese men and women. Clin Chem Lab Med. 2010;48:849–854. doi: 10.1515/CCLM.2010.161. [DOI] [PubMed] [Google Scholar]
- 17.Bakuradze T, Boehm N, Janzowski C, Lang R, Hofmann T, Stockis JP, Albert FW, Stiebitz H, Bytof G, Lantz I, Baum M, Eisenbrand G. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol Nutr Food Res. 2011;55:793–797. doi: 10.1002/mnfr.201100093. [DOI] [PubMed] [Google Scholar]
- 18.Cao C, Wang L, Lin X, Mamcarz M, Zhang C, Bai G, Nong J, Sussman S, Arendash GW. Caffeine synergizes with another coffee component to increase plasma GCSF: Linkage to cognitive benefits in Alzheimer’s mice. J Alzheimers Dis. 2011;25:323–335. doi: 10.3233/JAD-2011-110110. [DOI] [PubMed] [Google Scholar]
- 19.Corley J, Jia X, Kyle J, Gow A, Brett C, Starr J, McNeill G, Deary I. Caffeine consumption and cognitive function at age 70: The Lothian birth cohort 1936 study. Psychosomatic Med. 2010;72:206–214. doi: 10.1097/PSY.0b013e3181c92a9c. [DOI] [PubMed] [Google Scholar]
- 20.Galvin J, Powlishta K, Wilkins K, McKeel D, Jr, Xiong C, Grant E, Storandt M, Morris J. Predictors of preclinical Alzheimer disease and dementia: A clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 21.Petersen R, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 22.Brooks L, Loewenstein DA. Assessing the progression of mild cognitive impairment (MCI) to Alzheimer’s disease: Current trends and future directions. Alzheimers Res Ther. 2010;2:28. doi: 10.1186/alzrt52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Interventions. The Haworth Press; NY: 1986. pp. 165–173. [Google Scholar]
- 24.Cummings J, Mega M, Gray K, Rosenberg-Thompson S, Carusi D, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of neuropathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 25.Morris J. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 26.Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the physician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Beekly D, Ramos E, Lee W, Deitrich W, Jacka M, Wu J, Hubbard J, Koepsell T, Morris J, Kukull W. NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21 doi: 10.1097/WAD.0b013e318142774e. 249-158. [DOI] [PubMed] [Google Scholar]
- 28.Fuld P. Fuld Object-Memory Evaluation. Illinois: Stoelting Co.; 1981. [Google Scholar]
- 29.Benedict R, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 30.Loewenstein D, Amarilis Acevedo A, Small B, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a two to three year follow-up period. Dement Geriatr Cogn Disord. 2009;17:437–440. doi: 10.1159/000211803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wechsler D. The Wechsler Adult Intelligence Scale. 3. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 32.Monsch A, Bondi M, Butters N, Salmon D, Katzman R, Thal L. Comparison of verbal fluency tasks in the detection of dementia of the Alzheimer’s type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 33.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2. Oxford University Press; New York: 1998. pp. 502–505. 1998. [Google Scholar]
- 34.Army Individual Test Battery. Manual of directions and scoring. Washington: War Department, Adjutant General’s Office; 1944. 1944. [Google Scholar]
- 35.Graham JE, Rockwood K, Beattie BL, McDowell I, Eastwood R, Gauthier S. Standardization of the diagnosis of dementia in the Canadian Study of Health and Aging. Neuroepidemiology. 1996;15:246–256. doi: 10.1159/000109914. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric, Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington: 1994. [Google Scholar]
- 37.Lee KS, Chung JH, Lee KH, Shin M-J, Oh BH, Hong CH. Bioplex analysis of plasma cytokines in Alzheimer’s disease and mild cognitive impairment. Immunol Lett. 2008;121:105–109. doi: 10.1016/j.imlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Olson L, Humpel C. Growth factors and cytokines/chemokines as surrogate biomarkers in cerebrospinal fluid and blood for diagnosing Alzheimer’s disease and mild cognitive impairment. Exp Gerontol. 2010;45:41–46. doi: 10.1016/j.exger.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Fredholm B, Battig K, Holmen J, Nehlig A, Zvartau E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 40.Culm-Merdek KE, von Moltke LL, Harmatz JS, Greenblatt DJ. Fluvoxamine impairs single-dose caffeine clearance without altering caffeine pharmacodynamics. Br J Clin Pharmacol. 2005;60:486–493. doi: 10.1111/j.1365-2125.2005.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera M, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol Lett. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Forlenza OV, Diniz BS, Talib LL, Mendoca VA, Ojopi EB, Gattaz WF, Teixeira AL. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;28:507–512. doi: 10.1159/000255051. [DOI] [PubMed] [Google Scholar]
- 43.Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Sockadar S, Richartz E, Koehler N, Bartels M, Buchkremer G, Schott K. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psych Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Laske C, Stellow K, Stransky E, Seizer P, Akcay O, Eschweiler GW, Leyhe T, Gawaz M. Decreased plasma and CSF levels of stem cell factor in patients with early Alzheimer’s disease. J Alzheimers Dis. 2008;15:451–460. doi: 10.3233/jad-2008-15311. [DOI] [PubMed] [Google Scholar]
- 45.Laske C, Stellos K, Stransky E, Leyhe T, Gawaz M. Decreased plasma levels of granulocyte-colony stimulating factor (G-CSF) in patients with early Alzheimer’s disease. J Alzheimers Dis. 2009;17:115–123. doi: 10.3233/JAD-2009-1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.