Abstract
Background
Development of new aminoacyl-tRNA synthetase (aaRS)•tRNA pairs is central for incorporation of novel non-canonical amino acids (ncAAs) into proteins via genetic code expansion (GCE). The Escherichia coli and Caulobacter crescentus histidyl-tRNA synthetases (HisRS) evolved divergent mechanisms of tRNAHis recognition that prevent their cross-reactivity. Although the E. coli HisRS•tRNAHis pair is a good candidate for GCE, its use in C. crescentus is limited by the lack of established genetic selection methods and by the low transformation efficiency of C. crescentus.
Methods
E. coli was genetically engineered to use a C. crescentus HisRS•tRNAHis pair. Super-folder green fluorescent protein (sfGFP) and chloramphenicol acetyltransferase (CAT) were used as reporters for read-through assays. A library of 313 ncAAs coupled with the sfGFP reporter system was employed to investigate the specificity of E. coli HisRS in vivo.
Results
A genomically modified E. coli strain (named MEOV1) was created. MEVO1 requires an active C. crescentus HisRS•tRNAHis pair for growth, and displays a similar doubling time as the parental E. coli strain. sfGFP- and CAT-based assays showed that the E. coli HisRS•tRNAHis pair is orthogonal in MEOV1 cells. A mutation in the anticodon loop of E. coli tRNAHisCUA elevated its suppression efficiency by 2-fold.
Conclusions
The C. crescentus HisRS•tRNAHis pair functionally complements an E. coli ΔhisS strain. The E. coli HisRS•tRNAHis is orthogonal in MEOV1 cells. E. coli tRNAHisCUA is an efficient amber suppressor in MEOV1.
General significance
We developed a platform that allows protein engineering of E. coli HisRS that should facilitate GCE in E. coli.
Keywords: Genetic code expansion, Orthogonal pair, Non-canonical amino acids, Aminoacyl-tRNA synthetase, tRNA, Synthetic biology
1. Introduction
For over a decade, genetic code expansion (GCE) strategies have been used to incorporate a wide range of non-canonical amino acids (ncAAs) into proteins in living cells, making this technology a powerful tool for investigation of protein structure and function [1]. Incorporation of ncAAs via GCE relies on the reassignment of a codon (a stop codon is typically used) mediated by an aaRS•tRNA pair that functions to provide the ncAA-tRNA for ribosomal protein synthesis. The aaRS•tRNA pairs used in GCE must be orthogonal to the host organism; that is, the aaRS should exclusively interact with its cognate tRNA, and the tRNA should not be a substrate for the host’s aaRSs. To fulfil this requisite for GCE, most aaRS•tRNA pairs used in model organisms (e.g., E. coli, mouse, or human) are derived from organisms belonging to a different domain of life. Additionally, aaRSs with weak or no tRNA anticodon specificity are most attractive for GCE applications [2].
Tyrosyl- and pyrrolysyl-tRNA synthetase (TyrRS and PylRS) from Methanocaldococcus jannaschii and Methanosarcina barkeri/mazei, respectively, are the most commonly used aaRS•tRNA pairs in GCE as they and their engineered variants account for incorporation of more than 150 ncAAs [1, 3, 4]. However, despite their profound utility, the discovery and development of new aaRS•tRNA pairs remains imperative for GCE for two reasons [3]. First, new aaRS•tRNA pairs should expand the diversity of ncAAs in GCE. The scope of ncAAs incorporated by TyrRS and PylRS is biased towards aromatic, bulky, and large amino acids, in line with the their large amino acid binding pockets, limiting the repertoire of ncAAs they can be used to incorporate. Second, a set of diverse aaRS•tRNA pairs will be essential for the development of platforms to incorporate multiple ncAAs into a single polypeptide [5, 6].
Recently, histidyl-tRNA synthetases (HisRS) from Caulobacter crescentus and Escherichia coli were reported to be orthogonal to each other in vitro [7] and in vivo [8]. In E. coli, an unusual G-1 and the C73 base are identity elements for acylation by HisRS [9], whereas the C. crescentus tRNAHis lacks G-1 and contains A73, a base not recognized by C. crescentus HisRS [7]. Instead, C. crescentus HisRS recognizes an extended D-loop, U72, and the anticodon bases (GUG) as main identity elements for histidylation [7]. Thus, the introduction of E. coli HisRS and E. coli amber suppressor tRNAHisCUA enabled amber suppression in C. crescentus [8]. Despite this achievement it was clear that further development of the orthogonal E. coli pair was limited in Caulobacter, as this organism’s molecular genetic versatility is not as well understood as that of E. coli. Thus, we explored an alternative strategy to exploit E. coli HisRS•tRNAHis for GCE. We planned to switch E. coli’s housekeeping histidylation function from the E. coli HisRS•tRNAHis pair to that of C. crescentus in a genomically modified E. coli strain in which the original E. coli HisRS paired with its cognate amber suppressor tRNAHis would serve as an orthogonal histidine translational system.
2. Materials and Methods
2.1. Reagents and Strains
Restriction enzymes, T4 DNA ligase, and the Gibson Assembly mix were obtained from New England Biolabs. All amino acids were purchased from Sigma-Aldrich, Chem-Impex and Bachem. The Keio collection E. coli His-auxotroph strain JW2004-1 (ΔhisB270) was obtained from the Coli Genetic Stock Center (Yale University).
2.2. Plasmid construction
The C. crescentus histidyl-tRNA synthetase (hisS gene) and tRNAHis (hisT gene) expression cassette (CchisST) was constructed by first cloning hisT into the EcoRI and BamHI sites of the plasmid pGFib under the lpp promoter and rrnC terminator, and hisS (codon-optimized for expression in E. coli) under the constitutive promoter glnS into the NdeI and HindIII sites of the plasmid pGLN (Supplementary Information). The lpp.CchisT.rrnC fragment was then PCR-amplified from CchisT.pGFib, digested with NotI, and cloned into CchisS.pGLN. The resulting CcHisRS-tRNAHis expression cassette was later cloned into the pUC18 and pKD13 vectors to generate CchisST.pUC18 and CchisST.pKD13, respectively, using Gibson assembly. The T7 promoter of the multiple cloning sites of pRSFDuet1 and pCDFDuet1 (Novagen) were replaced with the tac promoter using inverse PCR resulting in tac.pRSF and tac.pCDF. Additionally, the lac repressor and the T7 promoter of pCDFDuet1 were replaced with the constitutive lpp promoter (lpp.pCDF). WTsfGFP was amplified and cloned into tac.pCDF. The E. coli tRNAHisCUA gene (EchisRam) was cloned into the EcoRI and BamHI sites of pGFib. The lpp.EchisRam.rrnC cassette from EchisRam.pGFib was cloned downstream of the T7 terminator of WTsfGFP.tac.pCDF. EchisRam was also cloned into the pACYC184-derived plasmid pCAT [10], which encodes the chloramphenicol acetyl-transferase gene (cat) with an amber stop codon at position 112. The E. coli HisRS gene (EchisS) was amplified from EchisS.pCA24N [11] and cloned into plasmids tac.pRSF and lpp.pCDF. EchisRam was cloned into pTECH to generate EchisRam.pTECH. Construction of sfGFP2TAG.pBAD was previously described [12]. Site-directed mutagenesis was carried out using the QuikChange kit from Agilent. A list of all plasmid used is shown in Supplementary Table 1.
2.3. E. coli MEOV1 strain construction
The E. coli MEOV0.1 strain was constructed by integrating the CchisST expression cassette into the chromosome of E. coli TOP10 cells using the λ-Red recombination system from the plasmid pKD46 as described by Datsenko and Wanner [13]. The CchisST cassette was integrated at the yohC I yohD locus together with a kanamycin (Kan) marker from pKD13 [13]. The Kan marker was later removed using the FLP recombinase from the plasmid pCP20. The Integration of CchisST and removal of the Kan marker was confirmed by PCR amplification of the corresponding genomic regions. Next, the plasmid CchisST.pUC18 was introduced into the resulting E. coli strain, TOP10.CchisST (or MEOV0.1), followed by transformation of the plasmid pSIM5 [14]. Chromosomal deletion of the EchisS from the E. coli MEOV0.1 strain was mediated by the λ-Red recombination system encoded in pSIM5 following a previously published protocol [14]. Briefly, MEOV0.1 cells with CchisST.pUC18 and pSIM5 were grown at 30 °C to an optical density at 600 nm (OD600) of 0.6, shifted to 42 °C for 20 min and the cells were made electrocompetent. MEOV0.1 cells were transformed with an EchisS knockout cassette amplified from pKD13 containing 50 base-pair homologous regions flanking the EchisS gene, targeting the genomic hisS region. After successful knock out of Ec HisRS, the Kan resistance marker was removed using pCP20. The resulting strain, TOP10.CchisST.ΔhisS, was named MEOV0.2. Finally, a mutagenic cassette targeting the only chromosomal copy of tRNAHis (hisR) in E. coli was used to mutate its anticodon sequence from GTG to CTA. The tRNAHis mutagenic cassette was fused to the Kan resistance cassette of pKD13 via overlapping PCR using three PCR fragments constructed as follows. PCR fragments 1 and 2 were amplified from E. coli genomic DNA and with primers (HISR_KFN and hisR.CTA_QR and hisR.CTA_QF and HISR_CAS_R, respectively, Supplementary Table 2) introducing the hisR anticodon change from GTG to CTA. Both fragments were digested with DpnI to remove residual template DNA and gel-purified. Fragment 3 was amplified from the plasmid pKD13 with primers PKD13.F2 and HISR_KRN. All 3 fragments were mixed and used for overlapping PCR with HISR_KFN and HISR_KRN to produce the mutagenic knock-in cassette. MEOV0.2 cells with pSIM5 were made λ-Red- and electrocompetent as described above, and transformed with the mutagenic knock-in cassette. Colony PCR was used to screen for the chromosomal GTG-to-CTA mutation indicated by a PCR product using the primers HISR_KFN and hisR.CTA_SCR. After pCP20-mediated removal of the Kan marker, the GTG to CTA mutation in hisR was verified by DNA sequencing. This strain was named MEOV1.
2.4. MEOV1 cells growth rate
Single colonies for MEOV1 and TOP10 cells were grown overnight at 37 °C in LB media without antibiotics. The overnight cultures were then diluted in LB media to an OD600 of ~0.01. 125 μL of the diluted cultures were transferred to a 96-well assay plate (Corning) and cell growth was monitored for 24 h at 37 °C in a Synergy HT plate reader (BioTek) without antibiotics. The final growth curves are the average of three independent experiments using three different colonies.
2.5. sfGFP reporter assays
MEOV1 cells harboring the plasmid EchisS.tac.pRSF were transformed with sfGFP2TAG.EchisRam.tac.pCDF, WTsfGFP.EchisRam.tac.pCDF, WTsfGFP.tac.pCDF, or sfGFP2TAG.tac.pCDF. Single colonies were grown overnight in LB medium with the corresponding antibiotics at 37 °C. Overnight cultures were diluted in LB media to an OD600 of ~0.01, and 125 μL of the cultures were then transferred to a 96-well assay plate. Protein expression was induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside. Additionally, 3 μL of the overnight cell cultures were spotted on LB-agar plates containing antibiotics and isopropyl β-D-1-thiogalactopyranoside. The plates were incubated for 24 h at 37 °C. Cell growth and sfGFP fluorescence (λex = 485 nm and λem = 528 nm) were monitored for 24 h at 37 °C in a Synergy HT plate reader (BioTek). Relative fluorescence was calculated by dividing the fluorescence arbitrary unit (a.u.) by the OD600 at a specific time point. The bars and standard deviations are the average of three assays from three distinct colonies.
2.7. sfGFP purification
0.5 ml of an overnight culture of MEOV1 cells expressing sfGFP2TAG, E. coli tRNAHis and HisRS was used to inoculate 1 L of LB media containing the appropriate antibiotics. Cells were grown to an A600 of 0.6 at 37 °C. sfGFP overexpression was induced with 0.1 mM IPTG for ~14 h at 25 °C. Cells were harvested and lysed with buffer containing 50 mM Tris (pH 8), 300 mM NaCl and 10 mM imidazole and sonication. Lysed cells were centrifuged at 11000 rpm for 35 min at 4 °C, and the supernatant was loaded on 1 mL of TALON (Takara) metal affinity resin. The resin was washed with lysis buffer and the protein was eluted with 250 mM imidazole.
2.6. Chloramphenicol resistance assay
MEOV1 cells were co-transformed with EchisRam.pCAT and EchisS.lpp.pCDF. Colonies were then grown overnight in 3 ml LB medium supplemented with the corresponding antibiotics at 37°C. Serial dilutions of the overnight cultures were prepared with OD600 of 0.1, 0.01, and 0.001 with fresh LB medium. 2 μL of the diluted cells were spotted on LB-agar plates with 100 μg/ml ampicillin, 10 μg/ml tetracycline, 100 μg/ml spectinomycin, and varying concentration of chloramphenicol. The plates were incubated at 37°C for 48 hours. Negative controls were carried out using the empty pCDFDuet1.
2.8. Amino acid library screening
The His-auxotrophic E. coli strain JW2004-1 (hisB−) from the Keio collection were co-transformed with the plasmids sfGFP2TAG.pBAD and EchisRam.pTECH [12, 15]. Cells were grown overnight at 37°C in LB medium with the corresponding antibiotics (kanamycin, ampicillin, and chloramphenicol). The cells were pelleted and washed three times with 1x M9 minimal salts (BD), and finally resuspended with M9 minimal media (1x M9 minimal salts, 0.1 mM CaCl2, 1mM MgSO4, and 1% glycerol), the appropriate antibiotics, and 0.2% arabinose. 50-μL aliquots of cell suspension were transferred to a 384-well plate containing 1 mM of different amino acids in each well [12]. Cell growth and sfGFP expression were monitored by measuring OD600 and fluorescence intensity, respectively, in a Synergy HT plate reader for 12 h at 37°C.
2.9. In vitro aminoacylation assays
E. coli tRNAHis was in vitro transcribed, purified, and 32P-labeled following previously described protocols [16]. E. coli HisRS was expressed and purified as previously described [17]. Aminoacylation reactions were performed using trace amounts of 32P-labeled tRNAHis with 2 μM unlabeled tRNAHis and 5 mM amino acid in buffer containing 50 mM HEPES (pH 7.5), 4 mM ATP, 10 mM MgCl2, 0.1 mg/mL BSA, and 1 mM Dithiothreitol. Reactions were initiated by addition of 50 nM E. coli HisRS. Time points were taken by quenching 2 μL of the reaction mixture into 5 μL of a solution containing 0.4 units/μL nuclease P1 (Sigma) in 200 mM sodium acetate (pH 5). 1 μL of quenched solution was run on polyethyleneimine-cellulose TLC plates to separate the aminoacyl-[32P]AMP and [32P]AMP products.
3. Results and discussion
3.1. Functional replacement of E. coli His-tRNAHis biosynthesis
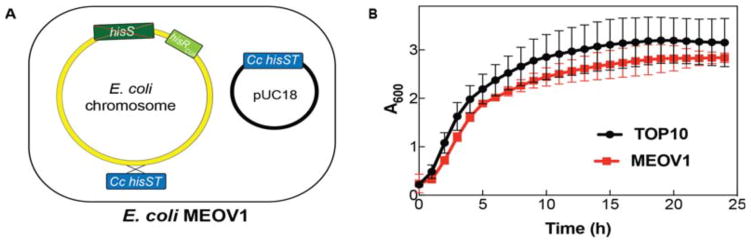
HisRS•tRNAHis pairs from E. coli and C. crescentus have orthogonal mechanisms of tRNA recognition that has been demonstrated in vitro and in vivo [7, 8]. A key distinction between E. coli and C. crescentus HisRS•tRNA recognition is the strong specificity of C. crescentus HisRS for the anticodon sequence GUG of tRNAHis [7]. This property of C. crescentus HisRS renders the enzyme unfit for use in genetic code expansion as it fails to aminoacylate C. crescentus tRNAHis with an altered anticodon [7]. In contrast, converting E. coli tRNAHis to an amber suppressor is well tolerated by E. coli HisRS, making it a more suitable candidate for the development of an E. coli HisRS•tRNA pair for GCE [8, 18]. We sought to genetically modify E. coli to free E. coli HisRS from its essential role in translation by functionally replacing it with C. crescentus HisRS•tRNAHis. To accomplish this, we first integrated a copy of C. crescentus HisRS and tRNAHis under constitutive promoters (glsS and lpp, respectively) into a permissive site of the chromosome of E. coli TOP10 using a λ-Red recombination system to generate the E. coli MEOV0.1 strain. However, attempts to delete the E. coli HisRS genomic copy failed. A possible explanation is that the expression level of the C. crescentus HisRS•tRNAHis pair was not sufficient to sustain deletion of E. coli hisS. To overcome this, we introduced the C. crescentus HisRS•tRNAHis expression cassette in the high-copy plasmid pUC18, and using λRed recombination, E. coli HisRS was deleted in E. coli MEOV0.1 to create E. coli MEOV0.2. We then tried to remove the single gene (hisR) encoding tRNAHis in E. coli, which is found in an operon with three other tRNA genes (argX, leuT and proM). We reasoned that deleting hisR may disrupt the expression of the entire locus, potentially leading to unintended consequences. To avoid this, E. coli hisR was converted to encode an amber suppressor tRNAHis by mutating the anticodon sequence (GTG to CTA), which produced the E. coli MEOV1 strain that exclusively relies on the activity of C. crescentus HisRS•tRNAHis for growth (Figure 1A).
Figure 1.
Design and growth rate of E. coli MEOV1. A. Schematic representation of MEOV1 cells. A copy of the C. crescentus HisRS and tRNAHis expression cassette (shown in the blue box as Cc hisST) was introduced into the E. coli TOP10 chromosome to give MEOV0.1. The high-copy plasmid pUC18 harboring a copy of the C. crescentus HisRS and tRNAHis expression cassette was transformed into MEOV0.1 followed by deletion of the E. coli HisRS gene (hisS, shown in dark green box) from the chromosome and mutagenesis of anticodon (GUG to CUA) of the E. coli tRNAHis gene (hisR, show in in light green box). B. Growth curves of E. coli TOP10 (black circles) and MEOV1 (red squares).
3.2. Cell growth evaluation of MEOV1
A previous attempt to functionally replace an endogenous E. coli aaRS•tRNA pair with an orthogonal pair produced an E. coli strain with a severe growth defect [19]. Therefore, to ensure that the MEOV1 strain is a viable system for GCE applications, we compared its growth to that of the parental TOP10 strain. MEOV1 and TOP10 cells showed very similar doubling times (Figure 1B), indicating the ability of the C. crescentus HisRS•tRNAHis to effectively replace the E. coli pair in the engineered MEOV1 strain.
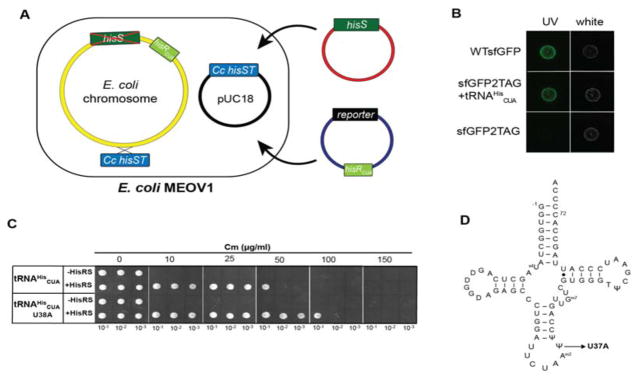
3.3. Probing the orthogonality of the Ec HisRS•tRNAHis pair in MEOV1 cells
To evaluate the orthogonality of E. coli HisRS•tRNAHis in the MEOV1 strain, we monitored the suppression of an amber codon at position 2 of super-folder green fluorescence protein (sfGFP) mRNA by E. coli tRNAHisCUA. Because MEOV1 encodes a copy of E. coli hisR with amber anticodon (tRNAHisCUA), we wondered whether its background expression was sufficient to promote suppression of the amber codon in sfGFP (sfGFP2TAG). Therefore, a plasmid harboring sfGFP2TAG was introduced into MEOV1 together with a plasmid containing E. coli HisRS (Figure 2A). Surprisingly, induction of sfGFP2TAG and E. coli HisRS showed no expression of sfGFP was observed (Figure 2B), indicating that the expression of the chromosomal E. coli hisRam (tRNAHisCUA) gene in MEOV1 is not sufficient to promote suppression of the amber codon in sfGFP. To probe this hypothesis, a copy E. coli hisRam under the constitutive promoter lpp was cloned into the plasmid containing sfGFP2TAG, and the resulting plasmid was introduced into MEVO1 cells together with E. coli HisRS. Expression of tRNAHisCUA under the lpp promoter together with E. coli HisRS resulted in expression of sfGFP (Figure 2B). In addition to sfGFP, we used a chloramphenicol (Cm)-resistance-based assay to confirm the orthogonality of the E. coli HisRS•tRNAHis pair. In the chloramphenicol acetyltransferase (cat) gene of pACYC184, an amber stop codon at the permissive site 112 was introduced by site-directed mutagenesis, and the E. coli tRNAHisCUA expression cassette was cloned into the same plasmid. MEOV1 cells expressing E. coli HisRS and tRNAHisCUA grew to a Cm concentration of 50 μg/mL (Figure 2C), while cells expressing only tRNAHisCUA did not survive even in low concentration of Cm. Together, these results demonstrate that the E. coli HisRS•tRNAHisCUA pair is orthogonal in E. coli MEOV cells.
Figure 2.
Reintroducing E. coli HisRS•tRNAHis as an orthogonal pair in MEOV1 cells. A. E. coli tRNAHisCUA was introduced in the same plasmid with sfGFP (reporter), while E. coli HisRS was introduced in a separate plasmid. B. MEOV1 cells harboring E. coli HisRS and either wild-type (WT) or sfGFP with a TAG codon at position 2. Cells were grown overnight and spotted on LB-agar. The plate was incubated overnight at 37 °C. “UV” indicates sfGFP fluorescence, whereas “white” corresponds to the growth on the plate visualized with white light. C. MEOV1 cells harboring a plasmid encoding the cat gene with an amber codon at position 112 were transformed with a plasmid with E. coli HisRS. Serial dilutions (OD600 0.1, 0.01, and 0.001) of overnight grown cell cultures were spotted on LB/agar plates with different concentration of chloramphenicol (Cm). D. Cloverleaf structure of E. coli tRNAHis. The arrow indicates position 37, which was mutated to adenosine.
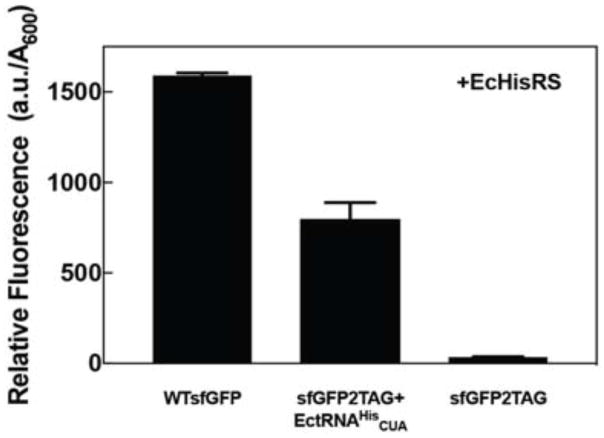
3.4. Suppression efficiency of E. coli HisRS•tRNAHisCUA
Previously reported suppression efficiencies mediated by the E. coli HisRS•tRNAHisCUA pair vary from 4% in C. crescentus [8] to 26–41% in E. coli [18]. To assess the E. coli HisRS•tRNAHisCUA efficiency in MEOV cells, we compared the florescence intensity from MEOV1 cells expressing wild-type sfGFP to cells expressing sfGFP2TAG in the presence of the E. coli HisRS•tRNAHisCUA pair (Figure 2A). Our results show that E. coli HisRS•tRNAHisCUA suppresses the amber codon in sfGFP with 50% efficiency relative to wild type (Figure 3), producing up to 7.5 mg of sfGFP per liter of MEOV1 culture. Additionally, we investigated the possibility of using E. coli tRNAHis as an opal (UCA) or ochre (UUA) suppressor. Using sfGFP with either TGA or TAA at position 2, we found that E. coli tRNAHisUUA suppresses an ochre codon with 33% efficiency relative to WTsfGFP fluorescence (Table 1). In contrast, tRNAHisUCA only poorly suppressed the opal codon (~6% efficiency). Based on these results, it appears that anticodon base U34 plays an important role in tRNAHis recognition by E. coli HisRS. Moreover, the anticodon changes did not affect the orthogonality of E. coli tRNAHis in MEOV1 as sfGFP fluorescence was only observed when both HisRS and the corresponding tRNAHis suppressor were expressed. However, while we observed high suppression efficiency by the E. coli HisRS•tRNA pair in the sfGFP experiments (Figure 3), the suppression efficiency appeared to be weaker in the chloramphenicol-based assays (Figure 2C). We sought to improve the suppression efficiency by introducing a mutation in the anticodon loop (U38 to A) of tRNAHis, which was previously reported to increase its suppression efficiency by up to 20 fold [20]. We introduced this mutation in E. coli tRNAHisCUA and found that cells with the mutant tRNA were able to grow to chloramphenicol concentrations two times higher than the “wild-type” tRNAHisCUA (up to 100 μg/mL). Overall, these results show that E. coli HisRS•tRNAHisCUA is an effective pair for stop codon suppression, although its suppression effectiveness varies depending on the targeted stop codon. However, low suppression efficiency can be countered by introducing a mutation in the anticodon loop of tRNAHis.
Figure 3.
MEOV1 cells harboring E. coli HisRS and either WT or sfGFP with an amber codon at position 2 (sfGFP2TAG) were grown overnight, diluted to an OD600 of 0.01, and grown for 24 h at 37 °C. Cell grow and fluorescence were monitored using a plate reader.
Table 1.
Suppression efficiency of amber (UAG), ochre (UAA), and opal (UGA) codons at position 2 of sfGFP by the E. coli HisRS and tRNAHis with the corresponding anticodon sequence.
| tRNAHisXXX | sfGFP2XXX | Suppression efficiency (%)* |
|---|---|---|
| GUG | CAC | - |
| CUA | UAG | 44.3 ± 11 |
| UUA | UAA | 33.1 ± 2.5 |
| UCA | UGA | 5.9 ± 2.6 |
Suppression efficiency is relative to wild-type sfGFP. The percentage values are the result of three independent assays, and the standard deviation is shown.
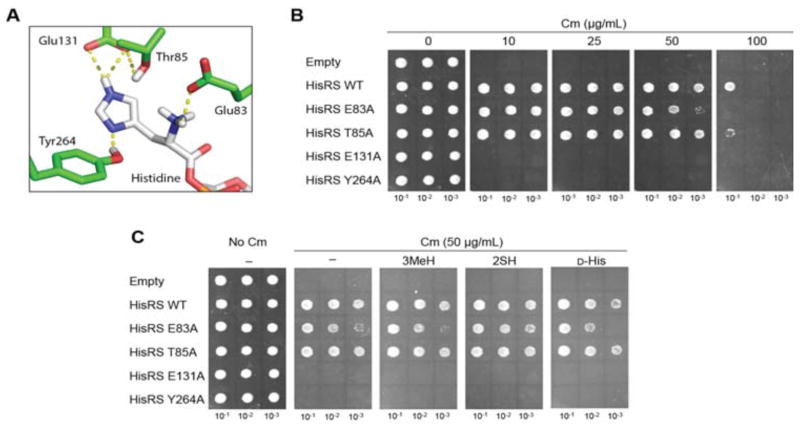
3.5. Dissecting the E. coli HisRS active site
After demonstrating that the E. coli HisRS•tRNAHis pair is orthogonal in MEOV1 cells, it can now be subjected to directed evolution methods to potentially generate novel HisRS variants with desired amino acid specificities. This process requires the randomization of HisRS residues involved in amino acid binding. Although there is a wealth of structural and biochemical information regarding the active site of HisRS that can be used to target specific residues, most of the data was obtained using in vitro experiments [21, 22]. Thus, we decided to explore the contribution of different E. coli HisRS residues in vivo using the Cm-based reporter assay. An analysis of the crystal structure of E. coli HisRS bound to His revealed a series of residues that has been predicted to be involved in binding (Figure 4A) [21]. E83 forms a salt bridge with α-amino group of His, and mutating the residue to Ala resulted in a 4500-fold decrease in the aminoacylation rate in vitro [22]. However, in vivo, the E83A HisRS mutant only showed a small growth defect in MEOV cells (Figure 4B). T85 helps to maintain the position of E131 through hydrogen bonds. However, its role aminoacylation seems dispensable since a T85A HisRS mutant grew as well as WT HisRS (Figure 4B). In accordance with previous work showing that E131 and Y264 determined the specificity of the His imidazole ring, we found that E131A and Y264A HisRS mutants were inactive (Figure 4B). Intrigued by these results, we decided to investigate whether these mutations in HisRS have an impact in its amino acid specificity. To test this hypothesis, the Cm-based assays was used in the presence of the His analogs: 2-thio-His (2SH), D-His, and 3-methyl-His (3MeH) (Figures 4C and 5A). Interestingly, neither E131 nor Y264 HisRS were able to support growth on Cm (Figure 4C), suggesting that these residues are essential for enzymatic activity of HisRS.
Figure 4.
Mutational analysis of E. coli HisRS active site residues. A. The structure of E. coli HisRS active site with Histidine (PDB: 1KMM). The HisRS residues that interact with the imidazole ring and amino group of His are shown. These residues were mutated to Ala. B. In vivo activity of E. coli HisRS mutants using the Cm-based assay as described for Figure 2C. C. Substrate specificity of HisRS mutants. Cm-based was used to explore potential changes in the substrate specificity of HisRS upon mutations. 1 mM 3mHis, 1 mM 2SH and 2 mM D-His were used. “Empty” and “–“ indicates plasmid without HisRS and no amino acid added, respectively.
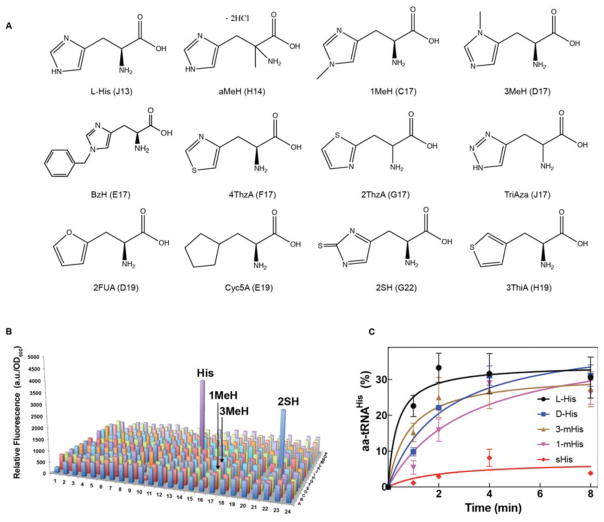
Figure 5.
E. coli HisRS substrate specificity in vitro and in vivo. A. Molecular structure of histidine analogs. aMeH (DL-α-methyl-His), 1MeH (1-methyl-His), 3MeH (3-methyl-His), BzH (Benzyl-L-His), 4ThzA (3-[4-Thiazolyl]-L-alanine), 2ThzA (2-[Thiazolyl]-L-alanine), TriAzA (1,2,4-Triazole-alanine), 2FUA (2-Furyl)-L-alanine), Cyc5A (3-Cyclopentane-L-alanine), 2SH (2-Thio-L-His), and 3ThiA (3-[3-Thienyl]-DL-alanine). The letter and number on each amino acid indicate the position in the 384-well plate. B. E. coli HisRS substrate specificity profile. 313 distinct amino acids (1 mM) were distributed in a 384-well plate. 50 μL of a His-auxotrophic E. coli strain JW2004-1 (hisB−) culture harboring sfGFP2TAG and E. coli tRNAHisCUA in minimal medium were added to each well and the plate was incubated at 37 °C with constant shaking. Cell growth and fluorescence were monitored and used to estimate relative fluorescence after 24 h. C. In vitro aminoacylation assays were carried out with E. coli HisRS and tRNAHis at 37 °C with 1 mM amino acid concentration. Each time point represents the average of three independent trials with the corresponding standard deviation shown.
3.6. Substrate selectivity of E. coli WT HisRS
To gain more insights into the amino acid substrate specificity of E. coli HisRS, we adapted a recently established amino acid screen that allows high-throughput assessment of aaRS specificity in vivo [12, 15]. This approach is based on the suppression of an amber codon in sfGFP by plasmid-encoded E. coli tRNAHisCUA and endogenously expressed HisRS in a His-auxotrophic E. coli strain grown in minimal media in the presence of a particular amino acid. The library consists of 313 amino acids, including canonical AAs and several His analogs, distributed in a 384-well plate (Figure 5A, Supplementary Information) [12]. To validate this experimental setup, we tested the expression of WT sfGFP and E. coli tRNAHis. As expected, the strongest fluorescence signal was observed from cells grown with His (Supplementary Figure 1). Only one additional florescence peak appeared when cells expressed sfGFP2TAG and tRNAHisCUA (Figure 5B), which originated from cells grown with 2SH. These results suggest that E. coli HisRS has a stringent amino acid selection mechanism (Figure 5B). However, our in vivo results contrast with previous reports that indicated that other His analogs present in our library are substrates of HisRS [23–25]. Among those His analogs are 1-methyl- (1MeH) and 3-methyl-His. To corroborate the results from the amino acid library, we carried out in vitro aminoacylation assays. Interestingly, in contrast to the in vivo results, purified E. coli HisRS aminoacylated tRNAHis with both 1MeH and 3MeH with similar efficiency as cognate His (Figure 5C). This discrepancy between in vivo and in vitro experiments might be explained by how well E. coli cells can tolerate widespread incorporation of these analogs into the proteome.
4. Conclusions
In this work, we were able to delete the essential hisS (HisRS) gene from E. coli by functionally replacing its activity with that of C. crescentus HisRS•tRNAHis. In addition, we showed that the E. coli HisRS•tRNAHis pair is orthogonal in MEOV1 cells demonstrating that MEOV1 can serve as a platform for the development of the E. coli HisRS•tRNAHis pair for genetic code expansion applications. Construction of E. coli HisRS libraries are being developed, which will be subjected to multiple rounds of selection and screening aimed at finding HisRS variants with desired ncAA-specificity. A drawback of our engineering system is that the resulting E. coli HisRS variants may only be used in a limited number of organisms (C. crescentus and MEOV1), due to the frequent occurrence of organisms encoding G-1-containing tRNAHis genes. To counter this limitation, the tRNAHis substrate specificity of E. coli HisRS could be altered, which has been an useful approach in the development of other aaRS•tRNA pairs [26].
Highlights.
The C. crescentus HisRS•tRNAHis pair functionally complements an E. coli ΔhisS strain.
The E. coli HisRS•tRNAHis pair is orthogonal and mediates suppression of UAG stop codons in E. coli MEOV1 cells.
E. coli HisRS active site residues essential for in vivo activity were identified.
Acknowledgments
We are grateful to Drs. Takahito Mukai and Ana Crnković for enlightening discussions. This work was supported by a grant from the National Institute of General Medical Sciences [GM22854 to DS].
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chin JW. Expanding and reprogramming the genetic code of cells and animals. Annu Rev Biochem. 2014;83:379–408. doi: 10.1146/annurev-biochem-060713-035737. [DOI] [PubMed] [Google Scholar]
- 2.O’Donoghue P, Ling J, Wang Y-S, Söll D. Upgrading protein synthesis for synthetic biology. Nature chemical biology. 2013;9:594–598. doi: 10.1038/nchembio.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumas A, Lercher L, Spicer CD, Davis BG. Designing logical codon reassignment–Expanding the chemistry in biology. Chem Sci. 2015;6:50–69. doi: 10.1039/c4sc01534g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan W, Tharp JM, Liu WR. Pyrrolysyl-tRNA synthetase: An ordinary enzyme but an outstanding genetic code expansion tool. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2014;1844:1059–1070. doi: 10.1016/j.bbapap.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Fredens J, Brunner SF, Kim SH, Chia T, Chin JW. Defining synonymous codon compression schemes by genome recoding. Nature. 2016;539:59–64. doi: 10.1038/nature20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrov N, Landon M, Guell M, Kuznetsov G, Teramoto J, Cervantes N, Zhou M, Singh K, Napolitano MG, Moosburner M, Shrock E, Pruitt BW, Conway N, Goodman DB, Gardner CL, Tyree G, Gonzales A, Wanner BL, Norville JE, Lajoie MJ, Church GM. Design, synthesis, and testing toward a 57-codon genome. Science. 2016;353:819–822. doi: 10.1126/science.aaf3639. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Gogakos T, Babina AM, Soll D, Randau L. Change of tRNA identity leads to a divergent orthogonal histidyl-tRNA synthetase/tRNAHis pair. Nucleic Acids Res. 2011;39:2286–2293. doi: 10.1093/nar/gkq1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko JH, Llopis PM, Heinritz J, Jacobs-Wagner C, Soll D. Suppression of amber codons in Caulobacter crescentus by the orthogonal Escherichia coli histidyl-tRNA synthetase/tRNAHis pair. PLoS One. 2013;8:e83630. doi: 10.1371/journal.pone.0083630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Himeno H, Hasegawa T, Ueda T, Watanabe K, Miura K, Shimizu M. Role of the extra G-C pair at the end of the acceptor stem of tRNAHis in aminoacylation. Nucleic acids research. 1989;17:7855–7863. doi: 10.1093/nar/17.19.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umehara T, Kim J, Lee S, Guo LT, Soll D, Park HS. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 2012;586:729–733. doi: 10.1016/j.febslet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 12.Fan C, Ho JM, Chirathivat N, Soll D, Wang YS. Exploring the substrate range of wild-type aminoacyl-tRNA synthetases. Chembiochem. 2014;15:1805–1809. doi: 10.1002/cbic.201402083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta S, Costantino N, Court DL. A set of recombineering plasmids for gram-negative bacteria. Gene. 2006;379:109–115. doi: 10.1016/j.gene.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Ko J-h, Wang Y-S, Nakamura A, Guo L-T, Söll D, Umehara T. Pyrrolysyl-tRNA synthetase variants reveal ancestral aminoacylation function. FEBS Lett. 2013;587:3243–3248. doi: 10.1016/j.febslet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das M, Vargas-Rodriguez O, Goto Y, Suga H, Musier-Forsyth K. Distinct tRNA recognition strategies used by a homologous family of editing domains prevent mistranslation. Nucleic Acids Res. 2014;42:3943–3953. doi: 10.1093/nar/gkt1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth EC, Francklyn CS. Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Mol Cell. 2007;25:531–542. doi: 10.1016/j.molcel.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan W, Francklyn C. Cytosine 73 is a discriminator nucleotide in vivo for histidyl-tRNA in Escherichia coli. J Biol Chem. 1994;269:10022–10027. [PubMed] [Google Scholar]
- 19.Iraha F, Oki K, Kobayashi T, Ohno S, Yokogawa T, Nishikawa K, Yokoyama S, Sakamoto K. Functional replacement of the endogenous tyrosyl-tRNA synthetase–tRNATyr pair by the archaeal tyrosine pair in Escherichia coli for genetic code expansion. Nucleic Acids Res. 2010;38:3682–3691. doi: 10.1093/nar/gkq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleina LG, Masson JM, Normanly J, Abelson J, Miller JH. Construction of Escherichia coli amber suppressor tRNA genes. II. Synthesis of additional tRNA genes and improvement of suppressor efficiency. J Mol Biol. 1990;213:705–717. doi: 10.1016/S0022-2836(05)80257-8. [DOI] [PubMed] [Google Scholar]
- 21.Arnez JG, Augustine JG, Moras D, Francklyn CS. The first step of aminoacylation at the atomic level in histidyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1997;94:7144–7149. doi: 10.1073/pnas.94.14.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guth E, Connolly SH, Bovee M, Francklyn CS. A substrate-assisted concerted mechanism for aminoacylation by a class II aminoacyl-tRNA synthetase. Biochemistry. 2005;44:3785–3794. doi: 10.1021/bi047923h. [DOI] [PubMed] [Google Scholar]
- 23.Freist W, Verhey JF, Ruhlmann A, Gauss DH, Arnez JG. Histidyl-tRNA synthetase. Biol Chem. 1999;380:623–646. doi: 10.1515/BC.1999.079. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Kawahara Si, Taki M, Kuno A, Hasegawa T, Taira K. Synthesis of a novel histidine analogue and its efficient incorporation into a protein in vivo. Protein eng. 2003;16:699–706. doi: 10.1093/protein/gzg084. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger S, Schlesinger MJ. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12 : I. Substitution of triazolealanine for histidine. J Biol Chem. 1967;242:3369–3372. [PubMed] [Google Scholar]
- 26.Chatterjee A, Xiao H, Schultz PG. Evolution of multiple, mutually orthogonal prolyl-tRNA synthetase/tRNA pairs for unnatural amino acid mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 2012;109:14841–14846. doi: 10.1073/pnas.1212454109. [DOI] [PMC free article] [PubMed] [Google Scholar]