Abstract
Nearly 60% of patients with head and neck squamous cell carcinoma (HNSCC) die of metastases or locoregional recurrence. Metastasis is mediated by cancer cell migration and invasion, which are in part dependent on extracellular matrix degradation by matrix metalloproteinases. Osteoactivin (OA) overexpression plays a role in metastases in several malignancies, and has been shown to upregulate matrix metalloproteinase (MMP) expression and activity.
Objectives
To determine how OA modulates MMP expression and activity in HNSCC, and to investigate OA effects on cell invasion.
Materials and Methods
We assessed effects of OA treatment on MMP mRNA and protein expression, as well as gelatinase and caseinolytic activity in HNSCC cell lines. We assessed the effects of OA gene silencing on MMP expression, gelatinase and caseinolytic activity, and cell invasion.
Results
OA treatment had differential effects on MMP mRNA expression. OA treatment upregulated MMP-10 expression in UMSCC14a (p=0.0431) and SCC15 (p<0.0001) cells, but decreased MMP-9 expression in UMSCC14a cells (p=0.0002). OA gene silencing decreased MMP-10 expression in UMSCC12 cells (p=0.0001), and MMP-3 (p=0.0005) and -9 (p=0.0036) expression in SCC25 cells. In SCC15 and SCC25 cells, OA treatment increased MMP-2 (p=0.0408) and MMP-9 gelatinase activity (p<0.0001), respectively. OA depletion decreased MMP-2 (p=0.0023) and -9 (p<0.0001) activity in SCC25 cells. OA treatment increased 70 kDa caseinolytic activity in UMSCC12 cells consistent with tissue type plasminogen activator (p=0.0078). OA depletion decreased invasive capacity of UMSCC12 cells (p<0.0001).
Conclusion
OA’s effects on MMP expression in HNSCC are variable, and may promote cancer cell invasion.
Keywords: Squamous cell carcinoma, Cell line, Extracellular matrix, Gelatinases, Gene silencing, Human, Matrix metalloproteinase 10, Matrix metalloproteinase 2, Matrix metalloproteinase 9, Neoplasm invasiveness, Messenger RNA, Tissue plasminogen activator
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the 6th most common malignancy (Ruokolainen et al., 2004), affecting over 500,000 individuals yearly worldwide (Mehanna et al., 2010), with more than 45,000 cases occurring in the United States alone (Siegel et al., 2015). One of the more aggressive human malignancies, a high incidence of loco-regional metastasis characterizes HNSCC (Imanishi et al., 2000). Despite multimodality treatment, the disease’s overall 5-year survival has remained at about 40% for decades (Gregoire et al., 2010). The frequent recurrence of HNSCCs after treatment is closely associated with invasive and metastatic ability (Johansson et al., 1997).
The ability to degrade the extracellular matrix (ECM) is essential for cancer cell invasion. Cancer cells express a variety of proteases including the matrix metalloproteinases. The matrix metalloproteinases are a family of zinc-dependent endopeptidases that collectively are capable of degrading all ECM components (Johansson et al., 1997). The family currently consists of 28 members (Baker et al., 2006). Based on their structure and substrate specificities, MMPs can be divided into subgroups, including collagenases, stromelysins, stromelysin-like MMPs, matrilysins, gelatinases, membrane-type MMPs, and others (Vihinen and Kahari, 2002). Matrix metalloproteinase (MMP) upregulation occurs in a number of physiologic and pathophysiologic conditions involving tissue remodeling including morphogenesis, tissue repair, angiogenesis, inflammatory cell migration, rheumatoid arthritis, osteoarthritis, autoimmune skin blistering, chronic dermal and intestinal ulcers, atherosclerotic plaques, and periodontitis (Adler et al., 1990; Johansson et al., 1997; Meade-Tollin et al., 1999; Vihinen and Kahari, 2002). An important component of the metastatic cascade, MMP overexpression in HNSCC relative to normal mucosa has been demonstrated in several studies (Baker et al., 2006; Bogusiewicz et al., 2003; Cazorla et al., 1998; Hong et al., 2000; Imanishi et al., 2000; Johansson et al., 1997; Kusukawa et al., 1996; Kusukawa et al., 1995; Kusukawa et al., 1993; Li et al., 2015; Myoung et al., 2002; P et al., 2001; Shimada et al., 2000; Soni et al., 2003; Sutinen et al., 1998; Tokumaru et al., 2000; Yoshizaki et al., 2001). MMP overexpression has been shown to have prognostic significance in HNSCC, having been associated with lymph node metastases, poor survival and drug resistance (Bogusiewicz et al., 2003; de Vicente et al., 2005; Hong et al., 2000; Johansson et al., 1997; Kurahara et al., 1999; Kusukawa et al., 1996; Kusukawa et al., 1993; Li et al., 2015; Myoung et al., 2002; Ondruschka et al., 2002; P et al., 2001; Ruokolainen et al., 2004; Shimada et al., 2000; Soni et al., 2003; Tokumaru et al., 2000; Yoshizaki et al., 2001).
Growth factors, cytokines, physical stress, and cell-matrix and cell-cell interactions induce MMP expression and activation (Heino, 1996; Johansson et al., 1997; P et al., 1999; Sundelin et al., 2005; Vihinen and Kahari, 2002; Werb et al., 1989; Yu and Stamenkovic, 1999). OA (also known as gpnmb (Anderson et al., 2006; Anderson et al., 2002; Pollack et al., 2007; Tse et al., 2006), dendritic cell heparin sulfate proteoglycan integrin dependent ligand (Chung et al., 2007; Shikano et al., 2001), and human hematopoietic growth factor inducible neurokinin (Bandari et al., 2003)) was first identified as a gene differentially expressed in melanoma cells with high metastatic potential (Tse et al., 2006). OA exists as a 65 kDa transmembrane protein and a 115 kDa excreted isoform (Abdelmagid et al., 2008). OA overexpression contributes to metastasis in breast carcinomas (Metz et al., 2007; Rose et al., 2010a; Rose et al., 2010b; Rose et al., 2007), melanoma cells (Pollack et al., 2007; Tse et al., 2006), hepatic carcinoma (Tse et al., 2006), lung carcinoma (Oyewumi et al., 2016; Tse et al., 2006), prostate carcinoma (Fiorentini et al., 2014), pancreatic cancer (Torres et al., 2015; Torres et al., 2014) and an experimental glioma (Rich et al., 2003). Osteoactivin (OA) has been shown to upregulate matrix metalloproteinase-3 (MMP-3) and MMP-9 expression in fibroblasts and skeletal muscle (Furochi et al., 2007; Ogawa et al., 2005; Tonogai et al., 2015); MMP-2 and -9 in prostate cancer cell lines (Fiorentini et al., 2014); and MMP-3 in breast carcinoma cell lines (Rose et al., 2007). We previously demonstrated that OA is overexpressed in oral squamous cell carcinoma (OSCC) cell lines, and that OA in the ECM promotes migration of these cells through its interactions with integrins(Arosarena et al., 2016). In this study, we demonstrate that OA upregulates expression and activity of MMPs shown to be overexpressed in HNSCC cells (Baker et al., 2006; Bogusiewicz et al., 2003; Cazorla et al., 1998; Hong et al., 2000; Imanishi et al., 2000; Johansson et al., 1997; Kusukawa et al., 1996; Kusukawa et al., 1995; Kusukawa et al., 1993; Li et al., 2015; P et al., 2001; Shimada et al., 2000; Soni et al., 2003; Sutinen et al., 1998; Tokumaru et al., 2000; Yoshizaki et al., 2001).
Materials and Methods
This study was granted exempt status from Temple University Institutional Review Board (protocol #13653) because commercially-available cells lines were used.
Cell culture
Cell lines representing different head and neck subsites, and derived from tumors at different stages were used (Table 1). Cell lines derived from oropharyngeal cancers were not included in this study as human papilloma virus infection can be a confounding variable in these malignancies (Mehanna et al., 2010). Similarly, cell lines derived from nasopharyngeal cancers were not used because Epstein Barr virus infection can be a confounding variable in these cancers (Mehanna et al., 2010). UMSCC-12 and -14a cells were obtained from the Head and Neck Cancer Biology Laboratory at the University of Michigan (Ann Arbor). SCC-15 and -25 cells were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia).(Lin et al., 2007) UMSCC-12 and -14a cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin/streptomycin and 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37°C. SCC-15 and -25 cells were maintained as previously described (Arosarena et al., 2016). UMSCC12, SCC15 and SCC25 cells were used in this study because of their invasive capacity demonstrated in previous studies (Lin et al., 2007; Liu et al., 2002; Zhang et al., 2016). UMSCC14a cells were used as a model of early-stage, noninvasive HNSCC (Henson et al., 2007).
Table 1.
Cell lines representing different head and neck subsites, and derived from tumors at different stages were used. “T” designates the stage of the primary tumor based on size. “N” represents nodal stage based on size and number of lymph node metastases to the neck. “M” represents distant metastatic stage. The overall stage (combination of T, N and M) is given in parentheses (Gregoire et al., 2010).
| Cell Line | Stage | Anatomic Site of Origin |
|---|---|---|
| SCC15 | T4N1M0 (IV) | Tongue |
| SCC25 | T2N1M0 (III) | Tongue |
| UMSCC12 | T2N1M0 (III) | Larynx |
| UMSCC14a | T1N0M0 (I) | Oral cavity |
Treatment with recombinant human OA (rhOA) in the ECM
Tissue culture plates were coated with rhOA (10 μg/mL, Acro Biosystems, Newark, Delaware) (Arosarena et al., 2016) or 0.1% bovine serum albumin (BSA, Sigma Chemical Company, St. Louis, Missouri) in phosphate-buffered saline (PBS) overnight at 4°C. Excess solution was aspirated from the plates, and the plates were seeded with cells at sub-confluence in media with 2.5% FBS (Day 0). On Days 2, 4 and 7, cellular lysates were harvested for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Similarly treated cells were harvested on Day 2 for Western blot analyses and zymography because we previously demonstrated maximal MAPK activation in HNSCC cell lines by 48 hours (Arosarena et al., 2016).
qRT-PCR
To assess effects of OA in the ECM on MMP expression, RNA harvest and reverse transcription were performed as previously described (Arosarena et al., 2011). qRT-PCR was performed twice with three replicates using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, California) according to the manufacturer’s recommendations. Primer sequences are listed in Table 2. Threshold cycles were used to normalize samples to β-actin, the endogenous control (Arosarena et al., 2016).
Table 2. Primer sequences used for qRT-PCR.
Primers for MMP-3 were designed using the National Center for Biotechnology, National Library of Medicine (National Institutes of Health, NIH) Primer Basic Local Alignment Search Tool (BLAST). All other primers were used as described previously.
| Gene | Primer Sequences (5′ – 3′) | |
|---|---|---|
| MMP-2 (Piotrowski et al., 2011) | Forward | ACTGTTGGTGGGAACTCAGAAG |
| Reverse | CAAGGTCAAT GTCAGGAGAGG | |
| MMP-3 | Forward | GGCAAGACAGCAAGGCATAG |
| Reverse | GGAACCGAGTCAGGTCTGTG | |
| MMP-7 (Piotrowski et al., 2011) | Forward | CGGATGGTAAGCAGTCTAGGG |
| Reverse | AGGTTGGATACATCACTGCATTAG | |
| MMP-9 (Ye et al., 2008) | Forward | GCACGACGTCTTCCAGTACC |
| Reverse | TCAACTCACTCCGGGAACTC | |
| MMP-10 (Vendelin et al., 2006) | Forward | GACCTGGGCTTTATGGAGATATT |
| Reverse | GCTTCAGTGTTGGCTGAGTGAA | |
| MMP-11 (Piotrowski et al., 2011) | Forward | TGGGTGTACGACGGTGAAAA |
| Reverse | CATGGGTCTCTAGCCTGATA | |
| MMP-13 (Winter et al., 2005) | Forward | AGTGGTAAGAATAGTAGATGTG |
| Reverse | GGCCGATCATATATTCAATAAGT | |
| β-Actin (Gao et al., 2011) | Forward | AGGTCATCACCATTGGCAAT |
| Reverse | ACTCGTCATACTCCTGCTTG |
Knock down of OA expression
OA knock down was performed in UMSCC12, SCC15 and SCC25 cells with siRNA transfection as previously described (Arosarena et al., 2016) to investigate effects of OA depletion on MMP expression and activity. We previously demonstrated that these cell lines express OA to a greater extent than immortalized oral keratinocytes (Arosarena et al., 2016). OA knock down was also used in UMSCC12 cells to study effects of OA depletion on in vitro invasion. Untreated control cells were exposed to transfection reagent only. OA knock down was confirmed in the cell lines with Western blot analysis (Figure 1).
Figure 1.
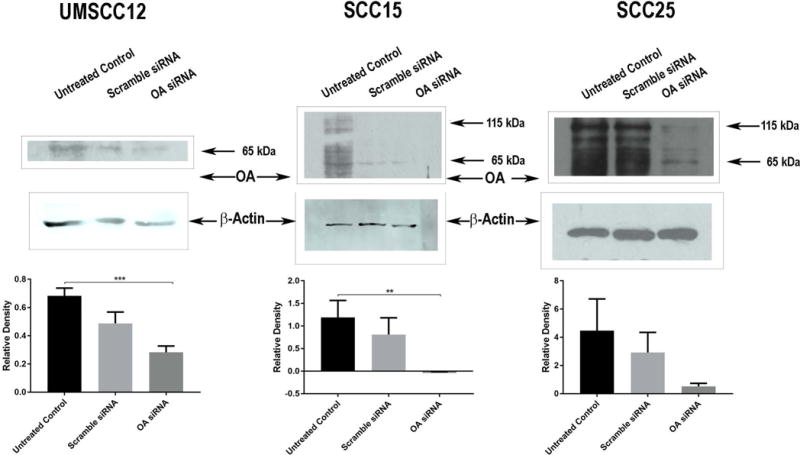
Western blot analyses
OA Immunoblots
To confirm OA knock down, cells were lysed in RIPA buffer with protease inhibitor cocktail (Sigma Chemical Company). Protein concentrations were measured with a Pierce bicinchonic acid kit (Thermo Fisher Scientific, Waltham, Massachusetts). Protein extracts (20 μg) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Resolved proteins were transferred to polyvinylidene fluoride (PVDF) membranes, and the membranes were probed with an antibody to OA (Santa Cruz Biotechnology, Santa Cruz, California). β-actin antibody (Sigma Chemical Company) was used as the endogenous control (Figure 1).
MMP Immunoblots
To confirm effects of OA treatment and OA knock down on MMP expression, protein extracts (10 μg) were resolved by SDS-PAGE, transferred to PVDF membranes and probed with antibodies to MMP-2 (72-kDa type IV collagenase, gelatinase A), -3 (stromelysin-1), -7 (matrilysin-1), -9 (92-kDa type IV collagenase, gelatinase B), -10 (stromelysin-2) and -13 (stromelysin-3). The MMP-9 antibody was obtained from Santa Cruz Biotechnology. The remaining MMP antibodies were obtained from R&D Systems (Minneapolis, Minnesota).
Blots were developed by enhanced chemiluminescence or infrared fluorescence imaging. At least three independent experiments were performed for Western blot analyses and densitometry was performed with LI-COR (Lincoln, Nebraska) Image Studio Lite version 4.0 software. β-actin was used as the endogenous control, and MMP band densities were normalized to β-actin. Representative experiments are presented.
Zymography
To assess ECM degradation activity related to OA treatment or knock down, cells were lysed in M-PER mammalian protein reagent (Thermo Fisher Scientific). Lysates were flash frozen and stored at −80°C. Native protein extracts (4 μg) were resolved on 8% SDS-PAGE gels with 0.05% gelatin or casein. Following electrophoresis, gels were washed in several changes of water, followed by several changes of 2.5% Tween-20 and several changes of reaction buffer (Tris-buffered saline with 5 mM CaCl2 and 2 μM ZnCl2, 5 minutes each). Gels were incubated for 7 days in reaction buffer at 37°C and then stained with amido black (Frankowski et al., 2012). At least three independent experiments were performed per sample for zymography and densitometric analyses were performed. Representative experiments are presented.
Viability assays
UMSCC12 cells were harvested 24 hours after treatment with OA- or scramble-siRNA, and seeded in 96-well tissue culture-treated plates at a density of 500 cells/well. Cell densities were measured with an MTT assay at 6 hours, and at days 2, 4 and 6 after seeding. Assays were performed twice, each time with 12 replicates.
Tumor spheroid invasion assays
UMSCC12 cells were used for this assay because proliferation assays revealed that OA knock down did not affect viability of these cells, which could affect quantitation of invasion. We previously demonstrated that OA knock down inhibits proliferation of SCC15 and SCC25 cells (Arosarena et al., 2016). UMSCC12 cells were harvested 24 hours after treatment with OA or scramble siRNA, and seeded in 96-well ultra-low attachment round-bottom plates at a density of 104 cells/well in 200 μL complete culture medium. After 48 hours spheroid formation was confirmed, and 100 μL of the culture medium was aspirated from each well. Ice-cold Matrigel® basement membrane matrix (100 μL diluted to 3.2 mg/mL in PBS) was added to each well and the matrix was allowed to solidify in a 5% CO2 atmosphere at 37°C for one hour. 100 μL fresh complete growth medium was added to the wells, and spheroid images were recorded daily for 6 days (Vinci et al., 2015). Spheroid areas were measured with NIH ImageJ software. Invasion assays were performed twice, each time with six replicates.
Statistical analyses
Prism GraphPad version 7.01 (La Jolla, California) statistical software was used for statistical analyses. The D’Agostino-Pearson Omnibus K2 test was used to determine normality of data. For comparisons of MMP expression and activity between OA-treated and BSA-treated samples, two-way analyses of variance was used for normally-distributed data and the Mann-Whitney U test was used for data that were not normally distributed. Two-way analyses of variance were used to compare means for MMP mRNA expression, spheroid areas and cell proliferation data. The Tukey post-hoc analysis was used to determine pairwise differences. The independent samples Kruskal-Wallis test (for data that were not normally distributed) and two-way analyses of variance (for normally distributed data) were used to compare sample means for MMP expression and activity between OA knock down samples and controls. A P value <0.05 was considered statistically significant.
Results
OA in the ECM differentially affects MMP mRNA expression
MMP-3 mRNA expression was not detected in the cell lines. MMP-7 mRNA was significantly decreased in SCC15 cells in the presence of OA. OA in the ECM resulted in MMP-2 and MMP-9 mRNA upregulation in SCC15 cells, and this upregulation was evident by day 2. MMP-10 and MMP-13 mRNA upregulation in UMSCC12 cells was noted by day 2 for MMP-10 and by day 4 for MMP-13. UMSCC14a cells upregulated MMP-11 and MMP-13 mRNAs by day 4 with OA treatment (Table 3).
Table 3. MMP mRNA expression in OA-treated cells relative to controls.
UMSCC12 cells were used as model of stage III HNSCC. UMSCC14a and SCC15 cells were used as models of stage I and IV HNSCC, respectively. The ΔΔCt method was used to compare MMP mRNA expression between rhOA-treated cells and controls. mRNA upregulation was considered significant if mean fold change was ≥1.5 and p<0.05.
| MMP | Cell Line | |||||
|---|---|---|---|---|---|---|
| UMSCC12 | UMSCC14a | SCC15 | ||||
| Mean Fold Change | p | Mean Fold Change | p | Mean Fold Change | p | |
| MMP-2 | 1.43 | <0.0001 | 0.66 | 0.1914 | 2.93 | 0.0158 |
| MMP-7 | 1.45 | 0.0003 | 1.30 | 0.2530 | 0.55 | 0.0088 |
| MMP-9 | 1.40 | 0.4334 | 1.09 | 0.4745 | 5.70 | 0.0002 |
| MMP-10 | 21.09 | 0.0086 | 1.11 | 0.0005 | 9.33 | 0.4435 |
| MMP-11 | 1.07 | 0.0851 | 1.56 | <0.0001 | 0.96 | 0.6191 |
| MMP-13 | 18.69 | 0.0057 | 6.69 | <0.0001 | 6.49 | 0.5417 |
OA treatment effects on MMP expression
OA in the ECM did not significantly change MMP expression in UMSCC12 and SCC25 cells (data not shown). In UMSCC14a cells, MMP-9 expression was decreased, but MMP-10 expression was increased in the presence of OA (p=0.0002 and p=0.0431, respectively, Figure 2A), and the expression of other MMPs did not differ between OA-treated cells and controls. OA in the ECM increased MMP-10 expression in SCC15 cells (p<0.0001, Figure 2B).
Figure 2.
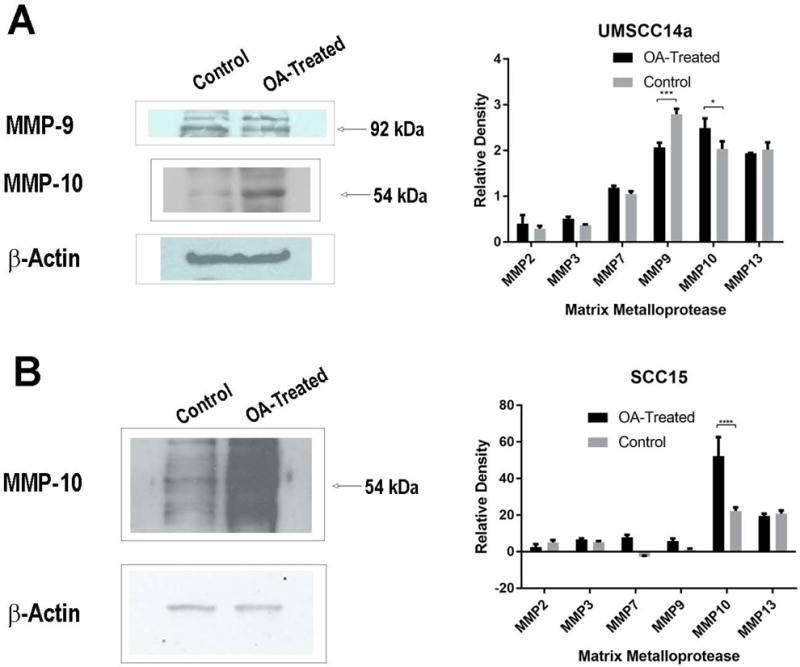
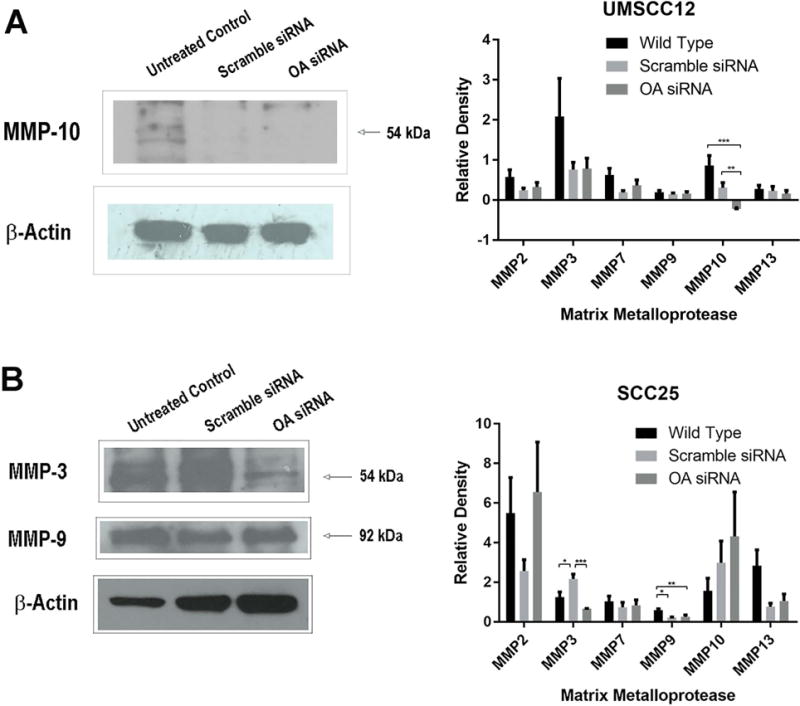
OA knock-down effects on MMP expression
OA knock-down decreased MMP-10 expression in UMSCC12 cells (p=0.0001, Figure 3A). There was decreased MMP-3 (p=0.0005) and MMP-9 (p=0.0036) expression with OA knock-down in SCC25 cells (Figure 3B). OA knock-down did not significantly change MMP expression in SCC15 cells (data not shown).
Figure 3.
OA promotes ECM degradation
Zymography was used to assess OA treatment and OA knock down effects on activity of ECM-degrading enzymes.
Gelatin zymography
OA in the ECM increased MMP-2 activity in SCC15 cells (p=0.0408). While MMP-9 activity was also increased with OA in the ECM, this was not statistically significant (p=0.4744). MMP-9 activity in SCC25 cells was significantly increased in the presence of OA (p<0.0001, Figure 4A). OA increased expression of both the latent (92 kDa) and active (83–84 kDa) (Matsuzawa et al., 1996; Meade-Tollin et al., 1999) forms of the enzyme in this cell line. OA knock down decreased MMP2 (p=0.0023) and MMP9 (p<0.0001) activity in this cell line (Figure 4B). OA treatment and depletion did not significantly affect gelatinase activity in UMSCC12 and UMSCC14a cells (data not shown).
Figure 4.
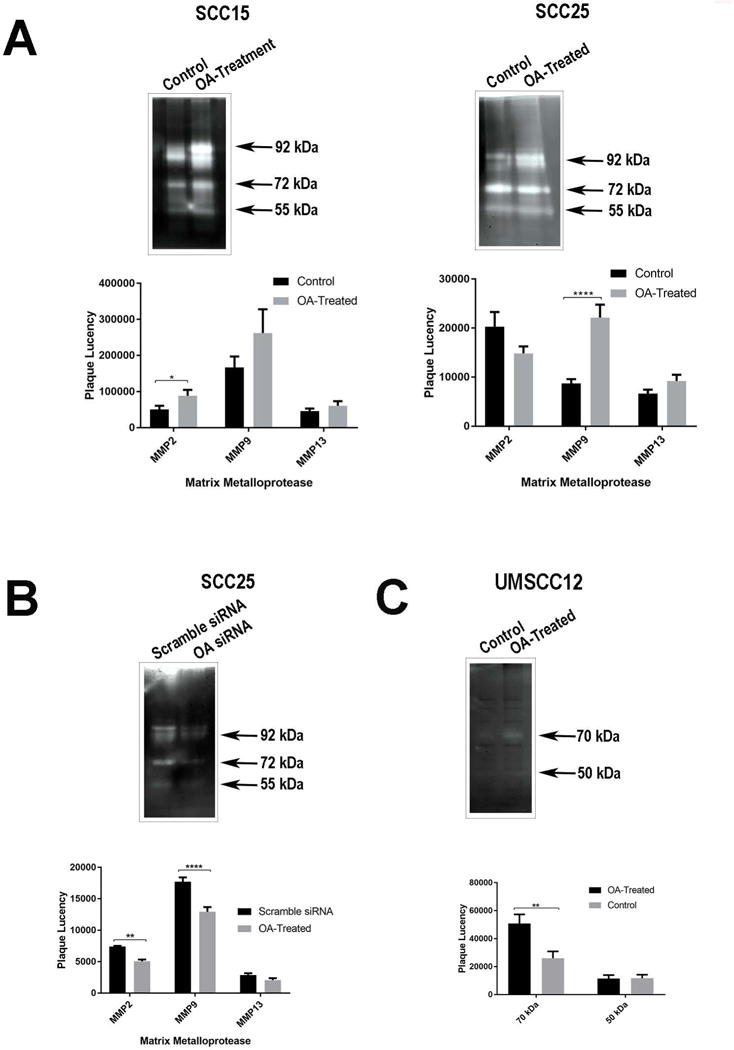
Casein zymography
Only the UMSCC12 cell line demonstrated caseinolytic activity. Two distinct bands were noted, the most prominent at about 70 kDa, which likely represents the activity of tissue-type plasminogen activator (tPA) (Ho et al., 2009). OA in the ECM significantly increased tPA activity in this cell line (p=0.0078). The less prominent 50 kDa band corresponded to the molecular weights of urokinase plasminogen activator (48 kDa) (Burggraf et al., 2004) or MMP-3 (Adler et al., 1990; Matsuzawa et al., 1996; Meade-Tollin et al., 1999), and OA treatment did not affect the intensity of this band. No bands were detected between 19 and 29 kDa to suggest MMP-7 activity (Adler et al., 1990; Nagashima et al., 1997). OA depletion with siRNA did not affect caseinolytic activity in this cell line (data not shown).
OA depletion decreases cell invasion in UMSCC12 cells
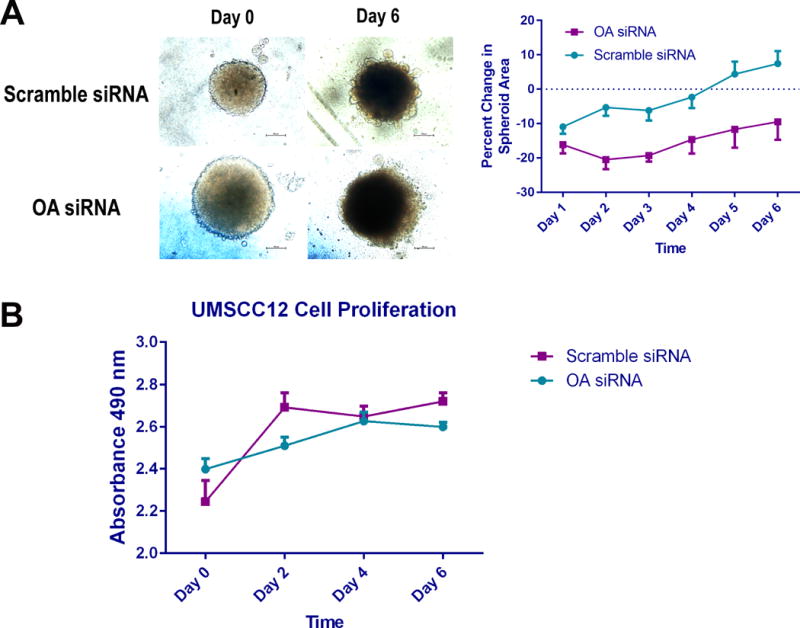
Scramble siRNA-treated UMSCC12 cells tended to form more compact spheroids, though the difference in spheroid areas between scramble siRNA- and OA siRNA-treated cells was not statistically significant (p=0.547). OA knock-down resulted in significantly decreased cell invasion by day 2 (p<0.0001, Figure 5A). OA depletion did not significantly affect cell viability in this cell line (p=0.2788, Figure 5B).
Figure 5.
Discussion
The cascade of cancer cell invasion and metastasis is a complex process involving cell attachment to the ECM, degradation of the ECM (including basement membrane components), detachment, and cell migration through the degraded matrix.(Johansson et al., 1997; Stetler-Stevenson et al., 1993) Moreover, neo-angiogenesis, and vascular intravasation and extravasation all require basement membrane digestion.(Cazorla et al., 1998; Imanishi et al., 2000; Vihinen and Kahari, 2002) Cancer cells facilitate ECM degradation by MMPs through co-clustering of cell surface receptors such as CD44 (the hyaluronan receptor) with ECM components and MMPs.(Yu and Stamenkovic, 1999) Integrin-ECM interactions also facilitate matrix degradation by MMPs that co-localize at the cell surface, and integrin interactions can upregulate MMP expression.(Brooks et al., 1996; Heino, 1996; Thomas et al., 2001; Werb et al., 1989)
OA exists as a 65 kDa transmembrane protein as well as a 115 kDa excreted isoform that can interact with cell surface receptors.(Abdelmagid et al., 2008) We have previously shown that OA in the ECM interacts with OSCC cell surface integrins, resulting in mitogen-activated protein kinase (MAPK) activation.(Arosarena et al., 2016) MMP upregulation occurs through activation of the MAPKs.(Furochi et al., 2007; Johansson et al., 1997; Vihinen and Kahari, 2002; Yu et al., 2011) We sought to determine if OA plays a role in MMP upregulation and activation in HNSCC. We studied the effects of OA in the ECM, as well as OA depletion in contrast to other studies that examined only protein depletion effects(Fiorentini et al., 2014).
Adhesion of HNSCC cells to OA in the ECM and OA depletion had differential effects on MMP mRNA and protein expression in the cell lines we tested. In a study of the effects of cytokines and hepatocyte growth factor on MMP expression in OSCC, Sundelin et al found that cell lines established from different tumors showed differential MMP expression patterns in response to stimulation.(Sundelin et al., 2005) Shimada et al were not able to detect MMP-7 expression in their study of human OSCCs(Shimada et al., 2000), while Impola et al detected MMP-7 in 73% of their OSCC samples(Impola et al., 2004). Other studies have confirmed that there is no characteristic MMP expression profile or activity pattern in HNSCC, and this may be due to the techniques used in the studies as well as to the fact that some of these studies lacked adequate sample numbers.(Werner et al., 2002)
While OA in the ECM increased MMP-10 and -13 mRNA expression in UMSCC12 cells, and increased MMP-11 and -13 mRNA expression in UMSCC14a cells, OA treatment did not result in increased expression of these proteins in the cell lines. Similarly, MMP-7 mRNA expression in UMSCC12 and SCC15 cells did not correlate with protein expression. These discrepancies between mRNA and protein expression may reflect differences in mRNA and protein degradation rates between OA-treated and control cells(Landry et al., 2013; Vogel et al., 2010), as well as mRNA translation repression by microRNAs(Bartel, 2009; Moore, 2013). Though a number of studies have demonstrated increased MMP mRNA expression in HNSCC cells as compared to normal upper aerodigestive tract mucosa(Cazorla et al., 1998; Cromer et al., 2004; Du et al., 1999; Gray et al., 1992; Imanishi et al., 2000; Impola et al., 2004; Kainuma et al., 2006; Magary et al., 2000; Muller et al., 1991; Muller et al., 1993; P et al., 2001; Stott-Miller et al., 2011; Suhr et al., 2007; Villaret et al., 2000; Ye et al., 2008; Yen et al., 2009; Ziober et al., 2006), the need to study these proteins at a molecular level is evidenced by a study of MMP expression in benign and malignant prostate tissue that revealed no correlation between mRNA and protein expression(Lichtinghagen et al., 2002).
In our study adhesion of cells to OA in the ECM increased MMP-10 expression in UMSCC14a and SCC15 cells, but decreased expression of MMP-9 in UMSCC14a cells. OA gene silencing decreased MMP-10 expression in UMSCC12 cells, and decreased MMP-3 and MMP-9 expression in SCC25 cells. These results indicate that OA can both promote and inhibit MMP expression in HNSCC. While differential responses in MMP expression to OA stimulation and OA gene silencing could relate to differences in function of the transmembrane and excreted forms of the protein (UMSCC12 did not express the 115 kDa excreted form of OA), these responses appear to be cell line-specific. Differential responses in MMP profile expression to OA stimulation and depletion may represent the fact that these cells lines were derived from different individuals, and possibly by different mechanisms of carcinogenesis.
Post-transcriptional regulation of MMPs is related to the fact that they are secreted as pro-enzymes, requiring activation.(Kleiner and Stetler-Stevenson, 1999; Sundelin et al., 2005) The antibodies used in this study to detect MMPs do not distinguish between the proenzymes and their active forms, so zymography was used to assess MMP activity in the cell lines. Cellular adhesion to OA in the ECM and OA gene silencing significantly affected gelatinase activity only in SCC15 and SCC25 cells, indicating that OA effects on MMP activation may be cell line-dependent. OA in the ECM increased MMP-2 activity in SCC15 cells. While MMP-9 activity was increased in both cell lines, this was only statistically significant for SCC25 cells. OA knock down decreased both MMP-2 and -9 activities in SCC25 cells. Cellular adhesion to OA in the ECM and OA gene silencing did not affect 54-kDa gelatinase activity (which could be attributed to MMP-3, -10 and/or -13, all 54 kDa) in any of the cell lines.(Vihinen and Kahari, 2002)
Only UMSCC12 cells demonstrated caseinolytic activity, and OA treatment significantly upregulated the activity of tPA in this cell line. The plasmin-dependent pathway is a significant pathway for the initiation of MMP-mediated matrix degradation, particularly in the context of oral inflammation(Birkedal-Hansen et al., 1993; Chang et al., 2006; Ho et al., 2009; Huang et al., 2008), and may be one mechanism of OA-mediated MMP upregulation. tPA upregulation in oral keratinocytes also occurs downstream of MAPK activation.(Chang et al., 2006) This is the first study, to our knowledge, demonstrating increased tPA activity in the presence of OA.
OA gene silencing in UMSCC12 cells inhibited matix invasion by tumor spheroids. The spheroid invasion assay was used to mimick the hypoxic conditions encountered in the tumor microenvironment, and because of its simplicity.(Vinci et al., 2015) Unlike the transwell invasion assay, the spheroid invasion assay is not dependent on tumor cell diameter, which varies significantly within the SCC15 and SCC25 cell lines. Spheroids formed by OA siRNA-treated cells had fewer invadopodia than those formed by control cells (Figure 5). Moreover, the spheroids formed by OA-depleted cells were less cohesive and started disintegrating by day 4.
Tissue-specific inhibitors of MMPs (TIMPs) modulate MMP activity, and acquisition of the invasive phenotype has been attributed to an imbalance between MMPs and TIMPs.(Kleiner and Stetler-Stevenson, 1999; Sundelin et al., 2005) However, the relationship between MMPs and TIMPs is more complicated, as it has become clear that TIMPs can form quaternary structures with membrane-type MMPs that activate secreted MMPs, and that TIMP overexpression is also associated with a more aggressive OSCC phenotype.(Kurahara et al., 1999; Yoshizaki et al., 2001) We did not assess TIMP expression in our cell lines, which is a limitation of this study. We also did not assess the expression of MMPs that have been primarily associated with carcinoma-associated fibroblasts (CAFs) in HNSCC (e.g., MMP-1).(Johansson et al., 1997)
Assessment of MMP expression in HNSCC cells in isolation is another limitation of this study. While MMP-2, -3, -9, -11 and -13 localize to tumor cells at the invading fronts in some studies(de Vicente et al., 2005; Hong et al., 2000; Ikebe et al., 1999; Impola et al., 2004; Johansson et al., 1997; Shimada et al., 2000; Soni et al., 2003), they have also been detected in stromal cells(de Vicente et al., 2005; Impola et al., 2004; Johansson et al., 1997; Soni et al., 2003; Sutinen et al., 1998; Yoshizaki et al., 2001). Tumor stromal cells have been typically identified as CAFs, but Soni et al demonstrated that epithelial cells that have undergone mesenchymal transformation can be found in the stroma and can overexpress MMPs(Soni et al., 2003). CAF-tumor cell interactions induce epithelial-to-mesenchymal transformation in epithelial malignancies, and these interactions also mediate MMP expression in both cell types. Co-culture of SCC25 cells with periodontal ligament fibroblasts upregulated MMP-1 and -9 expression by the cancer cells, and upregulated MMP-1, -2 and -3 production by the fibroblasts. Co-culture also increased MMP-2 and -9 activity.(Fullar et al., 2012) Similarly, CAFs protect HNSCC cells from the effects of cetuximab by causing upregulation of MMP expression by the tumor cells.(Johansson et al., 1997) Tumor-infiltrating lymphocytes(Vihinen and Kahari, 2002) and vascular endothelial cells(Nagashima et al., 1997) are other sources of MMP production within the tumor microenvironment. Further studies are needed to assess the effect of OA-mediated MMP expression in tumor cell-fibroblast and –endothelial cell co-culture models.
Despite the significance of MMPs in mediating tumor invasion and neo-angiogenesis, oncologic clinical trials of synthetic MMP inhibitors have had disappointing results, with some trials terminated for lack of efficacy and because patients receiving the MMP inhibitors had worse outcomes.(Coussens et al., 2002; Vihinen and Kahari, 2002) Increased understanding of the role of MMPs in tumor progression reveals that they are involved in a wide range of biological activity, including cell death, cell proliferation, cell differentiation, tumor-associated angiogenesis, angiogenesis inhibition and malignant conversion.(Coussens et al., 2002) Moreover, it has been recognized that MMPs, including MMP-3 and MMP-9 can have protective roles in tumor progression (e.g., angiogenesis inhibition by MMP-9).(Martin and Matrisian, 2007) Our results suggest that OA may be a more promising target for cancer therapy.
In conclusion, OA in the ECM upregulated MMP-10 expression in UMSCC14a and SCC15 cells, but decreased MMP-9 expression in UMSCC14a cells. OA gene silencing decreased MMP-10 expression in UMSCC12 cells, but increased MMP-9 expression in SCC15 cells. OA knock down decreased MMP-3 and -9 expression in SCC25 cells. In SCC15 and SCC25 cells, OA in the ECM increased gelatinase activity, and OA depletion decreased gelatinase activity in SCC25 cells. OA increased caseinolytic activity in UMSCC12 cells. OA depletion decreased invasive capacity of UMSCC12 tumor spheroids. Therefore, OA’s effects on MMP expression in HNSCC are variable, and may promote cancer cell invasion.
Acknowledgments
This work was supported by the Eugene N. Myers, MD Head and Neck Cancer Research Fund, Department of Otolaryngology – Head and Neck Surgery, Temple University School of Medicine.
Contract grant sponsor: National Institutes of Health; Contract grant number: NIH-NCI 1R21CA167126-01A1 (Dr. Arosarena)
References
- Abdelmagid SM, Barbe MF, Rico MC, Salihoglu S, Arango-Hisijara I, Selim AH, Anderson MG, Owen TA, Popoff SN, Safadi FF. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Experimental cell research. 2008;314(13):2334–2351. doi: 10.1016/j.yexcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Adler RR, Brenner CA, Werb Z. Expression of extracellular matrix-degrading metalloproteinases and metalloproteinase inhibitors is developmentally regulated during endoderm differentiation of embryonal carcinoma cells. Development. 1990;110(1):211–220. doi: 10.1242/dev.110.1.211. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30(1):81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Arosarena OA, Del Carpio-Cano FE, Dela Cadena RA, Rico MC, Nwodim E, Safadi FF. Comparison of bone morphogenetic protein-2 and osteoactivin for mesenchymal cell differentiation: Effects of bolus and continous administration. Journal of cellular physiology. 2011 doi: 10.1002/jcp.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosarena OA, Dela Cadena RA, Denny MF, Bryant E, Barr EW, Thorpe R, Safadi FF. Osteoactivin Promotes Migration of Oral Squamous Cell Carcinomas. Journal of cellular physiology. 2016;231(8):1761–1770. doi: 10.1002/jcp.25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EA, Leaper DJ, Hayter JP, Dickenson AJ. The matrix metalloproteinase system in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2006;44(6):482–486. doi: 10.1016/j.bjoms.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bandari PS, Qian J, Yehia G, Joshi DD, Maloof PB, Potian J, Oh HS, Gascon P, Harrison JS, Rameshwar P. Hematopoietic growth factor inducible neurokinin-1 type: a transmembrane protein that is similar to neurokinin 1 interacts with substance P. Regul Pept. 2003;111(1–3):169–178. doi: 10.1016/s0167-0115(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bogusiewicz M, Stryjecka-Zimmer M, Szymanski M, Rechberger T, Golabek W. Activity of matrix metalloproteinases-2 and -9 in advanced laryngeal cancer. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003;128(1):132–136. doi: 10.1067/mhn.2003.8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85(5):683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Burggraf D, Martens HK, Wunderlich N, Jager G, Hamann GF. Rt-PA causes a significant increase in endogenous u-PA during experimental focal cerebral ischemia. Eur J Neurosci. 2004;20(11):2903–2908. doi: 10.1111/j.1460-9568.2004.03757.x. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Hernandez L, Nadal A, Balbin M, Lopez JM, Vizoso F, Fernandez PL, Iwata K, Cardesa A, Lopez-Otin C, Campo E. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. The Journal of pathology. 1998;186(2):144–150. doi: 10.1002/(SICI)1096-9896(1998100)186:2<144::AID-PATH147>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Chang YC, Ho YC, Chou LS, Chou MY, Huang FM. Signal transduction pathways involved in the stimulation of tissue type plasminogen activator by interleukin-1alpha and Porphyromonas gingivalis in human osteosarcoma cells. Journal of periodontal research. 2006;41(5):374–380. doi: 10.1111/j.1600-0765.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- Chung JS, Sato K, Dougherty, Cruz PD, Jr, Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109(10):4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Cromer A, Carles A, Millon R, Ganguli G, Chalmel F, Lemaire F, Young J, Dembele D, Thibault C, Muller D, Poch O, Abecassis J, Wasylyk B. Identification of genes associated with tumorigenesis and metastatic potential of hypopharyngeal cancer by microarray analysis. Oncogene. 2004;23(14):2484–2498. doi: 10.1038/sj.onc.1207345. [DOI] [PubMed] [Google Scholar]
- de Vicente JC, Fresno MF, Villalain L, Vega JA, Hernandez Vallejo G. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral oncology. 2005;41(3):283–293. doi: 10.1016/j.oraloncology.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Du B, Wang P, Guo X, Du B. Expression of membrane type 1-matrix metalloproteinase in laryngeal carcinoma. Pathology oncology research : POR. 1999;5(3):214–217. doi: 10.1053/paor.1999.0217. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Bodei S, Bedussi F, Fragni M, Bonini SA, Simeone C, Zani D, Berruti A, Missale C, Memo M, Spano P, Sigala S. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Experimental cell research. 2014;323(1):100–111. doi: 10.1016/j.yexcr.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Frankowski H, Gu YH, Heo JH, Milner R, Del Zoppo GJ. Use of gel zymography to examine matrix metalloproteinase (gelatinase) expression in brain tissue or in primary glial cultures. Methods in molecular biology. 2012;814:221–233. doi: 10.1007/978-1-61779-452-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullar A, Kovalszky I, Bitsche M, Romani A, Schartinger VH, Sprinzl GM, Riechelmann H, Dudas J. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Experimental cell research. 2012;318(13):1517–1527. doi: 10.1016/j.yexcr.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furochi H, Tamura S, Mameoka M, Yamada C, Ogawa T, Hirasaka K, Okumura Y, Imagawa T, Oguri S, Ishidoh K, Kishi K, Higashiyama S, Nikawa T. Osteoactivin fragments produced by ectodomain shedding induce MMP-3 expression via ERK pathway in mouse NIH-3T3 fibroblasts. FEBS letters. 2007;581(30):5743–5750. doi: 10.1016/j.febslet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, Zhang X, Wang L, Tao H. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ST, Wilkins RJ, Yun K. Interstitial collagenase gene expression in oral squamous cell carcinoma. The American journal of pathology. 1992;141(2):301–306. [PMC free article] [PubMed] [Google Scholar]
- Gregoire V, Lefebvre JL, Licitra L, Felip E, Group E-E-EGW Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 5):v184–186. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- Heino J. Biology of tumor cell invasion: interplay of cell adhesion and matrix degradation. International journal of cancer Journal international du cancer. 1996;65(6):717–722. doi: 10.1002/(SICI)1097-0215(19960315)65:6<717::AID-IJC1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Henson B, Li F, Coatney DD, Carey TE, Mitra RS, Kirkwood KL, D’Silva NJ. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2007;36(6):363–370. doi: 10.1111/j.1600-0714.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Ho YC, Yang SF, Huang FM, Chang YC. Up-regulation of osteolytic mediators in human osteosarcoma cells stimulated with nicotine. Journal of periodontal research. 2009;44(6):760–766. doi: 10.1111/j.1600-0765.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Hong SD, Hong SP, Lee JI, Lim CY. Expression of matrix metalloproteinase-2 and -9 in oral squamous cell carcinomas with regard to the metastatic potential. Oral oncology. 2000;36(2):207–213. doi: 10.1016/s1368-8375(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Huang FM, Yang SF, Chang YC. Up-regulation of gelatinases and tissue type plasminogen activator by root canal sealers in human osteoblastic cells. J Endod. 2008;34(3):291–294. doi: 10.1016/j.joen.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Ikebe T, Shinohara M, Takeuchi H, Beppu M, Kurahara S, Nakamura S, Shirasuna K. Gelatinolytic activity of matrix metalloproteinase in tumor tissues correlates with the invasiveness of oral cancer. Clinical & experimental metastasis. 1999;17(4):315–323. doi: 10.1023/a:1006642428826. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Fujii M, Tokumaru Y, Tomita T, Kanke M, Kanzaki J, Kameyama K, Otani Y, Sato H. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31(8):895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- Impola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H, Isaka K, Saarialho-Kere U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. The Journal of pathology. 2004;202(1):14–22. doi: 10.1002/path.1479. [DOI] [PubMed] [Google Scholar]
- Johansson N, Airola K, Grenman R, Kariniemi AL, Saarialho-Kere U, Kahari VM. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. The American journal of pathology. 1997;151(2):499–508. [PMC free article] [PubMed] [Google Scholar]
- Kainuma K, Katsuno S, Hashimoto S, Oguchi T, Suzuki N, Asamura K, Usami S. Differences in the expression of genes between normal tissue and squamous cell carcinomas of head and neck using cancer-related gene cDNA microarray. Acta Otolaryngol. 2006;126(9):967–974. doi: 10.1080/00016480500546367. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- Kurahara S, Shinohara M, Ikebe T, Nakamura S, Beppu M, Hiraki A, Takeuchi H, Shirasuna K. Expression of MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of the oral cavity: correlations with tumor invasion and metastasis. Head & neck. 1999;21(7):627–638. doi: 10.1002/(sici)1097-0347(199910)21:7<627::aid-hed7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kusukawa J, Harada H, Shima I, Sasaguri Y, Kameyama T, Morimatsu M. The significance of epidermal growth factor receptor and matrix metalloproteinase-3 in squamous cell carcinoma of the oral cavity. Eur J Cancer B Oral Oncol. 1996;32B(4):217–221. doi: 10.1016/0964-1955(96)00016-4. [DOI] [PubMed] [Google Scholar]
- Kusukawa J, Sasaguri Y, Morimatsu M, Kameyama T. Expression of matrix metalloproteinase-3 in stage I and II squamous cell carcinoma of the oral cavity. J Oral Maxillofac Surg. 1995;53(5):530–534. doi: 10.1016/0278-2391(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am J Clin Pathol. 1993;99(1):18–23. doi: 10.1093/ajcp/99.1.18. [DOI] [PubMed] [Google Scholar]
- Landry JJ, Pyl PT, Rausch T, Zichner T, Tekkedil MM, Stutz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, Gagneur J, Korbel JO, Huber W, Steinmetz LM. The genomic and transcriptomic landscape of a HeLa cell line. G3. 2013;3(8):1213–1224. doi: 10.1534/g3.113.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ding S, Zhong Q, Li G, Zhang Y, Huang XC. Significance of MMP11 and P14(ARF) expressions in clinical outcomes of patients with laryngeal cancer. Int J Clin Exp Med. 2015;8(9):15581–15590. [PMC free article] [PubMed] [Google Scholar]
- Lichtinghagen R, Musholt PB, Lein M, Romer A, Rudolph B, Kristiansen G, Hauptmann S, Schnorr D, Loening SA, Jung K. Different mRNA and protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 in benign and malignant prostate tissue. European urology. 2002;42(4):398–406. doi: 10.1016/s0302-2838(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, Ferris RL, Lai SY. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head & neck. 2007;29(2):163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- Liu SC, Bassi DE, Zhang SY, Holoran D, Conti CJ, Klein-Szanto AJ. Overexpression of cyclin D2 is associated with increased in vivo invasiveness of human squamous carcinoma cells. Molecular carcinogenesis. 2002;34(3):131–139. doi: 10.1002/mc.10057. [DOI] [PubMed] [Google Scholar]
- Magary SP, Ryan MW, Tarnuzzer RW, Kornberg L. Expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases in laryngeal and pharyngeal squamous cell carcinoma: A quantitative analysis. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000;122(5):712–716. doi: 10.1016/S0194-5998(00)70202-6. [DOI] [PubMed] [Google Scholar]
- Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer metastasis reviews. 2007;26(3–4):717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- Matsuzawa K, Fukuyama K, Dirks PB, Hubbard S, Murakami M, Becker LE, Rutka JT. Expression of stromelysin 1 in human astrocytoma cell lines. J Neurooncol. 1996;30(3):181–188. doi: 10.1007/BF00177269. [DOI] [PubMed] [Google Scholar]
- Meade-Tollin LC, Way D, Witte MH. Expression of multiple matrix metalloproteinases and urokinase type plasminogen activator in cultured Kaposi sarcoma cells. Acta histochemica. 1999;101(3):305–316. doi: 10.1016/S0065-1281(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer–Part 1: Epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. doi: 10.1136/bmj.c4684. [DOI] [PubMed] [Google Scholar]
- Metz RL, Patel PS, Hameed M, Bryan M, Rameshwar P. Role of human HGFIN/nmb in breast cancer. Breast Cancer Res. 2007;9(5):R58. doi: 10.1186/bcr1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ. microRNAs: small regulators with a big impact on lipid metabolism. J Lipid Res. 2013;54(5):1159–1160. doi: 10.1194/jlr.E036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Breathnach R, Engelmann A, Millon R, Bronner G, Flesch H, Dumont P, Eber M, Abecassis J. Expression of collagenase-related metalloproteinase genes in human lung or head and neck tumours. International journal of cancer Journal international du cancer. 1991;48(4):550–556. doi: 10.1002/ijc.2910480412. [DOI] [PubMed] [Google Scholar]
- Muller D, Wolf C, Abecassis J, Millon R, Engelmann A, Bronner G, Rouyer N, Rio MC, Eber M, Methlin G, et al. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer research. 1993;53(1):165–169. [PubMed] [Google Scholar]
- Myoung H, Kim MJ, Hong SD, Lee JI, Lim CY, Hong SP. Expression of membrane type I-matrix metalloproteinase in oral squamous cell carcinoma. Cancer letters. 2002;185(2):201–209. doi: 10.1016/s0304-3835(02)00281-1. [DOI] [PubMed] [Google Scholar]
- Nagashima Y, Hasegawa S, Koshikawa N, Taki A, Ichikawa Y, Kitamura H, Misugi K, Kihira Y, Matuo Y, Yasumitsu H, Miyazaki K. Expression of matrilysin in vascular endothelial cells adjacent to matrilysin-producing tumors. International journal of cancer Journal international du cancer. 1997;72(3):441–445. doi: 10.1002/(sici)1097-0215(19970729)72:3<441::aid-ijc11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Nikawa T, Furochi H, Kosyoji M, Hirasaka K, Suzue N, Sairyo K, Nakano S, Yamaoka T, Itakura M, Kishi K, Yasui N. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am J Physiol Cell Physiol. 2005;289(3):C697–707. doi: 10.1152/ajpcell.00565.2004. [DOI] [PubMed] [Google Scholar]
- Ondruschka C, Buhtz P, Motsch C, Freigang B, Schneider-Stock R, Roessner A, Boltze C. Prognostic value of MMP-2, -9 and TIMP-1,-2 immunoreactive protein at the invasive front in advanced head and neck squamous cell carcinomas. Pathol Res Pract. 2002;198(8):509–515. doi: 10.1078/S0344-0338(04)70292-7. [DOI] [PubMed] [Google Scholar]
- Oyewumi MO, Manickavasagam D, Novak K, Wehrung D, Paulic N, Moussa FM, Sondag GR, Safadi FF. Osteoactivin (GPNMB) ectodomain protein promotes growth and invasive behavior of human lung cancer cells. Oncotarget. 2016;7(12):13932–13944. doi: 10.18632/oncotarget.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P Oc. Rhys-Evans P, Court WJ, Box GM, Eccles SA. Differential modulation of proliferation, matrix metalloproteinase expression and invasion of human head and neck squamous carcinoma cells by c-erbB ligands. Clinical & experimental metastasis. 1999;17(7):631–639. doi: 10.1023/a:1006751016860. [DOI] [PubMed] [Google Scholar]
- P OC. Rhys-Evans PH, Eccles SA. Expression of matrix metalloproteinases and their inhibitors correlates with invasion and metastasis in squamous cell carcinoma of the head and neck. Archives of otolaryngology–head & neck surgery. 2001;127(7):813–820. [PubMed] [Google Scholar]
- Piotrowski WJ, Gorski P, Pietras T, Fendler W, Szemraj J. The selected genetic polymorphisms of metalloproteinases MMP2, 7, 9 and MMP inhibitor TIMP2 in sarcoidosis. Med Sci Monit. 2011;17(10):CR598–607. doi: 10.12659/MSM.881987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack VA, Alvarez E, Tse KF, Torgov MY, Xie S, Shenoy SG, MacDougall JR, Arrol S, Zhong H, Gerwien RW, Hahne WF, Senter PD, Jeffers ME, Lichenstein HS, LaRochelle WJ. Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol. 2007;60(3):423–435. doi: 10.1007/s00280-007-0490-z. [DOI] [PubMed] [Google Scholar]
- Rich JN, Shi Q, Hjelmeland M, Cummings TJ, Kuan CT, Bigner DD, Counter CM, Wang XF. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. The Journal of biological chemistry. 2003;278(18):15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- Rose AA, Annis MG, Dong Z, Pepin F, Hallett M, Park M, Siegel PM. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PloS one. 2010a;5(8):e12093. doi: 10.1371/journal.pone.0012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AA, Grosset AA, Dong Z, Russo C, Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park M, Gaboury L, Siegel PM. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010b;16(7):2147–2156. doi: 10.1158/1078-0432.CCR-09-1611. [DOI] [PubMed] [Google Scholar]
- Rose AA, Pepin F, Russo C, Abou Khalil JE, Hallett M, Siegel PM. Osteoactivin promotes breast cancer metastasis to bone. Molecular cancer research : MCR. 2007;5(10):1001–1014. doi: 10.1158/1541-7786.MCR-07-0119. [DOI] [PubMed] [Google Scholar]
- Ruokolainen H, Paakko P, Turpeenniemi-Hujanen T. Expression of matrix metalloproteinase-9 in head and neck squamous cell carcinoma: a potential marker for prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(9):3110–3116. doi: 10.1158/1078-0432.ccr-03-0530. [DOI] [PubMed] [Google Scholar]
- Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. The Journal of biological chemistry. 2001;276(11):8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- Shimada T, Nakamura H, Yamashita K, Kawata R, Murakami Y, Fujimoto N, Sato H, Seiki M, Okada Y. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human oral squamous cell carcinomas: implications for lymph node metastasis. Clinical & experimental metastasis. 2000;18(2):179–188. doi: 10.1023/a:1006749501682. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Soni S, Mathur M, Shukla NK, Deo SV, Ralhan R. Stromelysin-3 expression is an early event in human oral tumorigenesis. International journal of cancer Journal international du cancer. 2003;107(2):309–316. doi: 10.1002/ijc.11366. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Stott-Miller M, Houck JR, Lohavanichbutr P, Mendez E, Upton MP, Futran ND, Schwartz SM, Chen C. Tumor and salivary matrix metalloproteinase levels are strong diagnostic markers of oral squamous cell carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(12):2628–2636. doi: 10.1158/1055-9965.EPI-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr ML, Dysvik B, Bruland O, Warnakulasuriya S, Amaratunga AN, Jonassen I, Vasstrand EN, Ibrahim SO. Gene expression profile of oral squamous cell carcinomas from Sri Lankan betel quid users. Oncology reports. 2007;18(5):1061–1075. [PubMed] [Google Scholar]
- Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cytokines on matrix metalloproteinase expression in oral squamous cell carcinoma in vitro. Acta Otolaryngol. 2005;125(7):765–773. doi: 10.1080/00016480510027484. [DOI] [PubMed] [Google Scholar]
- Sutinen M, Kainulainen T, Hurskainen T, Vesterlund E, Alexander JP, Overall CM, Sorsa T, Salo T. Expression of matrix metalloproteinases (MMP-1 and -2) and their inhibitors (TIMP-1, -2 and -3) in oral lichen planus, dysplasia, squamous cell carcinoma and lymph node metastasis. Br J Cancer. 1998;77(12):2239–2245. doi: 10.1038/bjc.1998.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GJ, Lewis MP, Whawell SA, Russell A, Sheppard D, Hart IR, Speight PM, Marshall JF. Expression of the alphavbeta6 integrin promotes migration and invasion in squamous carcinoma cells. J Invest Dermatol. 2001;117(1):67–73. doi: 10.1046/j.0022-202x.2001.01379.x. [DOI] [PubMed] [Google Scholar]
- Tokumaru Y, Fujii M, Otani Y, Kameyama K, Imanishi Y, Igarashi N, Kanzaki J. Activation of matrix metalloproteinase-2 in head and neck squamous cell carcinoma: studies of clinical samples and in vitro cell lines co-cultured with fibroblasts. Cancer letters. 2000;150(1):15–21. doi: 10.1016/s0304-3835(99)00371-7. [DOI] [PubMed] [Google Scholar]
- Tonogai I, Takahashi M, Yukata K, Sato R, Nikawa T, Yasui N, Sairyo K. Osteoactivin attenuates skeletal muscle fibrosis after distraction osteogenesis by promoting extracellular matrix degradation/remodeling. J Pediatr Orthop B. 2015;24(2):162–169. doi: 10.1097/BPB.0000000000000117. [DOI] [PubMed] [Google Scholar]
- Torres C, Linares A, Alejandre MJ, Palomino-Morales R, Martin M, Delgado JR, Martinez J, Perales S. The potential role of the glycoprotein osteoactivin/glycoprotein nonmetastatic melanoma protein B in pancreatic cancer. Pancreas. 2015;44(2):302–310. doi: 10.1097/MPA.0000000000000250. [DOI] [PubMed] [Google Scholar]
- Torres C, Perales S, Alejandre MJ, Iglesias J, Palomino RJ, Martin M, Caba O, Prados JC, Aranega A, Delgado JR, Irigoyen A, Ortuno FM, Rojas I, Linares A. Serum cytokine profile in patients with pancreatic cancer. Pancreas. 2014;43(7):1042–1049. doi: 10.1097/MPA.0000000000000155. [DOI] [PubMed] [Google Scholar]
- Tse KF, Jeffers M, Pollack VA, McCabe DA, Shadish ML, Khramtsov NV, Hackett CS, Shenoy SG, Kuang B, Boldog FL, MacDougall JR, Rastelli L, Herrmann J, Gallo M, Gazit-Bornstein G, Senter PD, Meyer DL, Lichenstein HS, LaRochelle WJ. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(4):1373–1382. doi: 10.1158/1078-0432.CCR-05-2018. [DOI] [PubMed] [Google Scholar]
- Vendelin J, Bruce S, Holopainen P, Pulkkinen V, Rytila P, Pirskanen A, Rehn M, Laitinen T, Laitinen LA, Haahtela T, Saarialho-Kere U, Laitinen A, Kere J. Downstream target genes of the neuropeptide S-NPSR1 pathway. Hum Mol Genet. 2006;15(19):2923–2935. doi: 10.1093/hmg/ddl234. [DOI] [PubMed] [Google Scholar]
- Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. International journal of cancer Journal international du cancer. 2002;99(2):157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- Villaret DB, Wang T, Dillon D, Xu J, Sivam D, Cheever MA, Reed SG. Identification of genes overexpressed in head and neck squamous cell carcinoma using a combination of complementary DNA subtraction and microarray analysis. The Laryngoscope. 2000;110(3 Pt 1):374–381. doi: 10.1097/00005537-200003000-00008. [DOI] [PubMed] [Google Scholar]
- Vinci M, Box C, Eccles SA. Three-dimensional (3D) tumor spheroid invasion assay. J Vis Exp. 2015;(99):e52686. doi: 10.3791/52686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Abreu Rde S, Ko D, Le SY, Shapiro BA, Burns SC, Sandhu D, Boutz DR, Marcotte EM, Penalva LO. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol Syst Biol. 2010;6:400. doi: 10.1038/msb.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JA, Rathcke IO, Mandic R. The role of matrix metalloproteinases in squamous cell carcinomas of the head and neck. Clinical & experimental metastasis. 2002;19(4):275–282. doi: 10.1023/a:1015531319087. [DOI] [PubMed] [Google Scholar]
- Winter S, Kohl A, Huppertz A, Herold-Mende C, Wiest T, Komposch G, Tomakidi P. Expression of mRNAs encoding for growth factors, ECM molecules, and MMP13 in mono-cultures and co-cultures of human periodontal ligament fibroblasts and alveolar bone cells. Cell and tissue research. 2005;319(3):467–478. doi: 10.1007/s00441-004-1026-z. [DOI] [PubMed] [Google Scholar]
- Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC genomics. 2008;9:69. doi: 10.1186/1471-2164-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CY, Chen CH, Chang CH, Tseng HF, Liu SY, Chuang LY, Wen CH, Chang HW. Matrix metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential oral cancer markers. Biomarkers. 2009;14(4):244–249. doi: 10.1080/13547500902829375. [DOI] [PubMed] [Google Scholar]
- Yoshizaki T, Maruyama Y, Sato H, Furukawa M. Expression of tissue inhibitor of matrix metalloproteinase-2 correlates with activation of matrix metalloproteinase-2 and predicts poor prognosis in tongue squamous cell carcinoma. International journal of cancer Journal international du cancer. 2001;95(1):44–50. doi: 10.1002/1097-0215(20010120)95:1<44::aid-ijc1008>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes & development. 1999;13(1):35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Wu Y, Helman JI, Wen Y, Wang C, Li L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Molecular cancer research : MCR. 2011;9(2):161–172. doi: 10.1158/1541-7786.MCR-10-0386. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu Y, Li YF, Yue Y, Yang X, Peng L. Targeting of Survivin Pathways by YM155 Inhibits Cell Death and Invasion in Oral Squamous Cell Carcinoma Cells. Cell Physiol Biochem. 2016;38(6):2426–2437. doi: 10.1159/000445594. [DOI] [PubMed] [Google Scholar]
- Ziober AF, Patel KR, Alawi F, Gimotty P, Weber RS, Feldman MM, Chalian AA, Weinstein GS, Hunt J, Ziober BL. Identification of a gene signature for rapid screening of oral squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(20 Pt 1):5960–5971. doi: 10.1158/1078-0432.CCR-06-0535. [DOI] [PubMed] [Google Scholar]


