Abstract
Fibrosis of the subsynovial connective tissue (SSCT) in carpal tunnel syndrome (CTS) patients is increasingly recognized as an important aspect of CTS pathophysiology. In this study, we evaluated the effect of blocking profibrotic pathways in fibroblasts from the SSCT in CTS patients. Fibroblasts were stimulated with transforming growth factor β1 (TGF-β1), and then treated either with a specific fibrosis pathway inhibitor targeting TGF-β receptor type 1 (TβRI), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) or vascular endothelial growth factor receptor (VEGFR). Fibrosis array and quantitative real-time polymerase chain reaction of fibrotic genes were evaluated. Array gene expression analysis revealed significant down-regulation of multiple fibrotic genes after treatment with TβRI, PDGFR and VEGFR inhibitors. No array fibrotic genes were significantly down-regulated with EGFR inhibition. Further gene expression analysis of known CTS fibrosis markers collagen type I A2 (Col1), collagen type III A1 (Col3), connective tissue growth factor (CTGF) and SERPINE1 showed significantly down-regulation after TβRI inhibition. In contrast, VEGFR inhibition significantly down-regulated CTGF and SERPINE1, whereas PDGFR and EGFR inhibition significantly down-regulated Col3. Taken together the inhibition of TβRI appears to be the primary mediator of fibrotic gene expression in fibroblasts from CTS patients. TGF-β/Smad activity was further evaluated, and as expected inhibition of Smad activity was significantly down-regulated after inhibition of TβRI, but not with PDGFR, VEGFR or EGFR inhibition. These results indicate that local therapies specifically targeting TGF-β signaling alone or in combination offer the potential of a novel local antifibrosis therapy for patients with CTS.
Keywords: Carpal tunnel syndrome, Subsynovial connective tissue, transforming growth factor β, Fibrosis
Introduction
Carpal tunnel syndrome (CTS) is the most common compression neuropathy (de Krom et al., 1992; Stevens et al., 1988). Non-inflammatory fibrosis of the subsynovial connective tissue (SSCT) is a characteristic of idiopathic CTS, with some studies suggesting that the fibrosis may be the cause, rather than a consequence of the nerve compression (Ettema et al., 2004; Freeland et al., 2002). Several studies have reported that the up-regulation of transforming growth factor β1 (TGF-β1) signaling and other fibrosis-related cytokines plays an important role in the SSCT fibrosis associated with idiopathic CTS (Chikenji et al., 2014; Gingery et al., 2014).
TGF-β1 regulates the expression of many extracellular matrix proteins, such as collagen, fibronectin, and elastin, as well as down-regulating matrix metalloproteases (Branton and Kopp, 1999; Mogyorosi and Ziyadeh, 1998). Thus, TGF-β1 stimulation leads to a vicious cycle of fibrosis which may compromise normal organ function (Kagami et al., 1996; Leask and Abraham, 2004). Previous reports have also noted that TGF-β1 affects the expression of other profibrotic cytokine receptors, such as the platelet-derived growth factor receptor (PDGFR), the epidermal growth factor receptor (EGFR), and the vascular endothelial growth factor receptor (VEGFR) (Deharvengt et al., 2012; Ferrari et al., 2006; Trojanowska, 2008). However, the effect of inhibition of these cytokines on the metabolism of fibroblasts derived from the SSCT of patients with CTS is unknown.
This study tested the hypothesis that inhibition of profibrotic cytokine receptors would reduce the expression of profibrotic genes in fibroblasts derived from the SSCT of CTS patients, using target specific inhibitors of TGF-β receptor type1 (TβRI) (SD208, an ATP-competitive TβRI inhibitor), PDGFR (AG1296, a protein kinase inhibitor of PDGFR), EGFR (Lapatinib, a small molecule kinase inhibitor of EGFR1 and 2 (Her2)) and VEGFR (Axitinib, a small molecule tyrosine kinase inhibitor of VEGFR). In addition, since canonical TGF-β/Smad activation is an important mediator of fibrosis in CTS we also evaluated the effect of inhibition on Smad reporter activity.
Materials and Methods
This study was approved by our Institutional Review Board. SSCT was harvested from patients with CTS at the time of carpal tunnel release. The inclusion criteria for patients with CTS included a clinical diagnosis of idiopathic CTS, confirmatory electrodiagnostic studies performed in conformance with the American Association of Neuromuscular and Electrodiagnostic Medicine guidelines (Stevens, 1997) and a lack of response to nonsurgical treatment. Patients with a history of previous volar wrist surgery, hand/wrist tumor or deformity, cervical radiculopathy, inflammatory arthritis, wrist osteoarthritis, flexor tendinitis, hemodialysis, morbid obesity (body mass index > 40 kg/m2), sarcoidosis, peripheral nerve disease, metabolic disorders such as diabetes mellitus or thyroid disease, amyloidosis, or major trauma to the ipsilateral wrist were excluded. This study included 5 patients (2 males, 3 females; mean age 63 years; range 59 to 65 years) who had undergone carpal tunnel release surgery.
Cell culture
Primary SSCT fibroblasts were derived from harvested CTS patient SSCT tissue. SSCT was minced and cultured at 37°C in minimal essential medium with Earle’s salts supplemented with 10 % fetal bovine serum and 1 % antibiotic-antimycotic. Media were changed every 3 days and adherent cells were passaged after reaching 70% confluence. We previously reported that cultured SSCT fibroblasts maintained phenotypic gene expression at least until passage 5 (Gingery et al., 2014). Therefore, we used passage 4 or 5 cells in this study. Cells were seeded at 5×105 fibroblast cells per well in 6 well-plates and cultured overnight. In order to determine the role of TGF-β, PDGF, EGF and VEGF signaling plays a role in CTS fibrosis, we used chemical inhibitors that targeted receptor signaling in primary fibroblasts from CTS patients. SD208 (IC50: 49 nM) (Bio-Techne, Devens, MA, 3269) is an ATP-competitive TβRI inhibitor (Uhl et al., 2004). AG1296 (IC50: 0.3–0.5 μM) (Selleck Chemical, Houston, TX, S8024) is a protein kinase inhibitor that is specific for PDGFR (Kovalenko et al., 1997). Lapatinib (IC50: 10.8 nM) (Selleck Chemical, Houston, TX, S1028) is a small molecule kinase inhibitor that targets both EGFR1 and 2 (Her2) (Moy et al., 2007). Axitinib (IC50: 0.1–0.3 nM) (Selleck Chemical, Houston, TX, S1005) is a small molecule tyrosine kinase inhibitor that primarily targets VEGFR and with some to a lesser extent PDGFR and c-kit (Keating, 2015). Given that TGF-β1 is a known mediator of CTS (Chikenji et al., 2014; Gingery et al., 2014) inhibitor treatments were performed in the presence of 5 ng/mL TGF-β1 (R&D Systems, Inc., Minneapolis, MN, 240-B-010). Six experimental groups were used to evaluate the effect on fibrotic gene signaling; vehicle control (Veh), Veh with TGF-β1 (TGF-β1), SD208 with TGF-β1 (SD208), AG1296 with TGF-β1 (AG1296), Lapatinib with TGF-β1 (Lapatinib) and Axitinib with TGF-β1 (Axitinib). All cell cultures other than the Veh only groups were pretreated with an inhibitor (SD208, 1 μM; AG1296, 20 μM; Lapatinib, 2 μM; or Axitinib, 1 μM) for 60 minutes prior to experiments, and then cultured with TGF-β1 for 24 hours.
Fibrosis Array and Quantitative real-time polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen, Grand Island, NY, 15596026). RNA was quantitated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Equal amounts of total RNA were used to synthesize cDNA, using the iScriptTM cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, 170-8891) as previously reported (Gingery et al., 2014).
Gene expression analysis was completed using Human Fibrosis polymerase chain reaction (PCR) arrays (SA Biosciences, Frederick, MD, PAHS-120Z) according to the manufacturer’s protocol. The geometric mean of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and ribosomal protein lateral stalk subunit P0 (RPLP0) served as an internal control. Fold changes were calculated on a log 2 scale, comparing treatment groups.
Additionally, genes whose expression were up-regulated in CTS patient SSCT fibroblasts compared to control normal human SSCT fibroblasts; collagen type I A2 (Col1), collagen type III A1 (Col3) and connective tissue growth factor (CTGF) (Gingery et al., 2014), were analyzed using quantitative real-time PCR (qRT-PCR). SERPINE1, a TGF-β responsive gene and a genes highly regulated in our arrays were also analyzed (Kutz et al., 2001). GAPDH served as a housekeeping gene for the qRT-PCR studies. C1000 Touch™ Thermal Cycler (Bio-Rad Laboratories, Hercules, CA) was used for the quantification and analysis. All PCR primers were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/) and were purchased from Integrated DNA Technologies (Coralville, IA). The probe sequences are listed in Table 1.
Table 1.
Primer sequences used for qRT-PCR
| Gene | Forward | Reverse | Accession Number |
|---|---|---|---|
| Col1 | ttgaccctaaccaaggatgc | cagttcttggctgggatgtt | NM_000089 |
| Col3 | gatcaggccagtggaaatgt | gtgtgtttcgtgcaaccatc | NM_000090 |
| CTGF | tcccacccaattcaaaacat | tgctcctaaagccacacctt | NM_001901 |
| SERPINE1 | ctctctctgccctcaccaac | gtggagaggctcttggtctg | NM_000602 |
| GAPDH | cagcctcaagatcatcagca | tgtggtcatgagtccttcca | NM_001256799 |
Smad Luciferase Reporter Assay
SSCT fibroblasts were stably transfected with a reporter plasmid containing the Smad binding element. The Smad binding element comprises a CAGACA motif that serves as a direct binding site for Smad proteins using the Cignal Reporter Assay (Qiagen, Valencia, CA, CCS-017L) as the Smad binding element. Cells were plated at 1.3×105 fibroblast cells per well and the following day cells were transfected with pathway reporters using FuGENE 6 transfection reagent (Promega Corporation, Madison, WI, E2691) according to the manufacturer’s protocols. After 24 hours culture, the transfected cells were pretreated with each inhibitor for 60 minutes and then treated with ± TGF-β (5ng/mL) as noted. After 24 hours cells were lysed with passive lysis buffer (Promega Corporation, Madison, WI, E1941) and cell lysates luciferase activity was measured upon addition of luciferase assay reagent (Promega Corporation, Madison, WI, E1483) using the Dual-Luciferase® Reporter Assay System (Promega Corporation, Madison, WI, E1910) as previously reported (Gingery et al., 2014). Relative units of luciferase activity were reported after subtracting the basal expression levels observed in the Veh group.
Statistical analysis
The gene expression of the fibrosis array result was normalized to housekeeping genes as determined by SA Biosciences RT2 Profiler™ PCR Array Data Analysis software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). Statistical analysis for qRT-PCR gene expression experiments and Smad luciferase reporter assay was analyzed by an unpaired Student’s t-test. All measurements were expressed as mean values and standard error. The level of statistical significance was set at p < 0.05 or p < 0.01 (where noted). The statistical analysis was performed using Statistical Package for the Social Sciences 21.0 for Windows (SPSS Inc., Chicago, IL).
Results
Fibrosis array - regulation of fibrotic genes upon cytokine receptor inhibition
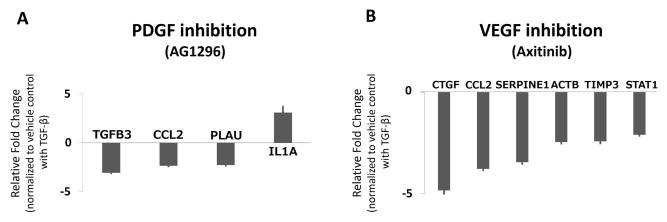
Multiple fibrotic genes were significantly (p < 0.05) up-regulated in SSCT cells treated with TGF-β1 as compared to Veh, including SERPINS, CTGF, VEGFA, PDGFA, TGF-β1 and TβRI. In addition, multiple genes were significantly (p < 0.05) down-regulated greater than 2-fold including integrins, TGF-β2, Smad6 and Smad3 (Fig. 1A, Suppl. Table 1). Upon inhibition of TβRI kinase activity with SD208 there were significant (p < 0.05) changes including down-regulation of SERPINE1, CTGF, VEGFA, PDGFA, TGF-β1, TβRI (Fig. 1B, Suppl. Table 2). Given that both PDGFA and VEGFA were significantly (p < 0.05) regulated by TGF-β1 and by inhibition of TβRI, and that EGFR has been shown to be an important fibrotic signal mediator (Liu et al., 2015; Peng et al., 2016; Wang et al., 2016) we explored whether inhibiting these cytokines would down-regulate pro-fibrotic genes. In both cases, we found that the number of fibrotic genes that were regulated by blocking PDGFR and VEGFR tyrosine kinase activity impacted fewer fibrotic target genes than TβRI inhibition. PDGFR inhibition resulted in down-regulation of TGF-β3, PLAU and CCL2 expression and increased IL1A expression (Fig. 2A, Suppl. Table 3). VEGFR inhibition resulted in down-regulation of multiple profibrotic genes including CTGF, SERPINE1, ACTB, STAT1, and TIMP3 (Fig. 2B, Suppl. Table 4). However, EGFR inhibitor had no significant effect on gene expression (Suppl. Table 5).
Figure 1.
Fibrotic array gene expression in fibroblasts derived from the SSCT in CTS patients treated with TGF-β1 and TβRI inhibitor (SD208). Significant (p < 0.05) 2-fold changes of fibrotic gene expression of TGF-β1 compared to vehicle control (Fig. A) and TGF-β1 + SD208 compared to vehicle control with TGF-β1 (Fig. B) in CTS fibroblast after 24 hours of treatment. All data are significant (p < 0.05) and reported as regulated 2-fold relative change mean ± SE. n=5.
Figure 2.
Fibrotic array gene expression in fibroblasts derived from the SSCT in CTS patients treated with PDGFR inhibitor (AG1296) and VEGRF inhibitor (Axitinib). Significant (p < 0.05) 2-fold changes of fibrotic gene expression of TGF-β1 + AG1296 (Fig. A) and TGF-β1 + Axitinib (Fig. B) in CTS fibroblast compared to vehicle control with TGF-β1. All data are reported as up-regulated more than 2-fold or down-regulated less than 0.5-fold significant regulation, mean ± SE. n=5.
Fibrotic expression of known SSCT CTS fibrotic markers
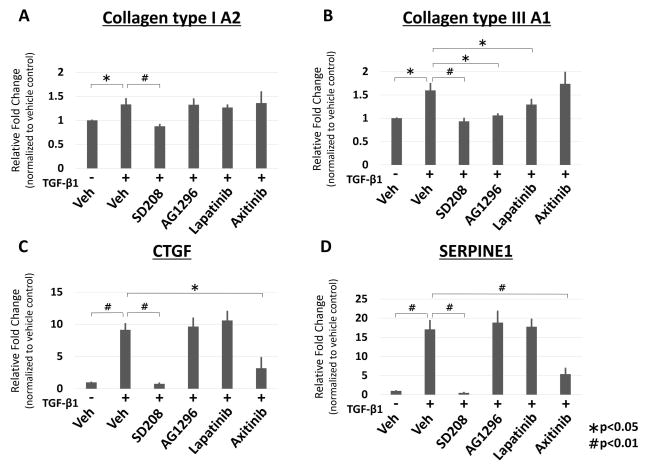
Inhibition with the TβRI inhibitor SD208, significantly down-regulated Col1 expression (p < 0.01), whereas all other receptor inhibitors did not regulate this gene (Fig. 3A). Col3 regulation was significantly decreased with TβRI (p < 0.01), PDGFR and EGFR (p < 0.05) inhibition; however VEGFR inhibition did not significantly regulate this gene expression (Fig. 3B). CTGF, another important marker of CTS fibrosis, was significantly down-regulated by TβRI (p < 0.01) and VEGFR (p < 0.05) inhibition; however inhibition of PDGFR and EGFR had no impact on CTGF expression (Fig. 3C). Finally, we evaluated SERPINE1 expression with inhibition and found, just as in the fibrosis arrays, that only TβRI and VEGFR significantly (p < 0.01) inhibited SERPINE1 expression (Fig. 3D).
Figure 3.
Gene expression analysis using qRT-PCR of known CTS fibrosis marker genes Col1, 3, CTGF, SERPINE1 upon cytokine receptor level inhibition. CTS fibroblasts were treated with TGF-β1 and TβRI (SD208), PDGFR (AG1296), EGFR (Lapatinib) and VEGFR (Axitinib) inhibitor for 24 hours. Gene expression is normalized to vehicle control. All data are expressed as mean ± SE. n=5. (* indicates p < 0.05, # indicates p < 0.01).
Smad Luciferase Reporter Assay
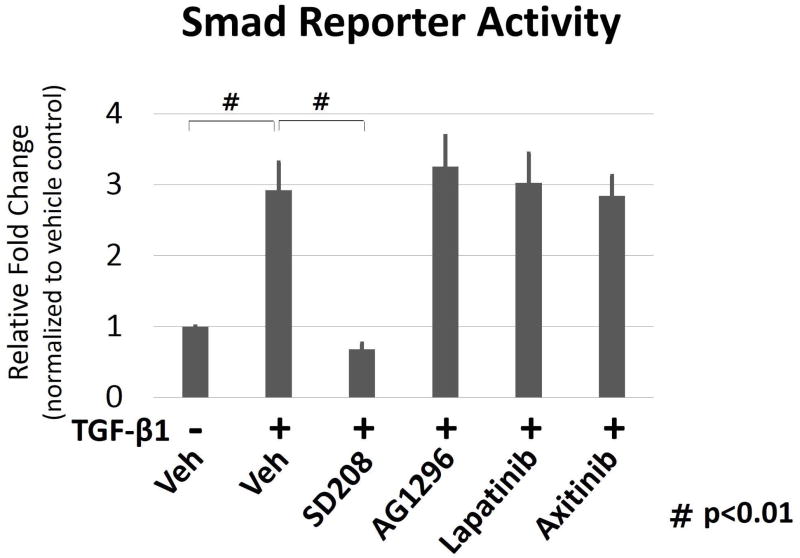
Smad reporter activity was significantly (p < 0.01) up-regulated in TGF-β1 treated cells; however inhibition with TβRI inhibitor significantly (p < 0.01) decreased reporter activity. Inhibitors to PDGFR, EGFR and VEGFR did not result in changes of Smad reporter activity (Fig. 4).
Figure 4.
Smad reporter activity comparing cytokine receptor inhibition. Comparison of CTS fibroblasts treated with TGF-β1 and TβRI (SD208), PDGFR (AG1296), EGFR (Lapatinib) and VEGFR (Axitinib) inhibitor for 24 hours. Data are expressed as relative fold change from vehicle control. All data are expressed as mean ± SE. n=3. (# indicates p < 0.01).
Discussion
TGF-β is a central mediator in fibrosis in many different organs, such as lung, liver and kidney (Kato et al., 2004; Krein and Winston, 2002; Meng et al., 2015; Pohlers et al., 2009). The effect of TGF-β on the cell is mediated by canonical TGF-β/Smad signaling as well as non-canonical pathways including mitogen-activated protein kinase (MAPK) pathways, Rho-like GTPase signaling pathways, and phosphatidylinositol- 3-kinase (PI3K) /Akt pathways (Rockey et al., 2015; Zhang, 2009). Compared to normal SSCT fibroblasts, iSSCT fibroblasts from CTS patients have significantly increased TGF-β1 and CTGF protein levels and show significantly up-regulated fibrosis related genes (Chikenji et al., 2014; Gingery et al., 2014). Further the patients have increased collagen deposition, hypervascularity, decreased permeability and increased stiffness (Ettema et al., 2004; Werthel et al., 2014). In this study, we sought to assess the effect of inhibiting various components of fibrotic signaling on the expression of profibrotic genes. The chemical inhibitors targeted TβRI (SD208), PDGFR (AG1296), EGFR (Lapatinib), and VEGFR (Axitinib) were used. Inhibitor concentrations were selected based on effective doses in cell culture experiments from previous reports (Andrianifahanana et al., 2013; Baroni et al., 2006; Gingery et al., 2014; Hu-Lowe et al., 2008; Nahta et al., 2007; Uhl et al., 2004).
The fibrosis arrays showed that TGF-β1 activated the expression of several genes, including SERPINE1 and growth factors such as CTGF, VEGFA and PDGFA. SD208 acts by inhibiting the TβRI kinase (ALK5) (Nasim et al., 2012). We and others have reported that the inhibition of TβRI by SD208 can down-regulate the expression of fibrotic markers in fibrotic diseases (Akhurst and Hata, 2012; Chen et al., 2006; Gingery et al., 2014). SSCT markers of fibrosis include Col1, 3 and CTGF (Chikenji et al., 2014; Gingery et al., 2014). Our gene expression results confirm this finding, showing significant up-regulation of Col1, 3 and CTGF and SERPINE1 expression. This up-regulation of genes associated with fibrosis is significantly down-regulated by SD208 treatment, indicating that TGF-β signaling is an important regulator of CTS fibrosis. Consistent with this was the significant down-regulation of many profibrotic genes by treatment with SD208 in the fibrosis array.
PDGFA and B chain dimeric isoforms (PDGF-AA, -AB, and -BB) also play important roles in the pathogenesis of fibrosis. These isoforms bind PDGFR α and β, respectively, and stimulate the expression of collagen (Bonner, 2004). PDGF signaling involves multiple pathways including the MAPK pathway, protein kinase C and calcium, c-jun n-terminal kinase, PI3K and the signal transducers and activators of transcription (STAT) pathway (Demoulin and Essaghir, 2014). AG1296 can reduce pulmonary fibrosis in rats by acting as a selective inhibitor of autophosphorylation of PDGFR α and β (Baroni et al., 2006; Kovalenko et al., 1997; Rice et al., 1999). In this study, the PDGFR inhibitor, AG1296, had limited effects on regulating fibrotic genes down-regulated the expression of Col3, however had limited effects on regulating other fibrotic genes. This data suggests that PDGFR may not be a significant target for the treatment of fibrosis that is seen in patients with CTS.
EGF/EGFR signaling has been associated with several types of human organ fibrosis including renal fibrosis, pulmonary fibrosis, and liver fibrosis (Fuchs et al., 2014; Vallath et al., 2014; Zhuang and Liu, 2014). EGF is a protein with 53 amino acid polypeptide and three intramolecular disulfide bonds, with a molecular weight of 6045-Da (Harris et al., 2003). EGFR (also known as human epidermal growth factor receptor (HER) 1 or erbB-1) is a tyrosine kinase receptor that is frequently expressed on the cell surface and is activated by binding of its specific ligands, including EGF and TGF-α (Scaltriti and Baselga, 2006). Depending on the ligand-receptor combination, multiple downstream signaling pathways such as MAPK/Extracellular signal regulated kinase (ERK), PI3K/Akt and Janus kinase / STAT can be activated (Holbro and Hynes, 2004). Lapatinib (originally known as GW572016) is a small molecule that inhibits the intracellular tyrosine kinase domains of EGFR and HER2 (Moy et al., 2007), and an effect of Lapatinib on fibrotic disease has been demonstrated (Andrianifahanana et al., 2013; Beyer et al., 2010; Cho et al., 2009). In this study, Lapatinib was found to significantly suppress one gene Col3. Taken together EGFR inhibition appears to have a limited response blocking profibrotic gene expression in CTS fibroblasts.
VEGF, a crucial regulator of blood-vessel formation in adults is also involved in fibrotic signaling (Olsson et al., 2006). VEGF signaling stimulates cellular responses through activation of multiple signaling pathways, such as the MAPK/ ERK and PI3K/Akt signaling pathways (Ferrara et al., 2003). Some studies suggested a fibrogenic effect of VEGF through other mechanisms as well, including promotion of inflammation, release of fibrosis-enhancing molecules from VEGF-activated endothelial cells, and direct effects of VEGF on hematopoietic stem cells (Sahin et al., 2012; Yoshiji et al., 2003). These studies suggest that VEGF inhibition might also have beneficial effects on fibrosis resolution. Axitinib is a potent, selective inhibitor of VEGFR1, 2, 3, PDGFR-β, and c-kit (Keating, 2015). One report describes a beneficial effect of Axitinib on pulmonary fibrosis (Hillman et al., 2014). In our study Axitinib suppressed the expression of CTGF and SERPINE1, which are downstream of TGF-β1 signaling. However, Smad signaling was not suppressed by Axitinib. This result suggests that the suppression of CTGF and SERPINE1 by Axitinib involves a different mechanism, separate from canonical TGF-β/Smad signaling, and that therefore VEGFR targeted treatment may provide an additional effect beyond that of a TGF-β1 inhibitor such as SD208.
Smad reporter activity was significantly down-regulated by inhibition of TβRI signaling via SD208, no other receptor inhibitors blocked Smad activity. These results suggest that SD208 targeting of TβRI blocks TGF-β/Smad signaling, and suppresses not only the expression of growth factors but also several profibrotic genes.
The strength of this study is that we evaluated receptor level fibrosis inhibition in cells derived from patients with CTS on expression candidate fibrotic cytokine/receptor genes. As such, this study may be useful in helping to identify candidate therapeutic targets both alone and in combination for the treatment of fibrosis in CTS patients. The principal weakness of this study is in vitro analysis and not an exploration at the tissue or organism level. Additionally, this study was limited to gene expression changes and future work will need to confirm these effects in vivo. Finally, patients with CTS tend to be aged 50 and above (Bland and Rudolfer, 2003), and so the role of cellular senescence in these results should be investigated. Indeed, we plan this in future studies.
In conclusion, we believe that these results confirm the key role of TGF-β signaling in the fibrosis associated with CTS, and that further studies into the effect of TGF-β inhibition, alone or in combination with each other targeted inhibitors, are warranted. Such studies might offer, for the first time, a mechanism-specific targeted drug therapy for CTS.
Supplementary Material
Acknowledgments
Contract grant sponsor: NIH/NIAMS; Contract grant number: AR49823 and F32 AR063596, as well as by funds provided by Mayo Clinic.
This study was supported by grants from NIH/NIAMS, AR49823 and F32 AR063596, as well as by funds provided by Mayo Clinic.
References
- Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nature reviews Drug discovery. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianifahanana M, Wilkes MC, Gupta SK, Rahimi RA, Repellin CE, Edens M, Wittenberger J, Yin X, Maidl E, Becker J, Leof EB. Profibrotic TGFbeta responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(11):4444–4454. doi: 10.1096/fj.12-224907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, Fraticelli P, Sambo P, Funaro A, Kazlauskas A, Avvedimento EV, Gabrielli A. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. The New England journal of medicine. 2006;354(25):2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- Beyer C, Distler JH, Distler O. Are tyrosine kinase inhibitors promising for the treatment of systemic sclerosis and other fibrotic diseases? Swiss medical weekly. 2010;140:w13050. doi: 10.4414/smw.2010.13050. [DOI] [PubMed] [Google Scholar]
- Bland JD, Rudolfer SM. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991–2001. Journal of neurology, neurosurgery, and psychiatry. 2003;74(12):1674–1679. doi: 10.1136/jnnp.74.12.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine & growth factor reviews. 2004;15(4):255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1(15):1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shi-wen X, Eastwood M, Black CM, Denton CP, Leask A, Abraham DJ. Contribution of activin receptor-like kinase 5 (transforming growth factor beta receptor type I) signaling to the fibrotic phenotype of scleroderma fibroblasts. Arthritis and rheumatism. 2006;54(4):1309–1316. doi: 10.1002/art.21725. [DOI] [PubMed] [Google Scholar]
- Chikenji T, Gingery A, Zhao C, Passe SM, Ozasa Y, Larson D, An KN, Amadio PC. Transforming growth factor-beta (TGF-beta) expression is increased in the subsynovial connective tissues of patients with idiopathic carpal tunnel syndrome. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32(1):116–122. doi: 10.1002/jor.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Kang JH, Kim T, Park KK, Kim CH, Lee IS, Min KS, Magae J, Nakajima H, Bae YS, Chang YC. Suppression of PAI-1 expression through inhibition of the EGFR-mediated signaling cascade in rat kidney fibroblast by ascofuranone. Journal of cellular biochemistry. 2009;107(2):335–344. doi: 10.1002/jcb.22130. [DOI] [PubMed] [Google Scholar]
- de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: prevalence in the general population. Journal of clinical epidemiology. 1992;45(4):373–376. doi: 10.1016/0895-4356(92)90038-o. [DOI] [PubMed] [Google Scholar]
- Deharvengt S, Marmarelis M, Korc M. Concomitant targeting of EGF receptor, TGF-beta and SRC points to a novel therapeutic approach in pancreatic cancer. PloS one. 2012;7(6):e39684. doi: 10.1371/journal.pone.0039684. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine & growth factor reviews. 2014;25(3):273–283. doi: 10.1016/j.cytogfr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. The Journal of bone and joint surgery American volume. 2004;86-A(7):1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17260–17265. doi: 10.1073/pnas.0605556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeland AE, Tucci MA, Barbieri RA, Angel MF, Nick TG. Biochemical evaluation of serum and flexor tenosynovium in carpal tunnel syndrome. Microsurgery. 2002;22(8):378–385. doi: 10.1002/micr.10065. [DOI] [PubMed] [Google Scholar]
- Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T, Lanuti M, Schmitt AD, Gupta S, Crenshaw A, Onofrio R, Taylor B, Winckler W, Bardeesy N, Caravan P, Golub TR, Tanabe KK. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery A, Yang TH, Passe SM, An KN, Zhao C, Amadio PC. TGF-beta signaling regulates fibrotic expression and activity in carpal tunnel syndrome. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32(11):1444–1450. doi: 10.1002/jor.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Experimental cell research. 2003;284(1):2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Hillman GG, Lonardo F, Hoogstra DJ, Rakowski J, Yunker CK, Joiner MC, Dyson G, Gadgeel S, Singh-Gupta V. Axitinib Improves Radiotherapy in Murine Xenograft Lung Tumors. Translational oncology. 2014 doi: 10.1016/j.tranon.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annual review of pharmacology and toxicology. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O’Connor P, Shalinsky DR, Bender SL. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(22):7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- Kagami S, Kuhara T, Yasutomo K, Okada K, Loster K, Reutter W, Kuroda Y. Transforming growth factor-beta (TGF-beta) stimulates the expression of beta1 integrins and adhesion by rat mesangial cells. Experimental cell research. 1996;229(1):1–6. doi: 10.1006/excr.1996.0336. [DOI] [PubMed] [Google Scholar]
- Kato J, Ido A, Hasuike S, Uto H, Hori T, Hayashi K, Murakami S, Terano A, Tsubouchi H. Transforming growth factor-beta-induced stimulation of formation of collagen fiber network and anti-fibrotic effect of taurine in an in vitro model of hepatic fibrosis. Hepatology research : the official journal of the Japan Society of Hepatology. 2004;30(1):34–41. doi: 10.1016/j.hepres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Keating GM. Axitinib: a review in advanced renal cell carcinoma. Drugs. 2015;75(16):1903–1913. doi: 10.1007/s40265-015-0483-x. [DOI] [PubMed] [Google Scholar]
- Kovalenko M, Ronnstrand L, Heldin CH, Loubtchenkov M, Gazit A, Levitzki A, Bohmer FD. Phosphorylation site-specific inhibition of platelet-derived growth factor beta-receptor autophosphorylation by the receptor blocking tyrphostin AG1296. Biochemistry. 1997;36(21):6260–6269. doi: 10.1021/bi962553l. [DOI] [PubMed] [Google Scholar]
- Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122(6 Suppl):289S–293S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- Kutz SM, Hordines J, McKeown-Longo PJ, Higgins PJ. TGF-beta1-induced PAI-1 gene expression requires MEK activity and cell-to-substrate adhesion. Journal of cell science. 2001;114(Pt 21):3905–3914. doi: 10.1242/jcs.114.21.3905. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Liu N, Wang L, Yang T, Xiong C, Xu L, Shi Y, Bao W, Chin YE, Cheng SB, Yan H, Qiu A, Zhuang S. EGF Receptor Inhibition Alleviates Hyperuricemic Nephropathy. Journal of the American Society of Nephrology : JASN. 2015;26(11):2716–2729. doi: 10.1681/ASN.2014080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Zhang Y, Huang XR, Ren GL, Li J, Lan HY. Treatment of renal fibrosis by rebalancing TGF-beta/Smad signaling with the combination of asiatic acid and naringenin. Oncotarget. 2015;6(35):36984–36997. doi: 10.18632/oncotarget.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogyorosi A, Ziyadeh FN. Increased decorin mRNA in diabetic mouse kidney and in mesangial and tubular cells cultured in high glucose. The American journal of physiology. 1998;275(5 Pt 2):F827–832. doi: 10.1152/ajprenal.1998.275.5.F827. [DOI] [PubMed] [Google Scholar]
- Moy B, Kirkpatrick P, Kar S, Goss P. Lapatinib. Nature reviews Drug discovery. 2007;6(6):431–432. doi: 10.1038/nrd2332. [DOI] [PubMed] [Google Scholar]
- Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Molecular cancer therapeutics. 2007;6(2):667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- Nasim MT, Ogo T, Chowdhury HM, Zhao L, Chen CN, Rhodes C, Trembath RC. BMPR-II deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of TGFbeta-TAK1-MAPK pathways in PAH. Human molecular genetics. 2012;21(11):2548–2558. doi: 10.1093/hmg/dds073. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nature reviews Molecular cell biology. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Peng K, Tian X, Qian Y, Skibba M, Zou C, Liu Z, Wang J, Xu Z, Li X, Liang G. Novel EGFR inhibitors attenuate cardiac hypertrophy induced by angiotensin II. Journal of cellular and molecular medicine. 2016;20(3):482–494. doi: 10.1111/jcmm.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochimica et biophysica acta. 2009;1792(8):746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Rice AB, Moomaw CR, Morgan DL, Bonner JC. Specific inhibitors of platelet-derived growth factor or epidermal growth factor receptor tyrosine kinase reduce pulmonary fibrosis in rats. The American journal of pathology. 1999;155(1):213–221. doi: 10.1016/S0002-9440(10)65115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. The New England journal of medicine. 2015;372(12):1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, Nellen A, Schmitz P, Heinrichs D, Berres ML, Doleschel D, Scholten D, Weiskirchen R, Moeller MJ, Kiessling F, Trautwein C, Wasmuth HE. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology (Baltimore, Md) 2012;55(5):1610–1619. doi: 10.1002/hep.25545. [DOI] [PubMed] [Google Scholar]
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(18):5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine Muscle & nerve. 1997;20(12):1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Sun S, Beard CM, O’Fallon WM, Kurland LT. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38(1):134–138. doi: 10.1212/wnl.38.1.134. [DOI] [PubMed] [Google Scholar]
- Trojanowska M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology (Oxford, England) 2008;47(Suppl 5):v2–4. doi: 10.1093/rheumatology/ken265. [DOI] [PubMed] [Google Scholar]
- Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, Murphy A, Wong DH, Wick W, Higgins LS, Weller M. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer research. 2004;64(21):7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- Vallath S, Hynds RE, Succony L, Janes SM, Giangreco A. Targeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunities. The European respiratory journal. 2014;44(2):513–522. doi: 10.1183/09031936.00146413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu N, Xiong C, Xu L, Shi Y, Qiu A, Zang X, Mao H, Zhuang S. Inhibition of EGF Receptor Blocks the Development and Progression of Peritoneal Fibrosis. Journal of the American Society of Nephrology : JASN. 2016;27(9):2631–2644. doi: 10.1681/ASN.2015030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werthel JD, Zhao C, An KN, Amadio PC. Carpal tunnel syndrome pathophysiology: role of subsynovial connective tissue. Journal of wrist surgery. 2014;3(4):220–226. doi: 10.1055/s-0034-1394133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Yamazaki M, Tsujinoue H, Imazu H, Masaki T, Fukui H. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52(9):1347–1354. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell research. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Liu N. EGFR signaling in renal fibrosis. Kidney Int Suppl (2011) 2014;4(1):70–74. doi: 10.1038/kisup.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.