Abstract
Children’s early language environments are related to later development. Little is known about this association in siblings of children with autism spectrum disorder (ASD), who often experience language delays or have ASD. Fifty-nine 9-month-old infants at high or low familial risk for ASD contributed full-day in-home language recordings. High-risk infants produced more vocalizations than low-risk peers; conversational turns and adult words did not differ by group. Vocalization differences were driven by a subgroup of “hyper-vocal” infants. Despite more vocalizations overall, these infants engaged in less social babbling during a standardized clinic assessment and they experienced fewer conversational turns relative to their rate of vocalizations. Two ways in which these individual and environmental differences may relate to subsequent development are discussed.
Keywords: autism infant sibling, vocalization, babbling, social feedback loop, home environment, language recording
Early home language environments have a substantial influence on later cognitive development (Hart & Risley, 1995). One-on-one infant directed speech, where infants and adults are interacting, is especially predictive of emerging language skills (Ramírez-Esparza, García-Sierra, & Kuhl, 2014; Weisleder & Fernald, 2013). This strong association between infant directed speech and emerging language reflects a “social feedback loop” wherein speech related infant vocalizations (e.g., babbling) are more likely to elicit adult responses than non-speech related vocalizations (e.g., laughing, crying; Warlaumont, Richards, Gilkerson, & Oller, 2014), and thus infant vocalizations are continually reinforced by adult responses. Disruptions in this reward-based social feedback loop are hypothesized to lead to negative downstream effects on language development. In children with autism spectrum disorder (ASD), this loop could be negatively impacted by deficits in social motivation, reciprocal interaction, and reward processing (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012). Over time, a disrupted system of social feedback might contribute to the language and communication delays frequently observed in these children (Sigman et al., 1999). For example, when children with ASD produce fewer speech-related vocalizations, they provide fewer opportunities for a parent to respond and consequently receive less contingent social feedback (Warlaumont et al., 2014).
Two recent studies targeting infants at high risk for ASD in the first year of life demonstrate the effectiveness of very early intervention; both treatment studies utilized parent-mediated programs designed to optimize social interactions between the infant and parent, which resulted in the treatment groups exhibiting lower ASD behaviors than the control groups (Green et al., 2015; Rogers et al., 2014). These studies highlight the importance of examining the early dyadic interactions between caregivers and their infants during the first years of life. One approach is to prospectively study infant siblings of children with ASD. It is estimated that 10–20% of younger siblings of children with ASD will develop the disorder themselves (Ozonoff et al., 2011; Sandin et al., 2014). An additional 20% of high-risk siblings who do not develop ASD may experience clinically-significant language delays (Constantino, Zhang, Frazier, Abbacchi, & Law, 2010; Toth, Dawson, Meltzoff, Greenson, & Fein, 2007). Unaffected high-risk siblings have also been found to display broader autism traits as early as 12-months of age (Georgiades et al., 2013).
Research on the early language environments of infants at risk for ASD is mixed. Most studies find that mothers of high-risk and low-risk infants provide equally enriched linguistic environments (Campbell, Leezenbaum, Mahoney, Day, & Schmidt, 2015; Leezenbaum, Campbell, Butler, & Iverson, 2014; Northrup & Iverson, 2015; Talbott, Nelson, & Tager-Flusberg, 2015). However, subtle differences in parent-infant vocal and play interactions have also been reported. Among 9-month-old high-risk infants and their mothers, for example, a greater amount of infant simultaneous speech (e.g., the mother is speaking and the infant interrupts) was predictive of later language delay (Northrup & Iverson, 2015). Further, infants who went on to have language delay displayed less vocal coordination with their mother, and these dyads had longer latencies between speakers. In another report, the play of high-risk and low-risk dyad was found to be comparably synchronous when it was led by the mother; although the high-risk dyads were less synchronous than low-risk dyads during infant-led play interactions (Yirmiya et al., 2006). In contrast, Wan and colleagues (2012) did not find differences in dyadic synchrony; however, high-risk infants appeared less lively and their parents more directive during play as compared to parents of low-risk infants. Taken together, these results suggest that despite relatively comparable early language environments of high- and low-risk infants, some subtle differences in interaction timing and quality do exist and remain to be further explored.
Critical language processes are in full swing at 9 months, making this time period a rich target for research. For example, typically developing infants begin reduplicative babbling (e.g., “baba”, “gaga”, “bobo”) between 6 and 10 months, and many utter their first words a few months later (Iverson, Hall, Nickel, & Wozniak, 2007; Stark, 1980). While there is evidence that high-risk infants show behavioral and motor differences as early as six months of age, the literature regarding babbling and vocalizations is mixed (Estes et al., 2015; Gammer et al., 2015). Previous studies have found that high-risk infants were slower to begin reduplicative babbling compared with low-risk infants (Iverson & Wozniak, 2007; Patten et al., 2014). However, findings from other studies examining rates of babbling during short parent-child play interactions have been inconsistent, with one study reporting less babbling and others reporting no differences in babbling or vocalizations in high-risk groups relative to control groups (Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011, vs Northrup & Iverson, 2015; Talbott et al., 2015). Further research is needed to determine whether very early vocalizations and pre-verbal developmental milestones such as babbling, are early manifestations of ASD risk.
Characterizing the linguistic daily home life of infants at-risk for ASD may yield unique insights into the role of reciprocal social-communication during a pre-symptomatic or prodromal period. To date, studies of the home language environment of infants at high-risk for ASD have relied on methods that are limited in their ability to provide representative, naturalistic samples of vocal behavior (e.g., recordings were either relatively short, less than an hour, or recorded in the lab). Caregiver talk and infant volubility fluctuate throughout the day (Warren et al., 2010), so short recordings may not accurately represent the infant’s home language environment. Our goal was to characterize the early home language environment experienced by 9-month-old infants at high and low risk for ASD using unobtrusive full-day language recording in the home. This study addressed three primary questions: do infants at high and low risk for ASD differ in the number of (1) vocalizations produced? (2) adult words heard? (3) turn-taking vocal interactions engaged in?
Methods
Participants
This study included 59 infants from the Infant Brain Imaging Study (IBIS), an ongoing longitudinal study of infants at high and low familial risk for ASD. The IBIS Network is an Autism Center for Excellence funded by the National Institutes of Health (R01 HD055741). The network includes four clinical sites: University of North Carolina, Chapel Hill; University of Washington, Seattle; The Children’s Hospital of Philadelphia; and Washington University, St. Louis, and data coordination at Montreal Neurological Institute, McGill University. Parents provided written informed consent prior to participating in this study. Procedures for this study were approved by the Institutional Review Boards at each clinical data collection site.
High-risk (HR) infants, n= 40, had a sibling who met ASD criteria on the Social Communication Questionnaire (SCQ; Rutter, Bailey, Lord, Cianchetti, & Fancello, 2003), Autism Diagnostic Interview (ADI-R; Lord et al., 1994), and diagnosis was confirmed by medical records. Low-risk (LR) infants, n= 19, had typically developing older siblings as determined by the Family Interview for Genetics (FIGS; Maxwell, 1992). LR infants did not have first degree relatives with ASD or related psychiatric disorders. Demographic information appears in Table 1, including information on infant race and ethnicity. Further exclusionary criteria for all infants included: significant medical conditions known to affect brain development, sensory impairment, low birth weight (< 2,200 g) or prematurity (<36 weeks gestation), perinatal brain injury secondary to birth complications or exposure to specific medication or neurotoxins during gestation, non-English speaking immediate family, contraindication for MRI, adoption, and first degree relative with psychosis, schizophrenia, or bipolar disorder.
Table 1.
Participant Demographics
| Low-Risk | High-Risk | |
|---|---|---|
| Sex | ||
| Male | 47% | 65% |
| Female | 53% | 35% |
|
| ||
| Maternal education | ||
| High school diploma | 21% | 36% |
| College degree | 42% | 33% |
| Graduate degree | 32% | 26% |
| Missing | 5% | 5% |
|
| ||
| Child race | ||
| White | 53% | 70% |
| African American | 16% | 3% |
| Asian | 0% | 3% |
| More than one race | 21% | 12% |
| Not answered | 11% | 12% |
|
| ||
| Child ethnicity | ||
| Non-Hispanic | 90% | 88% |
| Hispanic | 0% | 0% |
| Not answered | 10% | 12% |
The present study included all IBIS infants who contributed language recordings at 9 months of age. At the time of home language recordings, the age range of the siblings of HR infants was 39–184 months (M= 77.85), these data were missing for 15% of the sample. HR infants had a median of two older biological siblings (range 1–4) and LR infants had a median of one older siblings (range 1–4). Data was collected between April 2012 and August 2015.
Procedures
As part of the larger, ongoing longitudinal study, infants and their families were assessed during a clinic visit at 6 or 9 months depending on their visit schedule. Language recordings are conducted in the home at 9 months for all infants regardless of visit schedule. Infants in the current sample have not yet reached the age at which diagnostic outcome for ASD is assessed, hence ASD outcome is unavailable.
Measures
Home Language Environment
Language samples for all infants were collected using LENA digital recorders (Xu, Yapanel, & Gray, 2009). LENA recorders weigh 2 ounces and are worn by the infant using clothing specifically designed to provide optimal acoustic properties and to hold the recorder. Families were mailed packets containing LENA materials or provided packets at the 9-month clinic visit, if applicable. Packets were provided −3 to +1 weeks surrounding the infant’s 9-month birthday (see Table 3 for age at recording). Language recordings were recorded continuously for 16 hours. Families were instructed to start the recording when the infant woke up for the first time in the morning and let the recording run uninterrupted throughout the day and into the night. The majority of families (52 of 59) recorded two full-days of recordings yielding an average of 15.84 hours of recording per day (SD= 0.97 hours). Four families recorded 1 full-day and three recorded three days. Previous research has found that data from full day recordings are highly stable even with only 1-day of recording, for both TD and ASD children (Yoder, Oller, Richards, Gray, & Gilkerson, 2013). On average there were three days in between recording days (SD = 4.08, range 1–18).
Table 3.
LENA System Variables, by Risk Group
| Low-Risk M (SD) |
High-Risk M (SD) |
|
|---|---|---|
| LENA system | n = 19 | n = 40 |
| Chronological age at recording (months) | 9.53 (0.51) | 9.45 (0.93) |
| Average daily length of recoding (hours) | 15.67 (1.43) | 15.92 (0.67) |
| Infant vocalization (count) | 1079 (278) | 1301 (525) |
| Adult word (count) | 15,297 (5,649) | 11,916(5,727) |
| Conversational turn (count) | 278 (76) | 272 (118) |
| Total duration of infant vocalizations (seconds) | 893 (282) | 1044 (437) |
| Average length of infant vocalization (seconds) | 0.82 (0.06) | 0.80 (0.09) |
| Total duration of infant non-speech vocalizations duration (seconds) | 855 (352) | 854 (374) |
| Percentage of hours that included analyzable infant vocalizations | 79.80 (7.88) | 80.41 (10.51) |
| Total duration of silence detected (seconds) | 18,965 (8786) | 17,542 (7812) |
| Total duration of electronic noise (seconds) | 3,425 (3442) | 4,413 (3334) |
A recording was considered valid if it contained at least 8 hours of audio data. This benchmark was selected as it represents both the average waking day and at least half of the maximum 16-hour recording time. Two recordings (1.6%) were excluded based on these criteria. Both of these recordings were from HR infants. Of the remaining recordings, 97% were 16 hours.
Software developed by LENA Research Foundation allows for automatic processing of infant and adult vocalizations (LENA Pro software suite V3.3.4; Oller et al., 2010; Xu et al., 2009). Infant vocalizations are speech segments that are at least 0.6 seconds in duration and do not include non-speech sounds (e.g., crying, laughing, burping). The duration of infant non-speech vocalizations (in seconds) are also generated for each recording day. Adult words are the number of adult words spoken to and near the infant wearing the speech recorder. Conversational turns are defined when the infant vocalizes and an adult responds within 5 seconds, or vice versa, an adult vocalizes and an infant responds. Each variable is summed across the 16-hour recording day and then averaged across both recording days to generate an average daily count of infant vocalizations, adult words, and conversational turns.
We were interested in the association between infant vocalizations and conversational turns. To assess if the ratio of conversational turns to vocalizations was consistent for different infant volubility levels we created a variable called “conversational turns per vocalization “ which was computed by dividing conversational turn counts by infant vocalization counts. Lower proportions likely suggest that fewer of an infant’s vocalizations were accompanied by adult speech (e.g., the infant has to produce many more vocalizations for a single conversational turn compared to an infant with a higher proportion). This variable does not directly measure infant vocalizations that occurred in conversational turns alone, and therefore is an index of the relative number of infant vocalizations occurring in the social context.
Cognitive Development
Infant cognitive development was measured using standard scores from the Mullen Scales of Early Learning (MSEL; Mullen, 1995), a normed developmental assessment applicable to children from birth through 68 months. The Early Learning Composite (Mullen ELC), as well as five subscales (visual reception, fine motor, gross motor, receptive language, expressive language), were assessed at either 6 or 9 months.
Early Behavioral Features of ASD
The Autism Observation Scale for Infants (AOSI; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008) examines 16 features associated with early ASD (e.g., social babbling, early social-communication behaviors, behavioral reactivity, visual tracking and disengagement). Items are scored 0 to 2 or 3, where 0 represents typical functioning and 3 represents total absence of that behavior. The AOSI also generates a total score (range 0–50), which takes into account both the number of symptoms and the severity of each symptom. In this study, we were particularly interested in the AOSI total score and the Social Babbling item, which ranges in value from 0 to 3, with 3 representing the absence of social babbling. This item is designed to measure the degree to which an infant is able to engage in reciprocal (back and forth) vocalizations with the examiner. Infants were assessed using the AOSI at either 6 or 9 months. Administration and scoring procedures were identical at both time points.
Analytic Approach
Data were available for 40 HR and 19 LR infant participants who enrolled in the study and completed home language recordings at 9 months of age. Our primary objective was to answer three main questions: do infants at high- and low-risk for ASD differ in the number of (1) vocalizations produced? (2) adult words heard? (3) turn-taking vocal interactions engaged in?
General linear models (GLM) were fit to address our primary research questions. We began this model fitting by first testing for potential confounding variables. Chronological age at clinic testing and chronological age at LENA recording did not contribute significantly to model fit and were thus excluded as covariates from our final models. Although we did not find significant group differences in maternal education and sex of the infant using initial Chi-square tests, both of these variables are known to contribute to individual differences in language development (Hart & Risley, 1995; Huttenlocher, Haight, Bryk, Seltzer, & Lyons, 1991). To ensure that these variables did not moderate or explain associations of interest, we tested the effects of maternal education and sex of the infant on our models. These potential covariates did not significantly alter results and did not contribute significantly to the models, and were thus excluded. Mullen ELC scores were added to all final GLM models to adjust for observed group differences in infant cognitive skills (Table 2).
Table 2.
Infant Assessment Scores, by Risk Group
| Low-Risk M (SD) |
High-Risk M (SD) |
df | t | p | Cohen’sd | |
|---|---|---|---|---|---|---|
| Mullen 6–9 months | n= 19 | n=40 | ||||
| Mullen chronological age | 7.81 (1.61) | 7.27 (1.47) | 57 | 1.28 | .20 | .35 |
| Mullen ELC | 104.20 (11.97) | 96.68 (10.35) | 57 | 2.48 | .01 | .67 |
| Expressive Language | 46.32 (7.78) | 43.65 (8.31) | 57 | 1.18 | .24 | .33 |
| Receptive Language | 46.84 (12.71) | 44.48 (8.67) | 26* | 0.73 | .46 | .21 |
| Visual Reception | 58.11 (8.64) | 54.00 (8.86) | 57 | 1.68 | .09 | .46 |
| Gross Motor | 50.26 (11.47) | 48.63 (9.14) | 57 | 0.59 | .55 | .15 |
| Fine Motor | 56.94 (10.51) | 50.95 (8.31) | 57 | 2.37 | .02 | .63 |
|
| ||||||
| AOSI 6–9 months | n= 19 | n= 40 | ||||
| AOSI chronological age | 7.74 (1.35) | 7.30 (1.48) | 57 | 1.10 | .27 | −.31 |
| AOSI total score | 5.63 (3.23) | 9.47 (4.88) | 57 | −3.11 | .002 | .92 |
| AOSI social babbling | 0.94 (0.91) | 1.80 (1.15) | 57 | −2.82 | .006 | .82 |
Equal variances not assumed
Mullen and AOSI scores were obtained during the clinic visit when infants were between the ages of 6 and 9 months. Regarding the Mullen, 28 infants from the HR group contributed data at 6 months whereas 12 infants contributed data at 9 months; within the LR group, 13 infants contributed data at 6 months and 6 contributed data at 9 months. For the AOSI, 29 HR infants contributed data at 6 months and 11 infants contributed data at 9 months; 12 LR infants contributed data at 6 months and 7 contributed data at 9 months.
Preliminary analyses were conducted to assess group differences in: Mullen ELC and Mullen subscale scores, number of biological siblings, chronological age at language recording, and a number of variables probing the relative quality of the language recording. Associations between (1) Mullen scores and (2) the number of biological siblings and the key language environment variables were also tested. To answer our three main research questions, we first conducted Welch’s ANOVA to compare HR and LR groups on infant vocalizations. Welch’s ANOVA was used to examine group differences in infant vocalizations due to heteroscedasticity in vocalization counts across LR and HR groups. Prior to the analysis, Mullen ELC was partialed out from individual infant vocalization data resulting in a residual infant vocalization variable. Next, GLM was used to compare HR and LR groups on adult words and conversational turns.
For secondary analyses three vocalization groups were created based on infant vocalization counts at 9 months. Infants who vocalized 2 SD or more above the mean of the LR group were considered part of the Hyper-Vocal group. HR infants who were below this cut-off were part of the HR-Average-Vocal group; likewise LR infants who were below this cutoff were part of the LR-Average-Vocal group. General linear models were used to test whether vocalization group was associated with the language environment variables including adult words, conversational turns, and conversational turns per vocalization. The omnibus vocalization group main effect was tested first and followed up with pair-wise comparisons using an adaptive false discovery rate procedure (Hochberg & Benjamini, 1990). Adjusted p-values are reported for all pair-wise comparisons. The general linear model testing group differences in conversational turns per vocalization controlled for Mullen ELC as well as the number of adult words. To determine if increased infant vocalization levels are associated with lower proportion of conversational turns per vocalization, partial correlations were conducted for each group controlling for Mullen ELC and adult words. To assess for group differences in social babbling the non-parametric Mantel-Haenszel test was used due to the ordinal nature of the social babbling scores. Lastly, to assess for group differences in AOSI total scores the non-parametric Welch’s ANOVA was used due to heteroscedasticity in AOSI scores across vocalization groups.
All analyses were done using SAS statistics software, version 9.3 (SAS Institute INC, Cary, NC, USA).
Results
The 19 LR and 40 HR infants in this study did not differ on sex, χ2= 1.65, p = 0.197, or maternal education, χ2= 1.63, p = 0.444. The proportion of HR and LR infants contributing data at 6 and 9 months did not significantly differ for the Mullen or AOSI, χ2= 0.01, p = .902, χ2= 0.53, p = .466, respectively.
The Home Language Environment and Infant Risk Group
Preliminary analyses revealed that Mullen ELC and Mullen Fine Motor t-scores both differed significantly between HR and LR groups (Table 2), however these two variables were not significantly correlated with infant vocalizations, adult words, or conversational turns, all p > .05. HR and LR infant participants did not differ on chronological age at the time of the home language recording, t(55)= −.40, p = .687, total length of recording hours per day, t(21) = .72, p = .479, number of recording days, χ2(2)= 3.53, p = .170, total duration of electronic noise, t(57) = 1.05, p = .296, total duration of silent segments, t(57) = −.63, p = .533, or number of hours that contained analyzable infant vocalization data, t(57) = .22, p = .824 (see Table 3 for means and standard deviations by risk group). We did not find significant associations between recording length and infant vocalizations, adult words, or conversational turns, all p > .05. HR and LR groups significantly differed in number of biological siblings, χ2= 9.62, p = .022, however the number of siblings was not significantly correlated with infant vocalizations, adult words, or conversational turns, all p > .05.
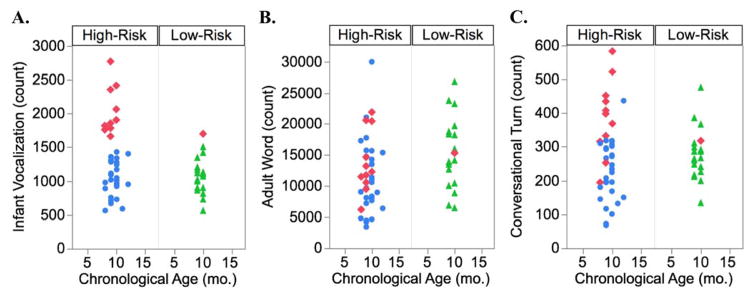
Nine-month old HR and LR infants differed significantly on number of infant vocalizations produced, F(1, 56)= 5.25, p = .025, with HR infants producing more vocalizations than LR infants (Figure 1a), controlling for Mullen ELC. HR and LR infants did not experience a significantly different number of adult words, F(1,56) = 3.35, p = .072, and Mullen ELC was not significant as a main effect, F (1,56) = 1.44, p = .234 (Figure 1b). Lastly, HR and LR infants did not engage in a significantly different number of conversational turns, F(1,56) = 0.00, p = .965, and Mullen ELC was not significant as a main effect, F (1,56) = 0.70, p = .407 (Figure 1c).
Figure 1.
Panel A, significant group differences in 9-month infant vocalizations. Panel B, adult word count by risk status. Panel C, conversational turn count by risk status. Red diamonds indicate Hyper-Vocal group. High-Risk-Average-Vocal infants are indicated with blue dots, Low-Risk-Average-Vocal infants with green triangles.
Secondary Analyses of Vocalization Groups
Visual inspection of the data revealed that a subgroup of infants vocalizing at an extremely high rate drove the significant risk group difference in infant vocalizations (Figure 1a). Operationally defining this group by vocalization counts of 2 standard deviations above the mean of the LR group yielded a sub-group of 12 “hyper-vocal” infants. This operational definition identified 1 LR infant and 11 HR infants. The LR infant was retained in the Hyper-Vocal group in all remaining analyses. Three vocalization groups were created based on this operational definition: LR Average-Vocal (n= 18), HR-Average-Vocal (n= 29), and Hyper-Vocal (n=12). On average, Hyper-Vocal infants produced 90% more vocalizations than LR-Average-Vocal infants, but they only experienced 36% more conversational turns and 8.5% fewer adult words.
Infants in these vocalization groups did not differ on chronological age at language recording, sex of the infant, maternal education, or number of siblings, all p > .05 (Table 4). The vocalization groups did differ significantly on Mullen ELC, F(2, 56)= 3.93, p = .025. Pair-wise comparisons indicated that the difference in Mullen ELC between the LR-Average-Vocal and HR-Average-Vocal groups was significant, t= 2.58, p = .024. The pair-wise comparison between LR-Average-Vocal and Hyper-Vocal did not survive the multiple comparison correction, t= 2.22, p = .060, and the HR-Average-Vocal and Hyper-Vocal did not significantly differ in Mullen ELC scores before or after multiple comparison corrections, t= 0.15, p = .878.
Table 4.
Participant Demographics, by Vocalization Group.
| LR-Average-Vocala (n= 18) | HR-Average-Vocalb (n= 29) | Hyper-Vocalc (n= 12) | Overall Group Comparison | Posthoc1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| M | SD | M | SD | M | SD | df | F/χ2 | p | ||
|
|
||||||||||
| Chronological age at recording (months) | 9.50 | 0.51 | 9.58 | 0.98 | 9.16 | 0.72 | 2, 56 | 1.51 | .328 | |
| Mullen ELC | 105.05 | 11.72 | 96.65 | 10.64 | 96.08 | 9.83 | 2,56 | 3.93 | .025 | a>b2 |
| AOSI Social Babbling | 0.89 | 0.90 | 1.66 | 1.23 | 2.17 | 0.83 | 2 | 9.43 | .002 | a<b,c |
| Conversational turns per vocalization | .253 | .240 | .182 | 4,54 | 10.68 | .0001 | a,b >c | |||
| Number of siblings (median, range)3 | 1 | 1–4 | 2 | 1–4 | 2 | 1–4 | 6 | 10.15 | .118 | |
| % Male | 50 | 65 | 58 | 2 | 1.14 | .578 | ||||
| % Maternal education4 | 18/47/35 | 33/37/30 | 58/25/17 | 2 | 5.22 | .264 | ||||
Pair-wise comparisons using an adaptive false discovery rate procedure
a>b, c before multiple comparison procedure
n=55
High school diploma/College Degree/Graduate Degree
We aimed to determine if adult words and conversational turns differed across the three vocalizations groups. Two linear models were used to investigate this question, first with adult words as the dependent variable, and then with conversational turns as the dependent variable. The model assessing associations between adult words and vocalization group did not indicate main effects that reached conventional levels of significance, including the main effect for vocalization group, F(2,55) = 3.13, p = .051, , and Mullen ELC, F(1,55) = 0.31, p = .580.
The next model testing association between conversational turns and vocalization group revealed a significant main effect for vocalization group, F(2,55) = 12.70, p < .0001, . The main effect for Mullen ELC was not significant, F(1,55) = 1.27, p = .265. Pair-wise comparisons revealed that the Hyper-Vocal group experienced more conversational turns when compared to LR-Average-Vocal group, t = 3.37, p = .001, and the HR-Average-Vocal, t = 5.05, p < .0001. The LR-Average-Vocal and HR-Average-Vocal group groups did not significantly differ on conversational turns, t = 1.34, p = .185.
Next, we were interested in determining if there were differences in the association between infant vocalizations and conversation turns across the three vocalization groups. To investigate this association we used the conversational turns per vocalization variable which measures the proportion of conversational turns that occur per infant vocalization. In addition to controlling for Mullen ELC we also controlled for number of adult words, since hearing more adult words would result in more opportunities for vocal interactions between the infant and adult. Results revealed that the proportion of conversational turns per vocalization significantly differed by vocalization groups, F(2,54) = 10.63, p = .0001, . The model also yielded a significant main effect for adult words, F(1,54) = 69.39, p < .0001, , however Mullen ELC was not significant as a main effect, F(1,54) = 1.22, p = .275. Table 4 includes least square means for conversational turns per vocalization. Pair-wise comparisons revealed that the Hyper-Vocal group had a lower proportion of conversational turns per vocalization when compared to both LR-Average-Vocal, t = 3.88, p = .0003, and HR-Average-Vocal, t = 3.49, p = .001. The LR-Average-Vocal and HR-Average-Vocal groups did not significantly differ in the proportion of conversational turns per vocalization, t = 0.88, p = .380. Together, these results suggest that while the Hyper-Vocal group generated more vocalizations and engaged in more raw conversational turns than the LR-Average-Vocal and HR-Average-Vocal groups in terms of raw numbers, a relatively lower than expected proportion of their vocalizations were associated with conversational turn taking.
In order to determine if the lower proportion of conversational turns per vocalization in the Hyper-vocal group was related to their overall higher levels of vocalization we computed partial correlations for each group, controlling for Mullen ELC and adult words. Results indicated that in the LR-Average-Vocal group infant vocalization count was negatively associated with the proportion of conversational turns per vocalization, r(18) = −.63, p = .008. However, this association was not significant in the HR-Average-Vocal group, r(29) = −.24, p = .222, nor the Hyper-Vocal group, r(12) = −.14, p = .703. Results were similar when correlations did not control for Mullen ELC and adult words.
Associations between Infant Vocalization Group and Early ASD Features
Lastly, we aimed to determine if increased vocalizations by the Hyper-Vocal group were reflected in differences on the AOSI, an early clinical assessment of ASD features. The test of AOSI social babbling revealed a significant main effect for vocalization group, χ2(2) = 9.61, p =.008. Follow-up pair-wise multiple comparisons indicated that the Hyper-Vocal group had higher social babbling scores (which indicate a lack of social babbling) when compared to the LR-Average-Vocal group, χ2(2) = 10.26, p = .001 (Figure 2 and Table 4). The HR-Average-Vocal groups and LR-Average-Vocal group also significantly differed on social babbling scores, χ2(2) = 4.77, p = .028. The Hyper-Vocal and HR-Average-Vocal did not significantly differ on social babbling scores, χ2(2) = 1.69, p = .193. Together, these results suggest that although infants in the Hyper-Vocal group generated a high volume of vocalizations, they were less likely to engage in social babbling.
Figure 2.
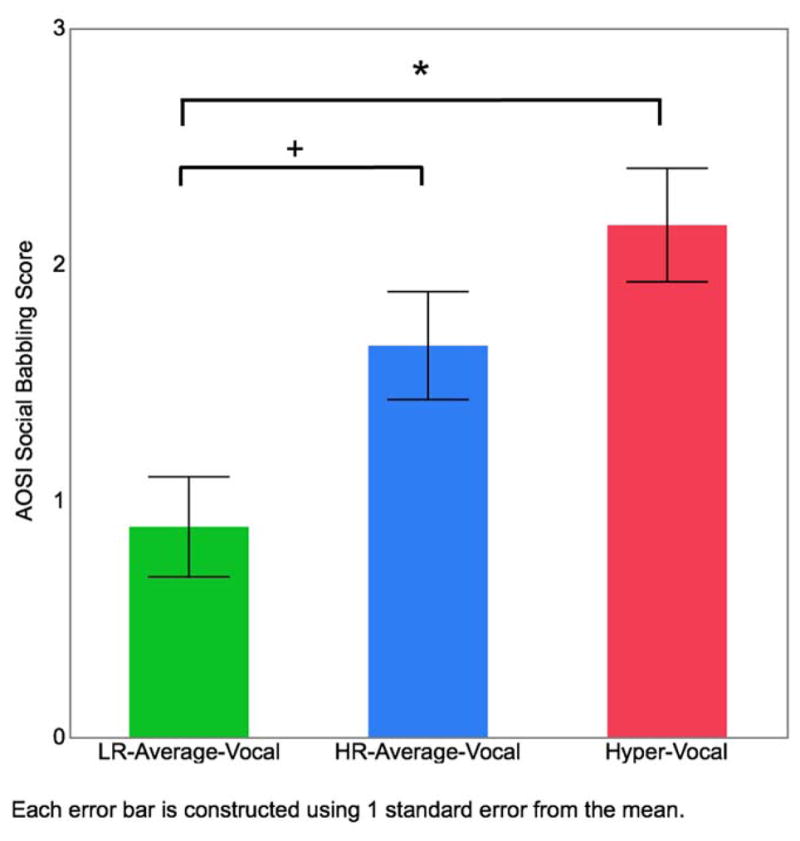
Vocalization group differences on AOSI social babbling scores. Higher AOSI social babbling scores indicate less social babbling directed to the examiner. * p < .001, + p < .05
As a final step AOSI total scores were investigated to determine if the findings associated with AOSI social babbling were reflective of general early ASD-related behaviors. The test of AOSI total scores revealed a significant main effects for vocalization group, χ2(2, 30) = 5.58, p =.008. Follow-up pair-wise comparisons indicated that the HR-Average-Vocal group displayed more early ASD symptoms than the LR-Average-Vocal group, t= 3.18, p = .004. The Hyper-vocal group did not differ significantly from the HR-Average-Vocal and LR-Average-Vocal groups on AOSI total scores, p > .05, suggesting that their profile of early ASD signs may not be general but rather specific to social communication difficulties.
Discussion
Consistent with prior research, our findings suggest that 9-month-old infants at high and low risk for ASD experience similarly rich home language environments, with equivalent numbers of conversational turn-taking. However, a substantial subgroup of infants (20%) produced significantly more vocalizations than expected (2 SD above the mean of low-risk infants). These “hyper-vocal” infants produced 90% more vocalizations than low-risk infants, comparatively they experienced only 36% more conversational turns and 8.5% fewer adult words. Further, hyper-vocal infants engaged in less social babbling and had fewer instances of conversational turn-taking for every vocalization when compared to their peers. Atypical early patterns, like hyper-vocalization, could lead to a diminished “social feedback loop” (Warlaumont et al., 2014) whereby increased vocalizations are not evoking reciprocal responses from adults. These cascading effects over developmental time could influence later language delay or ASD.
Given that difficulty with social communication is a hallmark of ASD, it seems intuitive to predict that all infants at high risk for ASD might vocalize less than their low-risk peers. In contrast, our data suggest that a significant subgroup of infants actually vocalize more than average. Hyper-vocal infants did not display the same exaggerated increase in conversational turns that they showed in vocalizations. Crucially, we found that hyper-vocal infants produced a lower proportion of conversational turns per vocalization than high-risk and low-risk peers. One interpretation of these findings is that, for hyper-vocal infants, a relatively fewer number of vocalizations occurred during a reciprocal interaction with a caregiver; meaning infants may have been vocalizing alone, outside of the social feedback loop. We aimed to determine if the lower proportion of conversation turns per infant vocalization was an effect of the higher number of vocalizations in the Hyper-vocal infants (e.g., the more infants vocalize the less parents and infants engage in conversation). Results did not provide support for this potential explanation, suggesting that hyper-vocal infants experience fewer conversational turns relative to their vocalization level due to a more nuanced reason. These hyper-vocal infants may be less sensitive to social reinforcement contingencies involving language and therefore less likely to have their language behavior shaped through dyadic interaction with a parent. In an independent confirmation of these results, a clinical assessment suggested that hyper-vocal infants were less likely to engage in social babbling (e.g., direct babbling to the examiner). Taken together, these data suggest that the increased vocalizations seen in the hyper-vocal infants were not, in part, socially directed.
If hyper-vocal infants are not engaging in as much conversational turn-taking as expected, it raises the question, what are they doing? One possibility is that their excess vocalizations may be characterized by features associated with repetitive or stereotypic vocal output. Indeed, recent research on high-risk infants that go on to develop ASD demonstrate high rates of restricted and repetitive behaviors at 12 months of age (Elison et al., 2014; Wolff et al., 2014). Similarly, Warren and colleagues found that toddlers with ASD engaged in more “monologues” than typically developing toddlers, meaning they had more vocalizations that were not accompanied by other adult speech (Warren et al., 2010). It is plausible that increased vocalizations in hyper-vocal infants precede the monologues reported by Warren and colleagues (2010). It is also plausible that hyper-vocal infants are not representative of infants who will later go on to have ASD, but rather un-affected infants that go on to have language delay or features of the broader autism phenotype (Constantino et al., 2010; Toth et al., 2007). Based on our current data, we are unable to determine if hyper-vocal infants demonstrate delayed reduplicative babbling or first-word acquisition; this is an important target for future research.
Alternatively, it is also plausible that hyper-vocal infants will go on to have better language skills compared to their high-risk peers who vocalized at average rates. Hyper-vocal infants heard more conversational turns in terms of raw numbers, albeit fewer than expected for how much they were vocalizing. Previous literature in normative development has indicated that increased adult-directed speech is associated with positive language outcomes (Ramírez-Esparza et al., 2014; Weisleder, & Fernald, 2013). The current study was limited in its ability to determine who, infant or adult, initiated conversational turn taking and if either infant or adult contributions facilitated or hindered these social interactions.
Hyper-vocal infants did not differ in 6–9 month AOSI total scores or Mullen ELC when compared to low-risk or high-risk infants that vocalized at average rates. However, previous work has shown that infants who go on to have ASD do not differ on AOSI total scores at 6 months, but they do at 12 months, so a lack of early group differences in total scores may be because global ASD risk signs are not present until 12 months (Bryson et al., 2008; Estes et al., 2015; Gammer et al., 2015; Zwaigenbaum, Bryson, Rogers, Roberts, Brian, & Szatmari, 2005). Previous research on early group differences in Mullen ELC scores is mixed, with some studies detecting group differences (Brian et al, 2015; Gammer et al., 2015) and others not detecting group differences (Estes et al., 2015; Ozonoff et al., 2010). Longitudinal follow up of these infants is needed to reveal the developmental influences of early hyper-vocal behavior on cognition and emerging ASD symptoms.
Broadly speaking, results from the current study showed that infants at high and low risk for ASD were provided with equivalently rich linguistic environments in terms of parent-infant conversational turns. We did, however, find areas of noteworthy variation. Studies by Siller and Sigman (2002, 2008) and Hirsh-Pasek and colleagues (2015) highlight the importance of this individual variation in their work on the effects of maternal responsiveness and communication development in children with ASD and children growing up in low-income households, respectively. Both studies reported that mothers who provided a responsive environment had children that went on to demonstrate superior language skills relative to peers whose mothers were less responsive (Hirsh-Pasek et al., 2015; Siller & Sigman, 2002, 2008). Non-human animal literature has further highlighted the importance of the early home environment. A rich social environment can extend the duration of the sensitive period for song learning in sparrows (Baptista & Petrinovich, 1986) and rodent studies have shown that maternal-infant interactions can modify brain development and alter gene expression (Cameron et al., 2005; Weaver, Meaney, & Szyf, 2006).
If the developing infant brain is shaped in part by the linguistic environment, then it follows that the home linguistic environment may be an ideal target for intervention in cases of high-risk infants. To date there have only been two small treatment studies of infants at high familial risk for ASD and both studies included some component that targeted sensitive parental responsiveness (Green et al., 2015; Rogers et al., 2014). The use of the LENA system as an effective change-agent for the language environment has received preliminary support. Nonparental caregivers increased their baseline words and conversational turns when provided with weekly feedback from the LENA system (Suskind et al., 2013). These findings are promising, and highlight the need for further studies investigating parental caregivers, post-intervention fadeout effects, and most importantly, intervention effects on infant development.
The current study is a departure from previous research involving infants at risk for ASD in that we used LENA language recorders to capture full-day recordings in the home environment. This departure in methodology could explain why we detected group differences in infant vocalizations where others groups did not (Northrup & Iverson, 2015; Talbott et al., 2015). The automated analysis developed by the LENA system eliminates the need for labor intensive hand-coding. Automated processes are accompanied by inherent limitations, however, including the system’s ability to accurately detect and label vocal behavior in a variety of settings. Initial validation reports of the LENA system indicated sensitivity of 82% for adult vocalizations and 76% for child vocalizations (Xu et al., 2009). An independent group conducted hand coding and compared LENA-based data to hand-coded data, with results indicating high correlation between hand-coding and LENA-based data (Soderstrom & Wittebolle, 2013). The researchers concluded that there was little evidence to suggest that the LENA system resulted in substantive artifacts in both home and daycare settings, although the LENA system tended to over-estimate adult words in the daycare setting (Soderstrom & Wittebolle, 2013). The chronological ages of infants in the current study fall within the ages of infants and children in the LENA validation study; however, we did not conduct an internal validation study using our own data.
Several limitations of this study warrant mention. First, as mentioned above, we did not conduct an internal validation study of the LENA system on our data, although the system has been found to be valid in two independent studies of similarly aged infants (Soderstrom & Wittebolle, 2013; Xu et al., 2009). Second, although the LENA feature detection algorithm is designed to detect adult words spoken to or near the infant wearing the recording as well as eliminate distant or faint voices, it is not well understood how the number of adults or children in the home environment influence the performance of the feature detection software. In the current study we did not ask families to report the number of adults or children present during the language recording session. Future efforts should weigh the value of this information with the burden on the family of collecting such data. Lastly, infants in this report are part of an ongoing study and have yet to reach a developmental and chronological age where a clinical diagnosis of ASD diagnoses may be ascertained. As such we are unable to determine whether early abnormal vocal behaviors are predictive of later ASD or related developmental concerns.
An important area of future investigation includes longitudinal follow-up which would provide additional information as to how vocalization patterns develop over time among low and high-risk infants. Warlaumont et al. (2014) reported children with ASD aged 16–48 months displayed lower proportion of speech versus non-speech vocalizations than typically developing peers. Interestingly, this group difference emerged overtime, with groups diverging at later time points. Continuing to follow infants in the current study may reveal complex patterns of change in communication over time, such that for a subgroup of infants, the first year of life may be a period of excess, but possibly non-socially contingent vocalizations, followed by a time of dynamic change resulting in overall lower levels of volubility.
Despite the noted limitations, our study has many strengths. Our use of language recorders allowed a window into the early home environment of infants at high and low familial risk for ASD. These full-day recordings potentially revealed meaningful new insights into the vocal behavior of high-risk infants, a substantial proportion of whom will go on to develop autism or specific language impairment. We found that high-risk infants vocalized more than their low-risk peers and that some high-risk infants showed highly atypical vocalizing behavior that did not appear to be socially directed. Further investigation of the development of these high-risk infants is necessary as is future independent studies to replicate our findings. On a final note, targeting hyper-vocal high-risk infants for personalized interventions (e.g., alerting parents to convert isolated infant vocalizations into parent-infant social exchanges) could potentially strengthen a diminished social feedback loop and alter aberrant developmental cascades.
Acknowledgments
The authors thank the children and their families for their ongoing participation in this longitudinal study, as well as the numerous research assistant and volunteers who have worked on this project.
This work was supported by grants through the National Institutes of Health (HD055741 PI Piven, HD055741-S1 PI Piven, HD003110 PI Piven, U54 EB005149 PI Kinnis), the National Science Foundation (IIS-1029679 PI Rehg), the Simons Foundation (SFARI Grant 140209). Dr. Swanson was supported by a Pathway to Independence Award (K99-MH108700) from NIMH and a National Research Service Award (T32-HD40127) from NICHD. Dr. Wolff was supported by a grant from the National Institute of Mental Health (K01-101653). LENA Research Foundation donated the clothing and digital recording devices used to collect the data presented herein. The funders had no role in study design, data collection, analysis, data interpretation, or the writing of the report.
References
- Baptista LF, Petrinovich L. Song development in the white-crowned sparrow: social factors and sex differences. Animal Behaviour. 1986;34:1359–1371. doi: 10.1016/S0003-3472(86)80207-X. [DOI] [Google Scholar]
- Brian AJ, Roncadin C, Duku E, Bryson SE, Smith IM, Roberts W, … Zwaigenbaum L. Emerging cognitive profiles in high-risk infants with and without autism spectrum disorder. Research in Autism Spectrum Disorders. 2014;8:1557–1566. doi: 10.1016/j.rasd.2014.07.021. [DOI] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The Autism Observation Scale for Infants: scale development and reliability data. Journal of autism and developmental disorders. 2008;38:731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–65. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Leezenbaum NB, Mahoney AS, Day TN, Schmidt EN. Social engagement with parents in 11-month-old siblings at high and low genetic risk for autism spectrum disorder. Autism: The International Journal of Research and Practice. 2015;19:915–24. doi: 10.1177/1362361314555146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16:231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. American Journal of Psychiatry. 2010;167:1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Reznick JS, Botteron KN, Estes AM, Gu H, … Piven J. Repetitive behavior in 12-month-olds later classified with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:1216–24. doi: 10.1016/j.jaac.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes AM, Zwaigenbaum L, Gu H, St John T, Paterson S, Elison JT, … Piven J. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders. 2015;7:24. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammer I, Bedford R, Elsabbagh M, Garwood H, Pasco G, Tucker L, … Charman T. Behavioural markers for autism in infancy: scores on the Autism Observational Scale for Infants in a prospective study of at-risk siblings. Infant Behavior & Development. 2015;38:107–15. doi: 10.1016/j.infbeh.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Zwaigenbaum L, Bryson S, Brian J, Roberts W, … Garon N. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. JAMA Psychiatry. 2013;70:42–8. doi: 10.1001/2013.jamapsychiatry.1. [DOI] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, … Johnson MH. Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. The Lancet Psychiatry. 2015;2:133–140. doi: 10.1016/S2215-0366(14)00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. 1995. [Google Scholar]
- Hirsh-Pasek K, Adamson LB, Bakeman R, Owen MT, Golinkoff RM, Pace A, … Suma K. The contribution of early communication quality to low-income children’s language success. Psychological Science. 2015;26:1071–83. doi: 10.1177/0956797615581493. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in Medicine. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: Relation to language input and gender. Developmental Psychology. 1991;27:236–248. doi.org/10.1037/0012-1649.27.2.236. [Google Scholar]
- Iverson JM, Hall AJ, Nickel L, Wozniak RH. The relationship between reduplicated babble onset and laterality biases in infant rhythmic arm movements. Brain and Language. 2007;101:198–207. doi: 10.1016/j.bandl.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–70. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leezenbaum NB, Campbell SB, Butler D, Iverson JM. Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism: The International Journal of Research and Practice. 2014;18:694–703. doi: 10.1177/1362361313491327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A, Disorders D, Hospital RF, Interview AD. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maxwell M. Family Interview for Genetic Studies (FIGS): a Manual for FIGS. Bethesda, MD: National Institutes of Mental Health; 1992. [Google Scholar]
- Mullen EME. Mullen scales of early learning. Circle Pines, MN: AGS; 1995. [Google Scholar]
- Northrup JB, Iverson JM. Vocal coordination during early parent-infant interactions predicts language outcome in infant siblings of children with autism spectrum disorder. Infancy. 2015;20:523–547. doi: 10.1111/infa.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller DK, Niyogi P, Gray S, Richards JA, Gilkerson J, Xu D, … Warren SF. Automated vocal analysis of naturalistic recordings from children with autism, language delay, and typical development. Proceedings of the National Academy of Sciences. 2010;107 doi: 10.1073/pnas.1003882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … Steinfeld MB. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:256–266. doi: 10.1016/j.jaac.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter AS, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–95. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, Oller DK. Vocal patterns in infants with autism spectrum disorder: canonical babbling status and vocalization frequency. Journal of Autism and Developmental Disorders. 2014;44:2413–28. doi: 10.1007/s10803-014-2047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52:588–98. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Esparza N, García-Sierra A, Kuhl PK. Look who’s talking: speech style and social context in language input to infants are linked to concurrent and future speech development. Developmental Science. 2014;17 doi: 10.1111/desc.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. Journal of Autism and Developmental Disorders. 2014;14:2981–2995. doi: 10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C, Cianchetti C, Fancello G. SCQ: Social Communication Questionnaire: manuale. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–7. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Ruskin E, Arbeile S, Corona R, Dissanayake C, Espinosa M, … Zierhut C. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64:1–114. doi: 10.1111/1540-5834.00002. Retrieved from http://www.jstor.org/stable/3181510. [DOI] [PubMed] [Google Scholar]
- Siller M, Sigman M. The behaviors of parents of children with autism predict the subsequent development of their children’s communication. Journal of Autism and Developmental Disorders. 2002;32:77–89. doi: 10.1023/A:1014884404276. [DOI] [PubMed] [Google Scholar]
- Siller M, Sigman M. Modeling longitudinal change in the language abilities of children with autism: parent behaviors and child characteristics as predictors of change. Developmental Psychology. 2008;44:1691–1704. doi: 10.1037/a0013771. [DOI] [PubMed] [Google Scholar]
- Soderstrom M, Wittebolle K. When do caregivers talk? The influences of activity and time of day on caregiver speech and child vocalizations in two childcare environments. PloS One. 2013;8:e80646. doi: 10.1371/journal.pone.0080646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark RE. Child Phonology. Elsevier; 1980. Stages of speech development in the first year of life; pp. 73–92. [DOI] [Google Scholar]
- Suskind D, Leffel KR, Hernandez MW, Sapolich SG, Suskind E, Kirkham E, Meehan P. An exploratory study of “quantitative linguistic feedback”: effect of lena feedback on adult language production. Communication Disorders Quarterly. 2013;34:199–209. doi: 10.1177/1525740112473146. [DOI] [Google Scholar]
- Talbott MR, Nelson CA, Tager-Flusberg H. Maternal Vocal Feedback to 9-Month-Old Infant Siblings of Children with ASD. Autism Research. 2015;9:460–470. doi: 10.1002/aur.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:145–57. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson MH, Charman T, Plummer F, Wai M. Parent–infant interaction in infant siblings at risk of autism. Research in Developmental Disabilities. 2012;33:924–932. doi: 10.1016/j.ridd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Warlaumont AS, Richards JA, Gilkerson J, Oller DK. A social feedback loop for speech development and its reduction in autism. Psychological Science. 2014;25:1314–24. doi: 10.1177/0956797614531023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SF, Gilkerson J, Richards JA, Oller DK, Xu D, Yapanel U, Gray S. What automated vocal analysis reveals about the vocal production and language learning environment of young children with autism. Journal of Autism and Developmental Disorders. 2010;40:555–569. doi: 10.1007/s10803-009-0902-5. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder A, Fernald A. Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychological Science. 2013;24:2143–52. doi: 10.1177/0956797613488145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, Gu H, … Ibis T. Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2014;55:945–53. doi: 10.1111/jcpp.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Yapanel U, Gray S. Reliability of the lena language environment analysis system in young children’s natural home environment. 2009 Retrieved from http://www.lenafoundation.org/wp-content/uploads/2014/10/LTR-05-2_Reliability.pdf.
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;5:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Oller DK, Richards JA, Gray S, Gilkerson J. Stability and validity of an automated measure of vocal development from day-long samples in children with and without autism spectrum disorder. Autism Research. 2013;6:103–107. doi: 10.1002/aur.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal Of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]