Abstract
Objective
This article describes the use of ultrasound measurements of physical strain within carotid atherosclerotic plaques as a measure of instability and the potential for vascular cognitive decline, microemboli and white matter changes.
Methods
Asymptomatic patients with significant (>60%) carotid stenosis were studied for dynamic measures of plaque instability, presence of microemboli, white matter changes, and vascular cognitive decline as measured by normative controls and premorbid state.
Results
Although classically asymptomatic, these patients showed vascular cognitive decline. The degree of strain instability measured within the atherosclerotic plaque directly predicted vascular cognitive decline in these patients felt previously to be asymptomatic by classical criteria. Further, 26 % of patients showed microemboli, and patients had twice as much white matter hyperintensities as controls.
Conclusions
These data show that physical measures of plaque instability are possible through interpretation of ultrasound strain data during pulsation which may be more clinically relevant than solely measuring degree of stenosis. The data also highlights the importance of understanding that the definition of symptoms should not be limited to motor, speech and vision but stresses the role of vascular cognitive decline in the pathophysiology of carotid atherosclerotic disease.
Keywords: carotid atherosclerosis, ultrasound, vascular cognitive decline, plaque instability, ultrasound strain, stroke
INTRODUCTION
Stroke remains a significant cause of disability worldwide, with considerable efforts ongoing, for the prevention of cerebrovascular events. Historically, ischemic stroke prevention has included reducing cardiac emboli, intensive medical therapy addressing coagulation, rheology, blood pressure, atherosclerosis-related risk factors, and the surgeries: carotid endarterectomy and carotid stenting. Continued advancements on all fronts have raised the question of the need for improvements in the diagnosis of the at-risk patient population. This is especially important in patients who present asymptomatically with atherosclerotic disease. Unlike the case of severe symptomatic carotid stenosis, the benefit of surgical intervention in asymptomatic carotid stenosis is less pronounced.1 These studies have suggested that the strategy of operating upon patients with asymptomatic, >60% stenosis would require an estimated 40 carotid endarterectomies to prevent one stroke in 5 years.28 Multiple studies have suggested that such aggressive treatment requires improved methods to identify patients with unstable plaques that are truly at risk.5,9,14,15,18,28 If the degree of stenosis in asymptomatic patients does not well predict carotid plaque thromboembolism, then surgeries guided by severity of luminal stenosis alone is a non-sustainable strategy into the future because of both cost and a poor risk-benefit ratio.23,27
A scientific approach to this clinical question would be the measurement of carotid plaque’s physical instability during pulsation. Such a measure would be a biomechanical analysis of the propensity for a plaque to fracture during pulsation which could result in creating an embolic or thromboembolic event. That biomechanical analysis must take into effect the morphology of the plaque, the peak stress within the cap of the plaque and its ability to create emboli and/or other clinically recognizable deficits.
Carotid Artery Plaque Instability and Cognitive Symptoms
The consequences of unrecognized emboli or silent strokes may be quite profound. Recent imaging studies suggest a far larger number of ischemic events are taking place than are recognized by our present clinical exams.20 Estimates are up to 11 million/year of so-called “silent strokes” in patients at risk.32,33 Silent stroke and vascular cognitive decline may not be easily detected by standard clinical exam but it may occur with concurrent subclinical emboli.8,17,3,37,38,39,40,41 Micro-emboli may cause cognitive impairment through rupture of vulnerable plaques 29, 37,38,39,40 either through rupture of the thin fibrous cap or by hemorrhage and thrombosis.37,38 Our studies of high grade stenosis including patients having prior stroke or TIA (Transient Ischemic Attack) suggest that vascular cognitive decline is seen in these patients and is directly related to the degree of physical instability of carotid atherosclerotic plaque.37
Measures of Plaque Instability
A parameter of physical stability of the carotid plaque may be far more important than its degree of stenosis in predicting patients at risk and has been a target of research regarding measures of plaque vulnerability. Recent studies13 have suggested the histopathologic classification of such plaques to be of importance post-operatively but have not found a good MRI (Magnetic Resonance Imaging) criteria for preop assessment of plaque stability. In the 1980’s and 1990’s imaging methods emphasized imaging lumen over vessel wall. Therefore, the available imaging studies primarily looked at the residual lumen as a surrogate for significance of plaque. With modern MRI and ultrasound imaging we can now look at the plaque and vessel wall in a more sophisticated fashion in an attempt to determine its physical instability or strain during pulsation.30 Deformation of the plaque with pulsation has been associated with cognitive decline through embolization.8,26,38,40 Shear strain elasticity index has been used as a potential assessment of vulnerability within the plaque.19,37 Such studies have been correlated with high resolution brain magnetic spectroscopy and magnetic resonance imaging.3,37 We have previously shown in patients that include these with strokes that this measure of strain in significant plaques correlates with MRI measures of brain white matter hyperintensities.3 This suggests that carotid atherosclerotic instability may be a marker not only of advanced plaque and potential emboli from that site, but also may be a marker of the systemic degree of atherosclerotic disease affecting both large and small vessels. Therefore, a carotid bifurcation deposit of atherosclerosis, which is accessible to noninvasive measurement may also act as a marker for patients at risk of total symptomatology as well as measure its individual stability. In this paper we present the utility of noninvasive ultrasound measurements of physical strain within the pulsating atherosclerotic plaque as a potential biomarker to determine which asymptomatic patients are at greatest risk for future symptomatology of all types, especially vascular cognitive decline. Previous work has looked at patients unspecified for previous symptomatology and has shown a significant correlation of increase in carotid strain and decrease in cognitive function.17,38 Such a correlation was not seen for degree of stenosis. In this paper we wish to analyze if such criteria are present in asymptomatic patients suggesting a potential utility of this method for early identifying of patients at greatest risk for eventual plaque rupture or systemic symptomatology. If such criteria can distinguish vulnerable from stable plaques through biomechanical stress analysis, we would be able to direct treatment, be it medical or surgical, to the patients at greatest risk and spare patients with asymptomatic but stable stenosis from unnecessary treatment.
METHODS
Participants
Twenty-seven asymptomatic subjects with significant (>60%) carotid plaque in the University of Wisconsin Atherosclerotic Plaque Study were studied for medical comorbidities, cognition, and an ultrasound-based biomechanical determination of strain within the plaque during pulsation. A subset of the group was studied for the presence of cerebral emboli, and MRI evidence of cerebral microvascular brain changes. All preoperative testing, was performed in accordance with the Health Sciences Institutional Review Boards of the University of Wisconsin - Madison. All patients provided written informed consent.
All patients were assessed by a faculty member of the University of Wisconsin Comprehensive Stroke Center to determine asymptomatic status relative to the carotid artery in question. All patients met the following inclusion criteria: age 18 years or older, native English speaking, and asymptomatic carotid stenosis as measured by the Asymptomatic Carotid Atherosclerotic Trial (ACAS criteria). Exclusion criteria were: patients with known dementia or inability to cooperate in the study, previous history of TIA or stroke, prior carotid procedures, cervical radiation, or other medical conditions which would interfere with the ability to cooperate with testing. Cognitive assessments and ultrasound images were conducted separately and were blinded to each other.
Neuropsychological Assessment
Each participant was administered the 60-minute neuropsychological test protocol recommended by the National Institute of Neurological Disorders (NINDS) and Canadian Stroke Network (CSN). This protocol was selected specifically for stroke patients and assesses several important functional domains with tests of executive function/attention, speeded psychomotor, verbal and nonverbal memory, language, and visuospatial skills. For the current study, widely-used verbal and performance IQ measures (WAIS-IV Information, Digit Span, and Block Design) were added.
All test scores were corrected for age and sex, published norms or covariate analysis. Scores on semantic and category fluency tasks, in addition to Trails A/B) were converted to standardized scores using the Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery. Results of other tests were age-normed using materials provided with each test.
Plaque Stability as Measured by Ultrasound Strain
Ultrasound data acquisition for Carotid Strain Imaging: Noninvasive clinical and research ultrasound acquisitions were performed on patients after obtaining informed consent, under a protocol approved by the UW-Madison IRB (Institutional Review Board). A standard Ultrasound clinical examination was performed on each patient followed by acquisition of research data. Typically, each carotid was scanned along the Common Carotid Artery (CCA), Internal Carotid Artery (ICA) and bulb, taking care to keep the vessel walls parallel to the two-dimensional imaging plane and the transducer.
Ultrasound radiofrequency echo signals were acquired and stored for offline processing over several cardiac cycles along with clinical B-mode images and color flow Doppler images using a Siemens S2000 Ultrasound system equipped with a 18L6 linear array transducer. A transmit center frequency of 11.4 MHz with a single transmit focus set at the depth of the plaque and sampling frequency of 40 MHz, was utilized for F data acquisition.
Plaque regions with adventitia were segmented using the Medical Imaging Interaction Toolkit (MITK) by a research sonographer on the end diastolic frame in B-mode images reconstructed from RF data. The segmentation was then transferred to the entire loop of RF data. Images were reviewed over the entire cardiac cycle to allow point-by-point comparison of vessel wall to plaque borders. A hierarchical block-matching motion tracking algorithm21 was used, by tracking the deformation over the segmented plaque region from the diastolic to systolic frame over two cardiac cycles.37 A dynamic frame skip methodology, with smaller frame skips during systole and larger frame skips during diastole for efficient strain estimation was incorporated. Local displacements were tracked using two-dimensional normalized cross correlation analysis with recursive Bayesian regularization21 over 3 iterations, and finally filtered with a 3 × 3 pixel (0.06 mm × 0.225 mm) median filter to eliminate any peak hopping artifacts. Local sub-sample displacements21 were accumulated over a cardiac cycle to determine the cumulative displacement and strain variations over a cardiac cycle. Local strain tensors were computed using a modified least squares fit over a 7-pixel linear segment. The accumulated maximum axial (MAAS), lateral (MALS) and shear (MASS) strain indices over a cardiac cycle were computed for each patient and utilized for the correlation with cognitive variables,39,40 respectively.
Transcranial Doppler Detection of Emboli
Transcranial Doppler (TCD) examination was performed with the SONORA Digital Bilateral Systems (Natus, Middleton, WI) using a 2.0 MHz transducer to image both middle cerebral arteries through the transtemporal window. Monitoring lasted for an hour and the total number of Highest Intensity Transient Signals (HITS) were recorded. These were reviewed after each session by a physician and two observers.
The TCD system utilized an emboli detection algorithm to identify high intensity transient MCA signals (HITS) suggestive of microemboli. The criteria utilized to distinguish HITS from artifacts were; 1) high intensity signal compared to the background blood flow, signal detected by system, 2) unidirectional signal within the Doppler velocity spectrum, 3) transient signal less than 300 ms in duration, the presence of an audible noise (moan, chirp, click, thud) (Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium, 1995 and 5) a change in complex mode. Two observers and a physician reviewed all HITS to distinguish HITS suggestive of microemboli from artifacts. The TCD examination was considered positive for the presence of microemboli if one or more HITS were detected during the 60-minute monitoring period.
MRI White Matter Hyperintensities
MRI measures of brain white matter changes or white matter hyperintensities seen on T-2 flair MRI signals are postulated to result from cumulative microvascular injury. We chose this measure as a measure of the target organ for both microvascular changes and subclinical emboli disease in these clinically asymptomatic patients.
A subset of participants who were free from MRI contraindications (non-removable metal implants, pregnancy, inability to lie still for approximately one hour) were scanned on a 3T GE ×750 MRI scanner. The scan sequences acquired relevant to the present paper include T1 and T2-FLAIR scans. The T1 scan parameters are as detailed herein: acquired in the axial plane with a 3D fast spoiled gradient echo sequence; inversion time (TI) = 450 ms; repetition time (TR) = 8.1 ms; echo time (TE) = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 mm; field of view (FOV) = 256 mm; slice thickness = 1.0mm. T2 fluid attenuated inversion recovery (FLAIR) scan parameters are as detailed herein: acquired in the sagittal plane; TI = 1868 ms; TR = 6000 ms; TE = 123 ms; flip angle = 90°; acquisition matrix = 256 × 256 mm; FOV = 256 mm; slice thickness = 2.0 mm, and no gap yielding a voxel resolution of 1 mm × 1 mm × 2 mm.3
As this project has a significant vascular focus, we decided to focus on white matter hyperintensities (WMH), a metric believed to represent cumulative vascular injury to the brain. In order to determine the extent/volume of WMH, Statistical Parametric Mapping Software (SPM8) with the Lesion Segmentation Tool (LST) extension developed by Schmidt in 2012 was used. The LST allows for computerized, as opposed to manual, tracing of the WMH lesions. This eliminates issues of inter-rater reliability and subjectivity. In validating the method, the developer applied it to a cohort of multiple sclerosis patients, and found an extremely high correlation with hand-tracing, which is evidence of the validity of this method (R2=0.94).31 We compared the subjects in the present study to previously acquired, age-matched, cognitively healthy participants from the Wisconsin Alzheimer’s Disease Research Center. These controls had the following demographics: N=64, mean age 69, 57.8% female, 45.3% on blood pressure medication, 15.6% diabetes, mean total Cholesterol 190.
RESULTS
27 asymptomatic patients with >60% carotid stenosis were studied in this analysis. The patients had an average age of 71.0 years (SD=7.22, range =57–84 years) 13 were female and 14 were male. Comorbid risk factors include the presence of known heart disease in 12 (52%), lipid abnormalities in 22 (82%), diabetes in 7 (26%), previous or current smoking (yes/no) in 18 (67.4%), and hypertension in 23 (85%). 89% were taking aspiring and 70% were being treated for lipid abnormalities.
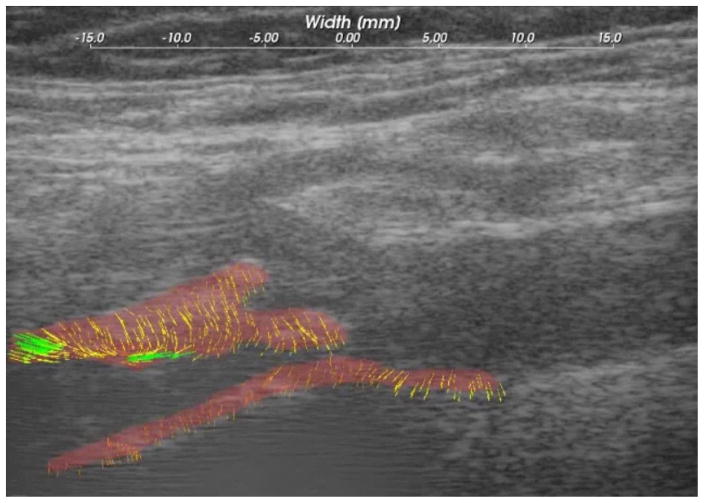
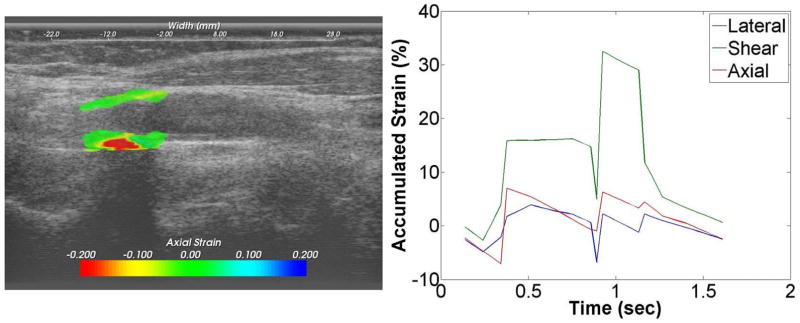
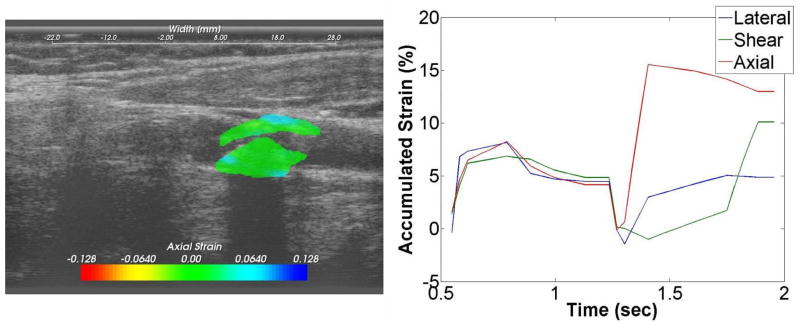
Ultrasound strain gave a dynamic picture of the presence and degree of instability during pulsation. Figure 1 shows the force lines of strain developing in a pulsating plaque at the origin of the internal carotid artery. These in turn are quantified as maximal axial, lateral and sheer strain. Figure 2 is a patient with less strain but high plaque stenosis. Figure 3 is a patient with higher strain but less plaque stenosis.
Figure 1.
B-mode image and segmented plaque with adventitia, showing strain line with pulsation.
Figure 2.
Strain images superimposed on ultrasound B-mode images for an asymptomatic patient with higher values of the accumulated strain. A plot of the accumulated strain over two cardiac cycles is shown on the right.
Figure 3.
Strain images superimposed on ultrasound B-mode images for an asymptomatic patient with lower values of the accumulated strain. A plot of the accumulated strain over two cardiac cycles is shown on the right.
The relationship between baseline cognition and strain was assessed using zero order and partial correlations. Significance was assessed at p<0.05. At least one maximum strain variable (lateral, axial, shear) predicted cognitive scores on 4 of 14 tests. Multiple strain measures shared overlapping variance predictive of cognition; the best predictor from the 3 strain variables is given after each variable, along with the correlation coefficient. As described above, all analyses control for age and sex (see Table 1).
Table 1.
High strain values are associated with low cognition scores. Each correlation coefficient is given, followed by the p-value in parentheses.
| Variable | Maximum Axial Strain | Maximum Lateral Strain | Maximum Shear Strain |
|---|---|---|---|
| Category Fluency | −.278 (.161) | −.355 (.069) | −.215 (.281) |
| Letter Fluency | .035 (.861) | −.039 (.849) | .028 (.891) |
| Confrontation Naming | −.242 (.245) | −.213 (.306) | −.121 (.564) |
| Trails A | −.412 (.033)* | −.468 (.014)* | −.398 (.040)* |
| Trails B | −.475 (.012)* | −.563 (.002)* | −.516 (.006)* |
| Digit Symbol | −.806 (<.001)* | −.721 (<.001)* | −.839 (<.001)* |
| Block Design | −.433 (.031) | −.236 (.256) | −.465 (.019)* |
| Information | −.359 (.078) | −.048 (.820) | −250 (.228) |
| Digit Span | −.194 (.352) | −.192 (.359) | −.087 (.681) |
| Verbal Memory-Immediate | −.294 (.145) | −.156 (.447) | −.313 (.120) |
| Verbal Memory-Delayed | −.189 (.355) | .051 (.820) | −.146 (.476) |
| Nonverbal Memory-Immediate | −.246 (.226) | −.062 (.763) | −.234 (.250) |
| Nonverbal Memory- Delayed | −.251 (.215) | −.106 (.605) | −.236 (.245) |
| Figure Copy | −.241 (.246) | −.195 (.350) | −.337 (.099) |
denotes significance at p = 0.05. The strongest significant correlation with strain is in bold for each cognitive variable.
Poor performance on tasks of simple motor ability (Trails A; r = −0.47, p =0.01) and complex motor/executive function (r = −0.564, p =0.002) was associated with increased maximum lateral strain. Low scores on another test of complex motor ability and executive function (Digit Symbol) were associated with increased maximum shear strain (r = 0.84, p< 0.001), as were visuomotor construction deficits (Block Design; r = −.47, p =0.019)
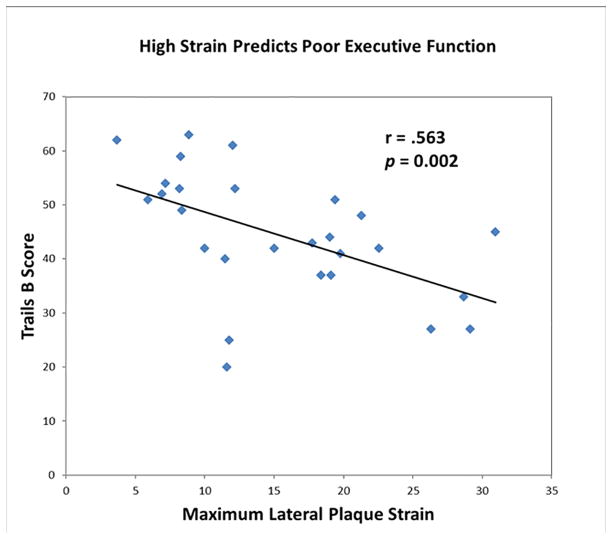
Nearly all relationships were negative: high strain was associated with poor cognition. (See Figure 4).
Figure 4.
Decline in Trails B Executive Function correlated with increase in maximal plaque p= 0.002
High axial strain in carotid atherosclerotic plaque as measured by ultrasound during pulsation is associated with decreased cognitive performance on tests of language, motor function, working memory, visuomotor construction, and executive function. This is true even in the absence of overt symptoms of TIA or stroke.
When evaluating the spectrum of vascular cognitive decline versus the maximum strain value within the plaque with pulsation, we see age-corrected negative correlations between maximum strain value and the following cognitive abilities: motor sequencing, executive function, and visual motor reproduction, general knowledge, as illustrated in Table 1. Several of the cognition variables had significant p-values <0.01 as shown in the table. Each of the 3 measures of the maximum strain predicts cognitive variables at p<0.05. Worse cognition is always associated with increased strain indices. For most variables, all 3 maximum strain variables predict cognitive decrement. All analyses control for age and sex. It is important to note that in the classically asymptomatic patient the loss of cognition is seen in executive cognitive function (Trails A, Trails B, Digit Symbol, Bloc Design) before marked changes are seen in memory parameters.
White Matter Hyperintensities and TCD Emboli Detection
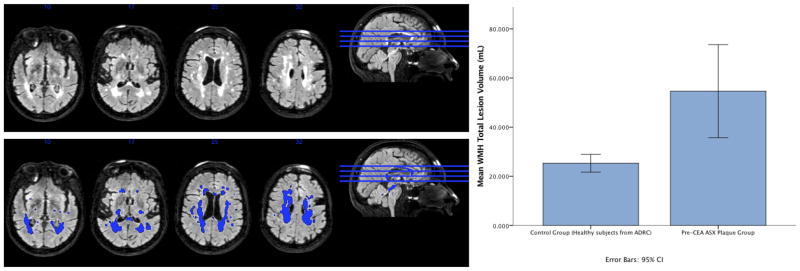
We saw mean white matter hyperintensity changes of 54.6 ml in 13 patients studied with MRI findings. In our age-matched control population N=64, WMH has a mean of 25.3 ml (p= 0.006) (Figure 5). Although in this small sample size, increasing white matter changes trended with but did not correlate with increasing strain, it is possible that would be the case in a larger sample size. The degree of hyperintensity changes was considered surprising given the classically asymptomatic nature of this population.
Figure 5.
White Matter Hyperintensities in an asymptomatic patient (left side) and WMH lesion volume in asymptomatic plaque subjects (n=13) (right side). Mean WMH TLV in the N=64 control group = 25.30639 ml (mean age: 69.06 ± 4.52 years). Mean WMH TLV in the N=13 ASX plaque subjects = 54.64323 ml (mean age: 69.62 ± 6.79 years). Independent two-tailed t-test for WMH TLV:p value = 0.006 --- ASX plaque group has statistically greater WMH TLV than the ADRC control group.
Similarly, in a period of only 1 hour of testing, 6 of 23 (26%) classically asymptomatic patients tested demonstrated high intensity transient signals (HITS) in the middle cerebral artery, showing microemboli, 9% had contralateral emboli. Although the degree of plaque strain trended with but could not significantly predict the presence or absence of emboli in this small sample group (p=0.15), this percentage of patients with emboli dramatically differs from what has been reported for normal control patients7,35 (zero) (p = 0.005) and is considered to be consistent with one etiology of vascular cognitive decline, subclinical emboli. Tong and Deklunder have both showed an expected zero rate of emboli in controls.4,7,12,16,24,25,34,35 The emboli detected in our study (Figure 6) were seen in only 1 hour of testing. It is unknown what data might result from longer periods of testing. HITS suggestive of microemboli were present in 26% of our asymptomatic patients, within the one-hour TCD monitoring time period, suggesting that while not presenting with classical TIA or stroke, the plaque in these subjects may not be stable and that microemboli from vulnerable plaque may be contributing to cognitive decline.
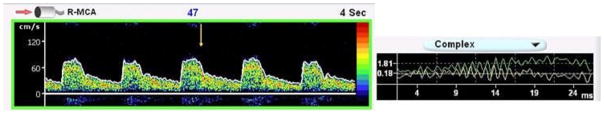
Figure 6.
Figure 6 demonstrates the Doppler signal from the RT MCA with the HIT identified by the yellow arrow. This HIT was also accompanied by an audible chirp, a change in complex, and was less than 300 ms in duration.
DISCUSSION
Stenosis – Emboli and Symptoms
Carotid artery surgery in response to cerebrovascular symptomatology dates back to the early work of Eastcott and Napaki.10 Over the subsequent decades, interventions, both by endarterectomy and by stenting have increased. The major landmark study, the North American Carotid Endarterectomy Trial (NASCET) first published in 1992 described benefit for symptomatic patients for surgery if they had significant carotid stenosis.2,6 These studies ushered in the use of carotid stenosis, defined in various studies as becoming significant at 50, 60, or 70% stenosis, as primary criteria for justification of surgery. Subsequent studies11 suggested that such parameters may be useful in asymptomatic disease and surgical intervention rapidly increased based on this parameter. It is important to understand that carotid atherosclerotic disease is primarily an embolic phenomena. While degree of stenosis may reach a point that would restrict flow, due to the individual characteristics of the Circle of Willis, flow characteristics of single vessel stenosis cannot reliably predict cerebral ischemia. At the same time, carotid plaque emboli generally are a size that often localize beyond the Circle of Willis, primarily in vessels with only pial-2-pial collateral. Such emboli may reliably result in loss of function to a region of brain which may or may not be clinically recognized due to its regional presence or absence of eloquence.
In our previous study plaque strain definitely affected by pulsation and instability of the plaques from our earlier stroke paper.36 We feel that this is related to neovascularity. The question is whether medications may affect this. Theoretically, antiplatelet therapy such as aspirin may decrease emboli but could theoretically increase plaque hemorrhage. Statins may theoretically stabilize plaque or stabilize the cholesterol deposits. In this study group 89% were taking aspirin and 70% were being treated for lipid abnormalities. The common use of both medications in atherosclerotic patients may preclude controlled studies of their effect on strain. The data from this paper does neither confirm nor denies either of these potential effects of the medications and indeed makes an interesting argument for future studies of medication effects versus strain. It is not known if medications such as aspirin or statins affect vessel strain or instability. A significant concern for loss of function that may not be clinically recognized is that of vascular cognitive decline. While this is of extraordinarily importance to the patients, it is not a standard part of the analysis of determining symptomatic or asymptomatic status. Yet, imaging studies suggest that several million “silent strokes” may be missed per year in patients at risk32,33 and further vascular cognitive decline has been shown to occur with concurrence subclinical emboli.8,26, 40,41 By instituting a standardized exam for vascular cognitive decline, we discovered that it exists in all of our study patients harboring large carotid bifurcation plaques in spite of their classically asymptomatic status.
Baseline cognitive decline was significant in all patients, but the degree of significance of the cognitive decline at presentation varied. Strain measurement also varied in the asymptomatic patients. However, the degree of strain or instability was directly correlated with the degree of cognitive decline. Our results also suggest that this effect is best seen in loss of executive function before memory is lost.
We estimate both the axial and lateral displacement vectors and corresponding strain tensor components (axial and lateral) within the 2D ultrasound imaging plane. Axial strains refer to the strain estimated along the direction of ultrasound beam propagation, while the lateral strains are estimated perpendicular to this direction, due to blood flow expanding and relaxing the vessel during systole as opposed to diastole. Note that for intact carotid plaque enclosed within a thicker fibrous cap, most of the deformation is anticipated along the axial or beam direction. For softer plaque or those with increased fissuring, increased lateral strains are observed, due to the movement of the plaque in the direction of blood flow during systole. Similar to symptomatic patients increased lateral strains were observed in asymptomatic patients with lower cognitive scores, possibly indicating that micro-embolization was occurring in these patients, with plaque being deformed along the direction of blood flow (instead of against the artery wall), which may lead to emboli getting released into the blood stream. Identification of this type of plaque deformation may be critical in the identification of vulnerable plaque, as this could be due to weakening of the plaque capsule, leading to fatigue failure or rupture even with the normal repeated alternating or cyclic stresses induced due to blood flow. Both the axial and lateral deformations contribute to the increased shearing strains in the artery. Increased shearing strains are an indicator of the presence of increased stress concentrations typically at boundary locations of the plaque with the artery wall, indicating regions at a potentially of high risk for rupture. In general, a higher probability of rupture would exist at locations with these stress concentrations.
The Mean Axial Strain index over a cardiac cycle varied in the 3.9% to 89.16% range (32.94 ±31.92), while the Mean Lateral Strain index ranged from 3.7% to 30.95% (15.98 ± 8.21), and the Mean Axial Strain index varied between 6.64 to 93.36% (36.33 ± 29.65), in these asymptomatic patients respectively. The significant variability in the Mean Axial Strain index in this group of patients might indicate that many of these patients could soon progress to gross symptoms due to fissuring or rupture of their plaque, while the others with lower strain indices may remain asymptomatic, due to the lower plaque deformations either due to the plaque being calcified or the presence of a thick fibrous cap. Relationships between other measures of the physics of vessel wall strain and anatomy need further study.
We saw a striking amount of WMH and emboli in these asymptomatic patients. We noted a doubling of the WMH change over control and 26% of these tested had emboli in only a one-hour sample. We have previously shown the importance of both white matter hyperintensity and TCD detected emboli in atherosclerotic patients not selected for symptomatic or asymptomatic status. In that group, we showed significant correlations between measurements of axial, lateral, peak-to-peak, shear plaque strain and the total amount of WMH (White Matter Hyperintensities) in the brain.3 In that prior study our subjects had a mean WMH lesion burden of almost two standard deviations greater than normal age-matched population (WMH Z-scores ranges from −0.76 to 5.96 with a mean of 1.81).3 In that study the presence of HITS was associated with an increase in the WHM lesion burden (beta of 0.346 (t[DF19] = 2.404, p= 0.027).3
We feel that the presence of some emboli in other distributions and the symmetry of the WMH showed that the presence of plaque instability not only suggest ipsilateral emboli but also is a marker of advanced systemic large and small vessel disease.
We did not expect to see such profound relationship between strain and cognitive decline in the present study since it only studied patients who were clinically asymptomatic. Although we did not see the degree of significance which was seen in the prior non-selected patients, we were surprised to see that these patient which were asymptomatic by classical criteria, were not only showing decrease in cognitive function, which correlated to strain or instability in the carotid plaque, but they were also showing a surprising amount of white matter hyperintensity and TCD hits as compared to controls.
This suggests that these plaques do indeed have a surprisingly high degree of instability even though the patients were classically asymptomatic. Ischemic cerebrovascular events are a complex process which takes place in a milieu of pre-existing small and large vessel diseases. To highlight only large vessel stenosis, is to underestimate the importance of these other factors or comorbidities which take place. Experience has shown that degree of stenosis is an inadequate screen for patients at risk without gross motor, speech, or visual symptoms. By incorporating parameters which assess large and small vessel disease as well as the presence or absence of physical instability in the plaque, we may work toward a better assessment of at-risk patients.
The limitations of this study are primarily in its sample size. We have chosen to carefully identify and clarify a small subset of patients with this presentation as to their cognitive function and carefully study their plaque stability characteristics as well as cognition over time. We feel that a small sample size well studies giving significant data is a better approach to this than imprecise measures. We have shown that the addition of additional patients does not change the positive result. One of the major problems with this field has been the inconsistency of diagnosis in asymptomatic disease. As our result show, when these patients have significantly unstable plaques, they are cognitively impaired. This would make the argument that the present limitations are primarily the classical definition of asymptomatic disease which emphasize motor, vision and speech. We believe that the emphasis on cognition is the major strength of this paper, as is the ability to detect the strain changes in the plaque and significantly relate that to cognitive decline.
CONCLUSION
This study shows that in classically asymptomatic patients with significant atherosclerotic plaques at the carotid bifurcation, that an ultrasound measure of the instability or strain with pulsation within that plaque correlates directly with vascular cognitive decline. The study further suggests that such unstable plaques are associated with white matter hyperintensities and subclinical microemboli.
The most significant information derived here is that rather than simply looking at degree of stenosis, which has always been only a surrogate for the relative amount of plaque at the bifurcation, we need to and can, be looking at the physical instability of the plaque in an attempt to predict, the risk of plaque rupture, emboli and progressive brain damage. Further, that damage which we call symptoms, must be defined beyond that of pure motor, vision, or speech symptomatology but also include cognitive decline. While studies of patients at the extreme, those symptomatic embolizing significantly enough to have major motor presentations, continue to justify interventions in those patients when they have significant plaques at the bifurcation, this is not the case in asymptomatic patients. Here a careful search for at-risk or unstable plaque is required. It is possible that those that do not have symptomatic motor presentations but do have early cognitive decline now may be diagnosed as to who is at risk by the presence or absence of physical instability within the plaque. Future studies will examine other genetic and biochemical markers for plaque instability to further define the population that would benefit from intensive medical or surgical interventions to prevent all stroke symptoms, both physical and cognitive. We recommend continued study to move toward a more rational basis of patient selection for intervention in patients who are not as yet showing clinical stroke by using physical measures of plaque strain to determine carotid atherosclerotic stability or lack of it.
Acknowledgments
This work was supported by:
The National Institutes of Health grant R01 NS064034, funded authors: Dr. Robert J. Dempsey, Dr. Bruce Hermann, Dr. Sterling Johnson, Dr. Stephanie Wilbrand, Dr. Carol Mitchell, Dr. Xiao Wang, Dr. Tomy Varghese, Dr. Daren Jackson, Nirvedh Meshram.
The National Institutes of Health grant R01 CA112192, funded authors: Dr. Tomy Varghese and Nirvedh Meshram.
The Rath Distinguished Graduate Foundation Research Award, Medical Scientist Training Program (University of Wisconsin) T32-T32GM008692, funded author: Sara Berman
The Wisconsin’s Alzheimer Disease Research Center P50-AG03351: Dr. Sterling Johnson and Sara Berman.
The authors would like to acknowledge the Clinical Research Unit of the Department of Neurosurgical Surgery for their help in coordinating the study. Furthermore, we would like to thank Linda van Brocklin for her assistance in draft preparation and Dr. Tom Cook for his insight regarding statistical analysis. Lastly, the authors would like to extend their deepest gratitude to all individuals who participated in this study.
Footnotes
ClinicalTrials.gov URL: https://clinicaltrials.gov/ct2/show/NCT02476396?term=Carotid+plaque&rank=3
DISCLOSURE:
R. Dempsey: None.
T. Varghese: Other; Siemens Ultrasound, Research Agreement for use of Ultrasound Research Interface. No financial benefit.
D.C. Jackson: None.
X. Wang: None.
N. Meshram: None.
C. Mitchell: Other; Davies Publishing, authorship for two echocardiography textbooks, currently under review, may have future royalties. Elsevier, author textbook chapters, may have future royalties.
B. Hermann: None.
S. Johnson: None.
S. Berman: None.
S. Wilbrand: None.
Contributor Information
Robert J. Dempsey, Department of Neurological Surgery, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison WI 53792. Phone: 608-265-5967, Fax 608-263-1728.
Tomy Varghese, Department of Medical Physics, University of Wisconsin School of Medicine and Public Health, 1111 Highland Avenue, Madison WI 53705. Phone: 608-265-8797, Fax: 608-262-2413.
Daren C. Jackson, Wisconsin Surgical Outcomes Research Program, Department of Surgery, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison WI 53705. Phone: 608-265-3749, Fax: 608-263-2354.
Xiao Wang, Rutgers Cancer Institute of New Jersey, 195 Little Albany Street, New Brunswick NJ 08903. Phone: 732-235-2465.
Nirvedh Meshram, Department of Medical Physics, University of Wisconsin School of Medicine and Public Health, 1111 Highland Avenue, Madison WI 53705. Phone: 608-265-8797, Fax: 608-262-2413.
Carol C. Mitchell, Department of Medicine, Cardiovascular Medicine Division, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue H6/377G, Madison WI 53792. Phone: 608-262-0680, Fax: 608-263-0405.
Bruce P. Hermann, Department of Neurology, University of Wisconsin School of Medicine and Public Health, 1685 Highland Avenue, Madison WI 53705. Phone: 608-263-5430, Fax: 608-263-0412.
Sterling C. Johnson, Alzheimer’s Disease Research Center, University of Wisconsin School of Medicine and Public Health, Waisman Laboratory for Brain Injury and Behavior, University of Wisconsin-Madison & Geriatric Research Education & Clinical Center, Wm S. Middleton Veterans Hospital. 600 Highland Avenue, Madison WI 53705. Phone: 608-262-9549.
Sara E. Berman, Alzheimer’ Disease Research Center, University of Wisconsin School of Medicine and Public Health, Neuroscience Training Program, University of Wisconsin – Madison & Medical Scientist Training Program, University of Wisconsin School of Medicine and Public Health 600 Highland Avenue, Madison WI 53705. Phone: 608-262-9549
Stephanie M. Wilbrand, Department of Neurological Surgery, University of Wisconsin School of Medicine and Public Health, Madison WI 53792. Phone: 608-265-9248, Fax: 608-263-1728.
References
- 1.Alonso F, Abhishek R, Bambakidis NC. Treatment of Symptomatic and Asymptomatic Carotid Stenosis: A review of the literature. UH Neurological Institute Journal. 8(1) [Google Scholar]
- 2.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 3.Berman SE, Wang X, Mitchell CC, Kundu B, Jackson DC, Wilbrand SM, et al. The relationship between carotid artery plaque stability and white matter ischemic injury. Neuroimage Clin. 2015;9:216–222. doi: 10.1016/j.nicl.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braekken SK, Reinvang I, Russell D, Brucher R, Svennevig JL. Association between intraoperative cerebral microembolic signals and postoperative neuropsychological deficit: comparison between patients with cardiac valve replacement and patients with coronary artery bypass grafting. J Neurol Neurosurg Psychiatry. 1998;65:573–576. doi: 10.1136/jnnp.65.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev. 2005:CD001923. doi: 10.1002/14651858.CD001923.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborators NASCET. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 7.Deklunder G, Roussel M, Lecroart JL, Prat A, Gautier C. Microemboli in Cerebral Circulation and Alteration of Cognitive Abilities in patients with mechanical prosthetic Heart Valves. Stroke. 1998;29:1821–1826. doi: 10.1161/01.str.29.9.1821. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey RJ, Vemuganti R, Varghese T, Hermann BP. A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurgery. 2010;67:484–493. doi: 10.1227/01.NEU.0000371730.11404.36. discussion 493–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doe C, Jethwa PR, Gandhi CD, Prestigiacomo CJ. Strategies for asymptomatic carotid artery stenosis. Neurosurg Focus. 2011;31:E9. doi: 10.3171/2011.9.FOCUS11206. [DOI] [PubMed] [Google Scholar]
- 10.Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;267:994–996. doi: 10.1016/s0140-6736(54)90544-9. [DOI] [PubMed] [Google Scholar]
- 11.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 12.Fearn SJ, Pole R, Wesnes K, Faragher EB, Hooper TL, McCollum CN. Cerebral injury during cardiopulmonary bypass: emboli impair memory. J Thorac Cardiovasc Surg. 2001;121:1150–1160. doi: 10.1067/mtc.2001.114099. [DOI] [PubMed] [Google Scholar]
- 13.Gijsen FJ, Nieuwstadt HA, Wentzel JJ, Verhagen HJ, van der Lugt A, van der Steen AF. Carotid Plaque Morphological Classification Compared with Biomechanical Cap Stress: Implications for a Magnetic Resonance Imaging-Based Assessment. Stroke. 2015;46:2124–2128. doi: 10.1161/STROKEAHA.115.009707. [DOI] [PubMed] [Google Scholar]
- 14.Hadar N, Raman G, Moorthy D, O’Donnell TF, Thaler DE, Feldmann E, et al. Asymptomatic carotid artery stenosis treated with medical therapy alone: temporal trends and implications for risk assessment and the design of future studies. Cerebrovasc Dis. 2014;38:163–173. doi: 10.1159/000365206. [DOI] [PubMed] [Google Scholar]
- 15.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 16.Horn J, Naylor AR, Laman DM, Chambers BR, Stork JL, Schroeder TV, et al. Identification of patients at risk for ischaemic cerebral complications after carotid endarterectomy with TCD monitoring. Eur J Vasc Endovasc Surg. 2005;30:270–274. doi: 10.1016/j.ejvs.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Jackson DC, Sandoval-Garcia C, Rocque BG, Wilbrand SM, Mitchell CC, Hermann BP, et al. Cognitive Deficits in Symptomatic and Asymptomatic Carotid Endarterectomy Surgical Candidates. Arch Clin Neuropsychol. 2016;31:1–7. doi: 10.1093/arclin/acv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakkos SK, Nicolaides AN, Charalambous I, Thomas D, Giannopoulos A, Naylor AR, et al. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg. 2014;59:956–967.e951. doi: 10.1016/j.jvs.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 19.Majdouline Y, Ohayon J, Keshavarz-Motamed Z, Roy Cardinal MH, Garcia D, Allard L, et al. Endovascular shear strain elastography for the detection and characterization of the severity of atherosclerotic plaques: in vitro validation and in vivo evaluation. Ultrasound Med Biol. 2014;40:890–903. doi: 10.1016/j.ultrasmedbio.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Markus HS, MacKinnon A. Asymptomatic embolization detected by Doppler ultrasound predicts stroke risk in symptomatic carotid artery stenosis. Stroke. 2005;36:971–975. doi: 10.1161/01.STR.0000162717.62684.40. [DOI] [PubMed] [Google Scholar]
- 21.McCormick M, Varghese T, Wang X, Mitchell C, Kliewer MA, Dempsey RJ. Methods for robust in vivo strain estimation in the carotid artery. Phys Med Biol. 2012;57:7329–7353. doi: 10.1088/0031-9155/57/22/7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadareishvili ZG, Rothwell PM, Beletsky V, Pagniello A, Norris JW. Long-term risk of stroke and other vascular events in patients with asymptomatic carotid artery stenosis. Arch Neurol. 2002;59:1162–1166. doi: 10.1001/archneur.59.7.1162. [DOI] [PubMed] [Google Scholar]
- 23.Naylor AR. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol. 2012;9:116–124. doi: 10.1038/nrcardio.2011.151. [DOI] [PubMed] [Google Scholar]
- 24.Purandare N, Voshaar RC, Morris J, Byrne JE, Wren J, Heller RF, et al. Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry. 2007;62:339–344. doi: 10.1016/j.biopsych.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Purandare N, Burns A. Cerebral Microemboli in the genesis of Dementia. J Neurol Sci. 2009;283:17–20. doi: 10.1016/j.jns.2009.02.306. [DOI] [PubMed] [Google Scholar]
- 26.Rocque BG, Jackson D, Varghese T, Hermann B, McCormick M, Kliewer M, et al. Impaired cognitive function in patients with atherosclerotic carotid stenosis and correlation with ultrasound strain measurements. J Neurol Sci. 2012;322:20–24. doi: 10.1016/j.jns.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 28.Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 2004;35:2425–2427. doi: 10.1161/01.STR.0000141706.50170.a7. [DOI] [PubMed] [Google Scholar]
- 29.Russell D. Cerebral microemboli and cognitive impairment. J Neurol Sci. 2002;203–204:211–214. doi: 10.1016/s0022-510x(02)00293-9. [DOI] [PubMed] [Google Scholar]
- 30.Shi H, Varghese T, Dempsey RJ, Salamat MS, Zagzebski JA. Relationship between ultrasonic attenuation, size and axial strain parameters for ex vivo atherosclerotic carotid plaque. Ultrasound Med Biol. 2008;34:1666–1677. doi: 10.1016/j.ultrasmedbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Smith CD, Snowdon DA, Wang H, Markesbery WR. White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology. 2000;54:838–842. doi: 10.1212/wnl.54.4.838. [DOI] [PubMed] [Google Scholar]
- 33.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 34.Stygall J, Newman SP, Fitzgerald G, Steed L, Mulligan K, Arrowsmith JE, et al. Cognitive change 5 years after coronary artery bypass surgery. Health Psychol. 2003;22:579–586. doi: 10.1037/0278-6133.22.6.579. [DOI] [PubMed] [Google Scholar]
- 35.Tong DC, Bolger A, Albers GW. Incidence of Transcranial Doppler-Detected Cerebral Microemboli in Patients referred for Echocardiography. Stroke. 1994;25:2138–2141. doi: 10.1161/01.str.25.11.2138. [DOI] [PubMed] [Google Scholar]
- 36.Tureyen K, Vemuganti R, Salamat MS, Dempsey RJ. Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurgery. 2006;58(5): 971–977. doi: 10.1227/01.NEU.0000210246.61817.FE. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Jackson DC, Mitchell CC, Varghese T, Hermann BP, Kliewer MA, et al. Estimation of ultrasound strain indices in carotid plaque and correlation to cognitive dysfunction. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5627–5630. doi: 10.1109/EMBC.2014.6944903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Jackson DC, Mitchell CC, Varghese T, Wilbrand SM, Rocque BG, et al. Classification of Symptomatic and Asymptomatic Patients with and without Cognitive Decline Using Non-invasive Carotid Plaque Strain Indices as Biomarkers. Ultrasound Med Biol. 2016;42:909–918. doi: 10.1016/j.ultrasmedbio.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Mitchell CC, Varghese T, Jackson DC, Rocque BG, Hermann BP, et al. Improved Correlation of Strain Indices with Cognitive Dysfunction with Inclusion of Adventitial Layer with Carotid Plaque. Ultrason Imaging. 2016;38:194–208. doi: 10.1177/0161734615589252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Jackson DC, Mitchell CC, Varghese T, Hermann BP, Kliewer MA, et al. Correlation of cognitive function with ultrasound strain in indices in carotid plaque. Ultrasound Med Biol. 2014;40(1): 78–89. doi: 10.1016/j.ultrasmedbio.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whisnant JP, Melton LI, 3rd, Davis PH, O’Fallon WM, Nishimaru K, Schoenberg BS. Comparison of case ascertainment by medical record linkage and cohort follow-up to determine incidence rates for transient ischemic attacks and stroke. J Clin Epidemiol. 1990;43(8): 791–797. doi: 10.1016/0895-4356(90)90239-l. [DOI] [PubMed] [Google Scholar]