Abstract
Background
Knowledge of hepatitis C virus (HCV) is believed to be important in altering risk behaviour, improving engagement in care, and promoting willingness to initiate HCV treatment. We assessed factors associated with HCV knowledge and treatment willingness amongst people who inject drugs (PWID) in an era of direct acting antivirals.
Methods
Data were derived from three prospective cohort studies of PWID in Vancouver, Canada, between June 2014 and May 2015. HCV knowledge and treatment willingness were assessed using a Likert scale. Multivariable linear regression identified factors associated with higher HCV knowledge and treatment willingness.
Results
Amongst 630 participants, mean scores for HCV knowledge and treatment willingness were 25.41 (standard deviation [SD]: 2.52) out of 30, and 6.83 (SD: 1.83) out of 10, respectively. In multivariable analyses, Caucasian ancestry (adjusted linear regression model estimate [β] 0.50; 95% confidence interval [CI] 0.17, 0.82), employment (β 0.76; 95% CI: 0.38, 1.13), diagnosed mental health disorder (β 0.44; 95% CI: 0.11, 0.78) and previous HCV treatment (β 0.94; 95% CI: 0.46, 1.43) were independently associated with higher knowledge. Downtown Eastside (DTES) residence (i.e., epicenter of Vancouver’s drug scene) was independently associated with lower knowledge (β −0.48; 95% CI: −0.81, −0.15). Greater HCV knowledge (β 0.12; 95% CI: 0.07, 0.17) was independently associated with higher HCV treatment willingness. DTES residence (β −0.31; 95% CI: −0.56, −0.06) and daily crack cocaine smoking (β −0.52; 95% CI: −0.92, −0.13) were independently associated with lower treatment willingness.
Conclusion
Socioeconomic factors, such as neighborhood residence and employment, were associated with HCV knowledge. Higher HCV knowledge was associated with more HCV treatment willingness. Our findings suggest that increasing HCV knowledge amongst PWID may be an integral component of the HCV cascade of care and that efforts might be best targeted to individuals with greater socioeconomic disadvantage.
Keywords: hepatitis c, knowledge, treatment, willingness, socioeconomic
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a leading cause of end-stage liver disease in North America (Kim et al., 2015). People who inject drugs (PWID) compose a large subset of individuals infected with HCV (Hajarizadeh, Grebely, & Dore, 2013). Among PWID, up to 90% of individuals may be HCV-seropositive (Ng et al., 2013). PWID populations face multiple barriers to accessing treatment, with treatment uptake rates ranging from 1.5% to 2.0% per year (Alavi et al., 2014; Iversen et al., 2014). Barriers to care are multifactorial at the patient, provider, treatment regimen, and health care system levels. Until recently, complex therapy based on interferon and ribavirin have presented a significant barrier to treatment. These medications have been associated with multiple toxicities and side effects, while associated with low rates of cure (Ray & Thomas, 2015).
Knowledge of HCV transmission, natural history and treatment is believed to be an important step in altering transmission risk behaviour, enhancing health maintenance and increasing engagement with HCV treatment (Kwiatkowski, Fortuin Corsi, & Booth, 2002). Studies have shown that lower HCV knowledge amongst PWID, is linked to socioeconomic factors such as lower levels of formal education and non-Caucasian ancestry (Surjadi, Torruellas, Ayala, Yee, & Khalili, 2011; Treloar et al., 2011). Conversely, factors associated with increased healthcare exposure such as opioid agonist treatment (OAT) and more frequent visits to general practitioners have been associated with increased HCV knowledge (Dunn et al., 2013; Treloar et al., 2011). An important finding for treatment programs is that willingness to undergo HCV therapy has been associated with higher baseline HCV knowledge (Surjadi et al., 2011; Zeremski et al., 2014). Understanding which factors are associated with HCV knowledge levels enables development of targeted interventions to enhance HCV knowledge with the ultimate goal of increasing treatment uptake and completion.
Much of the literature to date has examined factors associated with HCV knowledge and treatment willingness prior to the current direct acting antiviral (DAA) era. Interventions tailored to the needs of PWID will be an important component of efforts to roll out HCV treatment and prevention programs. As Interferon sparing DAA-based therapies became widely available in Vancouver, Canada beginning in the spring/summer of 2014, the objective of this study was to assess HCV knowledge in a cohort of PWID in this setting, between June 2014 and May 2015. We aimed to identify factors that were associated with greater HCV knowledge as well as a greater willingness to undergo HCV treatment in the current DAA era in order to better inform future education and treatment efforts.
METHODS
Study design
The Vancouver Injection Drug Users Study (VIDUS), AIDS Care Cohort to Evaluate exposure to Survival Services (ACCESS) and At-Risk Youth Study (ARYS) are open prospective cohorts of drug users in Vancouver, Canada. These cohorts, including detailed sampling and recruitment procedures, have been described elsewhere (Strathdee et al., 1998; Tyndall et al., 2003; Wood et al., 2006). VIDUS enrolls human immunodeficiency virus (HIV)-negative adults (≥18 years of age) who injected drugs in the month prior to enrolment; ACCESS enrolls HIV-positive adults (≥18 years of age) who used illicit drugs (other than or in addition to cannabis) in the month prior to enrolment; and ARYS enrolls street-involved youth aged 14–26 years who used illicit drugs in the previous month. The primary modes of recruitment in all cohorts are self-referral, word of mouth, and street outreach. Participants are required to reside in the Greater Vancouver region at enrolment and provide written informed consent.
The follow-up procedures for these studies were harmonized to allow for analyses of merged data. At baseline and semi-annually thereafter, participants completed an interviewer-administered questionnaire collecting data on demographics, drug use patterns, healthcare access, and other exposures. Venous blood samples were drawn at each visit for HCV and HIV serologic testing and HIV disease monitoring as appropriate. Referral for free HIV/AIDS care was provided to those found to be HIV positive, and these individuals were subsequently followed in ACCESS. In addition, a complete HIV-related clinical profile, including exposure to antiretroviral agents, was obtained for all ACCESS participants through a confidential linkage with the provincial Drug Treatment Program (Hogg et al., 1998, 2001). Participants were given a stipend ($40 CDN) at each study visit. The cohort studies have received annual approval from the University of British Columbia/Providence Healthcare Research Ethics Board.
Participants and HCV knowledge and willingness assessment
For this analysis, questionnaire responses collected from June 1, 2014 to May 30, 2015 were queried for all cohort participants who self-reported ever testing positive for HCV. Those who did not report a history of injection drug use or who reported being HCV-seronegative were excluded. Self-report was used for exclusion as only those respondents who self-reported being HCV-positive were asked specific HCV knowledge and treatment willingness questionnaire items.
HCV knowledge and treatment willingness were assessed with a series of questions (14 items) pertaining to HCV transmission, natural history and treatment willingness. These questions were developed by local healthcare providers who are involved in the treatment of individuals with HCV. Responses were recorded using a Likert scale from 1 (“strongly disagree”) to 5 (“strongly agree”) with most questions worded such that a higher score reflected a greater level of HCV knowledge or willingness to participate in HCV therapy. Questions that were worded such that a lower numeric response reflected more knowledge or willingness were imputed with a reversed scale to allow comparison between questions. Respondents were also permitted to decline answering a questionnaire component or to answer “not applicable” or “I don’t know.”
For the assessment of factors associated with HCV knowledge and treatment willingness, participants were excluded if they had missing or inadequate responses (responding “not applicable”, “I don’t know” or declining to respond) for at least one question, due to inability to calculate consistent total knowledge scores with these responses. For the assessment of factors associated with treatment willingness, individuals who had been successfully treated or who were on treatment at the time of the questionnaire were also excluded from further analysis. If a participant completed two study visits during the study period, we used the most recent observation only.
Study variables
The primary outcomes of interest were the composite scores for knowledge and treatment willingness, respectively, based on the summation of responses to the remaining questionnaire items following a factor analysis [Table 2].
Table 2.
Summary scores of the HCV knowledge and willingness to undergo HCV treatment scales amongst HCV positive people who inject drugs in Vancouver, Canada (n = 1192).
| Questionnaire Item | Mean (SD) | % answering “strongly agree” or “agree” |
|---|---|---|
|
HCV knowledge scale I know how HCV is transmitted |
4.09 (0.65) | 88.1% |
| Using clean needles, syringes and equipment reduces the risk of being infected with HCV | 4.30 (0.63) | 93.3% |
| People living with HCV must be careful about sharing toothbrushes or razors | 4.21 (0.61) | 91.7% |
| People living with HCV can live for many years without knowing they have it | 4.14 (0.61) | 88.8% |
| HCV can cause liver failure | 4.25 (0.51) | 93.1% |
| Once HCV has been cured, people could catch it again if they still share needles | 4.17 (0.55) | 88.0% |
| HCV treatment willingness scale | ||
| I would be willing to take treatment if it meant only taking pills and no injection | 3.29 (1.06) | 44.2% |
| I would be willing to consider starting treatment for HCV in the next year | 3.53 (1.08) | 53.5% |
| Other questionnaire items | ||
| I know a lot about hepatitis C in general | 3.57 (0.95) | 61.1% |
| My doctor has discussed treatment for hepatitis C with me | 3.50 (1.12) | 61.5% |
| Treatment for hepatitis C can cure the infection in most people | 3.78 (0.81) | 66.3% |
| Treatment for hepatitis C consists of a weekly injection and pills every daya | – | 51.3% |
| What I have heard about the side effects of treatment for hepatitis C scares me | 3.53 (1.03) | 56.9% |
| Treating my other illnesses would be more important than treating hepatitis C | 3.12 (1.08) | 31.0% |
SD: standard deviation. HCV: hepatitis C virus.
Where questionnaire scored from 1 (strongly disagree) to 5 (strongly agree). Responses of not available, I don’t know or refusal to answer the question were removed from the analysis for each question.
Response recorded as true, false or I don’t know. Proportion answering true reported, calculation of mean not performed.
We considered a range of explanatory variables. Sociodemographic characteristics included age (per year older), sex (male vs. female), Caucasian ancestry, ≥high school completion, homelessness, Downtown Eastside (DTES) residence, and employment. The DTES is an area in Vancouver with an increased density of poverty and illicit substance use. Drug use behaviours included ≥daily heroin injection, ≥daily cocaine injection, ≥daily crack smoking, years of injecting (per year longer), and heavy alcohol use as defined by the National Institute for Alcohol Abuse and Alcoholism (National Institutes of Health, 2015). Variables assessing healthcare access included receipt of OAT, accessing any health or social service, HIV coinfection (stratified by ≥95% or <95% antiretroviral adherence, as in previous studies) (Milloy et al., 2016), ever diagnosed with a mental health disorder, and ever taking HCV treatment. HCV knowledge scores were added to the analysis of treatment willingness. All behavioural variables referred to the past 6 months and were dichotomized as yes vs. no unless otherwise stated.
We also assessed attitudes towards HCV treatment by asking participants “Which is more important to you if you were to consider treatment?” with response options of “How long it lasts” or “Types of side effects”.
Statistical analysis
Exploratory factor analysis using varimax rotation was used to determine the number of factors present among the 14 items, using a maximum likelihood method. These calculations were performed using SAS software, version 9.3 (SAS Institute, Cary, North Carolina, USA). Factors were then retained if: they had an Eigenvalue of greater than 1 and preceded the elbow in a Scree plot, and if the set of items collectively accounted for 70–80% of the variance. Factor loadings were used to determine the number of items included within each factor and items with factor loading >0.4 were retained. In order to validate an adequate number of parameters for the final model, Tucker and Lewis’s reliability coefficient for the maximum likelihood was calculated and yielded a score of close to 1 (0.93). This measure is based on the residual correlations in the matrix after the effects of final factors are taken out. The coefficient ranges from 0 (poorest fit) to 1 (complete fit), therefore a score of 0.93 indicates successful choice of questionnaire items. We further calculated Cronbach’s alpha to measure internal consistency of the reduced groups, where alpha is a function of the number of items in a test. The final subscales of knowledge and treatment willingness were composed of 6 and 2 items, respectively, and had Cronbach’s alpha’s of 0.81 and 0.64. A Cronbach’s alpha of ≥ 0.7 is considered good and ≥ 0.6 acceptable (Lance, Butts, & Michels, 2006). The individual item scores were combined to come up with the total knowledge and willingness scores (maximum scores of 30 and 10, respectively). Both knowledge and willingness were assessed as continuous variables using these composite scores with higher scores corresponding to higher knowledge or willingness, respectively.
Next, bivariable and multivariable linear regression analyses were performed to assess factors associated with HCV knowledge and willingness to undergo therapy, respectively. We used an a priori-defined backward model selection procedure based on examination of the Akaike Information Criterion (AIC) and p-values to construct a multivariable model. Specifically, variables were included in the full multivariable model if they were significantly associated with knowledge or willingness in the bivariable analyses at a p-value of <0.1. After examining the AIC of the full model, we removed the variable with the largest p-value and built a reduced model. We continued this iterative process until no variables remained for inclusion. We selected the multivariable model with the lowest AIC score for each outcome, as previously described (Hayashi et al., 2012).
We examined differences between the sample eligible for the analyses of HCV knowledge levels and the group excluded due to inadequate or missing questionnaire responses, using Chi-squared and Mann-Whitney test. Further, two sensitivity analyses including the group who answered “I don’t know” to any knowledge or willingness item were performed by imputing “I don’t know” as corresponding to a response of either 1 (“strongly disagree”) or 3 (“neutral”), respectively. This was performed due to the concern that participants responding “I don’t know” represented a vulnerable subset of our population and that excluding them from the analysis may have biased our findings. This component of the statistical analysis was performed using RStudio, version 0.99.892 (R Foundation for Statistical Computing, Vienna, Austria). All p-values were two-sided.
RESULTS
As shown in Figure 1, 1192 PWID who self-reported being HCV-seropositive completed a study visit during the study period. Characteristics of the sample are shown in Table 1. Overall knowledge level in the total sample (n=1192) was high with mean responses above 4 (“agree”) for all six included items [Table 2]. More than 88% of participants correctly answered (4 “agree” or 5 “strongly agree”) each included knowledge question item. Mean composite score for HCV knowledge amongst 630 questionnaire respondents who answered all questions was 25.41 (standard deviation [SD]: 2.52) out of 30.
Figure 1.
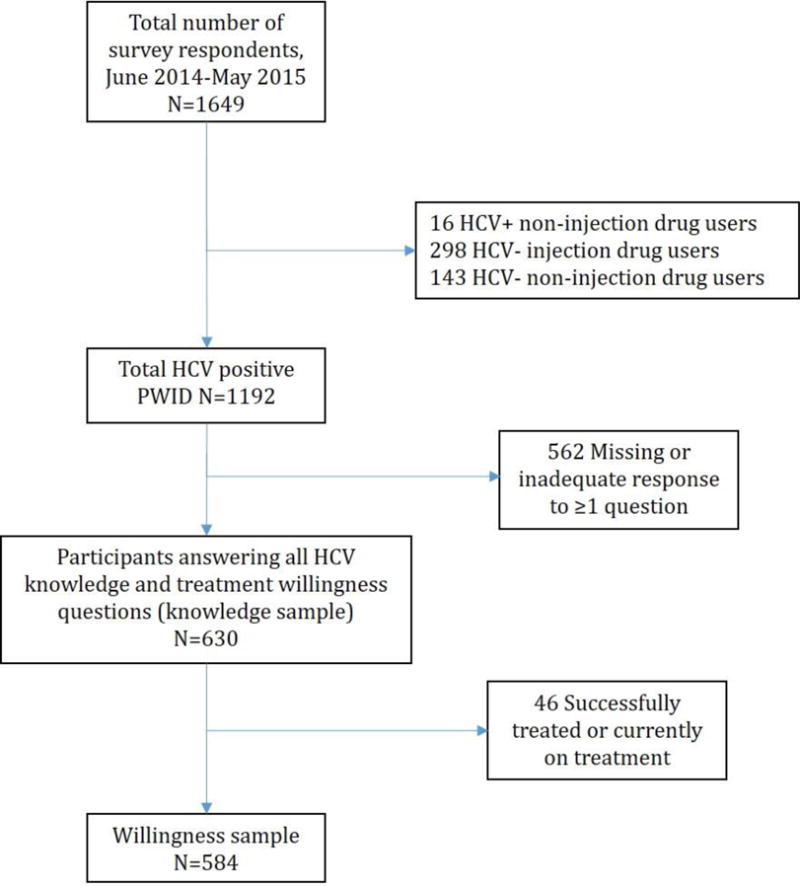
Determination of analytic samples.
Table 1.
Characteristics of self-reported HCV positive people who inject drugs in the VIDUS, ACCESS and ARYS cohorts, June 1, 2014 to May 30, 2015 (n = 1192).
| Characteristics | Knowledge Cohort n=630 N (%) |
Missing Responses n=562 N (%) |
p-value |
|---|---|---|---|
| Age (median, IQR) | 49.1 (42.2–54.9) | 48.9 (39.8–55.3) | 0.67 |
| Male | 422 (67.0) | 333 (59.3) | 0.01 |
| Ethnicity/Ancestry | |||
| Caucasian | 366 (58.1) | 316 (56.2) | 0.52 |
| Aboriginal/First Nations | 188 (29.8) | 184 (32.7) | |
| Asian | 7 (1.1) | 8 (1.4) | |
| Black/Caribbean/African American | 13 (2.1) | 6 (1.1) | |
| Other | 56 (8.9) | 48 (8.5) | |
| ≥High school diploma | 305 (48.4) | 249 (44.3) | 0.16 |
| Homelessa | 90 (14.3) | 106 (18.9) | 0.03 |
| DTES residencea | 343 (54.4) | 310 (55.2) | 0.80 |
| Employmenta | 155 (24.6) | 146 (26.0) | 0.59 |
| ≥Daily heroin injectiona | 131 (20.8) | 113 (20.1) | 0.77 |
| ≥Daily cocaine injectiona | 33 (5.2) | 31 (5.5) | 0.83 |
| ≥Daily crack smokinga | 65 (10.3) | 75 (13.4) | 0.11 |
| Heavy alcohol usea | 80 (12.7) | 83 (14.8) | 0.30 |
| Years of injection drug use (median, IQR) | 25.7 (18.1–35.7) | 27.5 (18.1–35.7) | 0.47 |
| Accessed any health or social servicesa | 503 (79.8) | 449 (79.9) | 0.98 |
| Opioid agonist therapya | 345 (54.8) | 306 (54.5) | 0.97 |
| HIV serostatus | |||
| HIV negative | 350 (55.6) | 354 (63.0) | 0.02 |
| HIV positive, ART adherence <95% | 111 (17.6) | 76 (13.5) | |
| HIV positive, ART adherence ≥95% | 169 (26.8) | 131 (23.3) | |
| History of mental health disorder | 408 (64.8) | 343 (61.0) | 0.18 |
| Seen by physician for HCV carea | 388 (61.6) | 204 (36.3) | <0.0001 |
| Seen by HCV specialista | 109 (17.3) | 53 (9.4) | <0.0001 |
| Ever undergone transient elastography | 53 (8.4) | 22 (3.9) | 0.001 |
| Ever offered HCV treatment | 344 (53.0) | 236 (42.0) | <0.0001 |
| Ever taken HCV treatment | 58 (9.2) | 68 (12.1) | 0.105 |
| Cohort | |||
| ACCESS | 280 (44.4) | 207 (36.8) | 0.01 |
| ARYS | 34 (5.4) | 44 (7.83) | |
| VIDUS | 316 (50.2) | 311 (55.3) |
IQR: interquartile range. DTES: Downtown Eastside. HIV: human immunodeficiency virus. ART: antiretroviral therapy. HCV: hepatitis C virus. VIDUS: Vancouver Injection Drug Users Study. ARYS: At-Risk Youth Study. ACCESS: AIDS Care Cohort to Evaluate exposure to Survival Services
Denotes activities in the previous six months.
Willingness to undergo treatment was somewhat lower (<4) compared to knowledge scores [Table 2]. Excluding “not applicable” respondents with a history of prior or current treatment, 61% of participants “strongly agreed” or “agreed” to consider starting HCV treatment within the next year. Of 584 respondents, excluding those previously successfully treated or currently on treatment, the mean composite score for treatment willingness was 6.83 (SD: 1.83) out of 10.
Participants were asked additional questions related to treatment willingness which were not included in the composite score. When asked if they prioritized treatment duration versus risk of side effects, 58% reported risk of side effects as a more important treatment consideration while only 16% were more concerned about treatment duration. Fifty-seven percent of participants reported that what they have heard about the side effects of HCV treatment scares them. Only 66.3% of respondents answered “agree” or “strongly agree” to the question “Treatment for hepatitis C can cure the infection in most people”, and 51.3% still believed that HCV treatment included a weekly interferon injection.
Factors Associated with HCV Knowledge
Six hundred and thirty (53%) participants were included in the analysis of factors associated with HCV knowledge level after exclusion of those with missing or inadequate responses. The majority of the knowledge sample were male (67%) with a median age of 49.1 years (interquartile range [IQR]: 42.2–54.9) and were more likely to be HIV-positive compared to the missing responses group (p = 0.02). The missing responses group was more likely to be homeless, and less likely to have been engaged in HCV care as demonstrated by less contact with a physician regarding HCV, fewer HCV specialist referrals, fewer transient elastography assessments and less offers of HCV treatment (all p <0.05). Among the 630 participants, 344 (53%) had ever been offered HCV treatment and of those offered therapy, 58 (17%) had ever initiated treatment.
Factors associated with HCV knowledge levels are shown in Table 3. In multivariable analyses, factors independently associated with increased level of HCV knowledge included: Caucasian ancestry (adjusted linear regression model estimate [β] 0.50; 95% Confidence Interval [CI]: 0.17, 0.82), being employed (β 0.76; 95% CI: 0.38, 1.13), a history of being diagnosed with a mental health disorder (β 0.44; 95% CI: 0.44, 0.11), and previous receipt of HCV treatment (β 0.94; 95% CI: 0.46, 1.43). Attainment of high school education or greater was not associated with HCV knowledge (unadjusted β 0.28; 95% CI: −0.06, 0.61). DTES residence was independently associated with a lower level of HCV knowledge (β −0.48; 95% CI: −0.81, −0.15).
Table 3.
Bivariable and multivariable linear regression of factors associated with HCV knowledge and willingness to undertake treatment among HCV-positive people who inject drugs in Vancouver, Canada (2014–2015).
| Characteristic | Knowledge (n = 630) |
Willingness (n = 584) |
||
|---|---|---|---|---|
| Unadjusted Model Estimate (β) (95% CI)a |
Adjusted Model Estimate (β) (95% CI)a |
Unadjusted Model Estimate (β) (95% CI)a |
Adjusted Model Estimate (β) (95% CI)a |
|
| Age (per year older) | 0.0001 (−0.02,0.02) | 0.0004 (−0.01, 0.01) | ||
| Male (yes vs. no) | 0.42 (0.07,0.77) | 0.28 (0.02, 0.55) | ||
| Caucasian ancestry (yes vs. no) | 0.67 (0.34,1.01))** | 0.50 (0.17, 0.82)* | 0.09 (−0.16, 0.35) | |
| ≥High school education (yes vs. no) | 0.29 (−0.06, 0.61) | −0.13 (−0.38, 0.12) | ||
| Homelessb (yes vs. no) | −0.01 (−0.19, 0.46) | −0.02 (−0.37, 0.33) | ||
| Downtown Eastside residenceb (yes vs. no) | −0.68 (−1.0, −.035)** | −0.48 (−0.81, −0.15)* | −0.40 (−0.65, −0.15)** | −0.31 (−0.56, −0.06)* |
| Employmentb (yes vs. no) | 0.86 (0.48, 1.24)** | 0.76 (0.38, 1.13)** | 0.27 (−0.02, 0.56) | |
| ≥Daily crack smokingb (yes vs. no) | −0.01 (−0.56, 0.53) | −0.55 (−0.96, −0.15)* | −0.52 (−0.92, −0.13)* | |
| ≥Daily heroin injectionb (yes vs. no) | −0.30 (−0.70, 0.11) | −0.14 (−0.45, 0.16) | ||
| ≥Daily cocaine injectionb (yes vs. no) | −0.27 (−1.02, 0.47) | 0.24 (−0.30, 0.78) | ||
| Heavy alcohol useb (yes vs. no) | 0.14 (−0.35, 0.64) | 0.44 (0.06, 0.82) | ||
| Years of IDU (per year longer) | 0.005 (−0.01, 0.02) | −0.003 (−0.01, 0.01) | ||
| Accessed health or social servicesb (yes vs. no) | 0.26 (−0.16, 0.67) | 0.21 (−0.10, 0.53) | ||
| OAT (yes vs. no) | 0.02 (−0.31, 0.36) | 0.01 (−0.24, 0.26) | ||
| HIV seropositive (yes vs. no) | −0.04 (−0.37, 0.30) | 0.23 (−0.02, 0.48) | ||
| ART adherence <95% vs. HIV negative | 0.17 (−0.28, 0.62) | 0.15 (−0.19, 0.49) | ||
| ART adherence ≥95% vs. HIV negative | −0.17 (−0.56, 0.22) | 0.28 (−0.02, 0.57) | ||
| History of mental health disorder (yes vs. no) | 0.46 (0.11, 0.80)* | 0.44 (0.11, 0.78)* | 0.12 (−0.14, 0.38) | |
| Previous HCV treatment (yes vs. no) | 1.08 (0.59, 1.57)** | 0.94 (0.46, 1.43)** | 0.64 (0.11, 1.18)* | |
| HCV Knowledge (per score increase) | – | – | 0.13 (0.08, 0.18)** | 0.12 (0.07, 0.17)** |
HIV: human immunodeficiency virus. OAT: opioid agonist therapy. IDU: injection drug use. HCV: hepatitis C virus.
Factors Associated with HCV Treatment Willingness
Assessment of factors associated with willingness to undergo HCV treatment was performed in 584 (49%) individuals after excluding those previously successfully treated or currently on treatment. As shown in Table 3, participants were less willing to undergo HCV treatment if they resided in the DTES (β −0.31; 95% CI: −0.56, −0.06) or reported at least daily crack cocaine smoking (β −0.52; 95% CI: −0.92, −0.13). A greater degree of HCV knowledge was associated with an increased willingness to pursue HCV treatment (β 0.12; 95% CI: 0.07, 0.17).
Sensitivity Analysis
The results of sensitivity analyses were largely unchanged from the planned analysis. The only difference was that older age was associated with lower HCV knowledge (β −0.02; 95% CI: −0.04, −0.01), and that DTES residence was no longer independently associated with low treatment willingness (β −0.23; 95% CI: −0.44, −0.03; p=0.06).
DISCUSSION
Overall, we found a high level of HCV knowledge amongst our sample of PWID. Caucasian ancestry, employment and residence outside the DTES were independently associated with greater HCV knowledge implying a possible impact of socioeconomic and structural barriers upon knowledge acquisition. Greater healthcare exposure was associated with higher HCV knowledge scores while DTES residence and daily crack cocaine smoking were associated with less willingness to initiate HCV treatment. A one point increase in the composite HCV knowledge score was associated with a 0.1 point increase in the score of willingness to initiate HCV treatment, however, study participants cited concerns regarding treatment side effects as being a major barrier. Knowledge of high sustained virologic response (SVR) rates and the interferon-sparing nature of DAA regimens appeared to be limited and this may have contributed to the cohort having only modest willingness to initiate HCV treatment.
Previous studies, mostly conducted prior to the current DAA era, have shown varying levels of HCV knowledge amongst their populations. These studies have used different questionnaires to attempt to assess HCV knowledge and have evaluated diverse populations with or without a history of HCV infection or injection drug use, respectively (Cohen-Moreno et al., 2010; Dunn et al., 2013; Stein, Maksad, & Clarke, 2001; Strauss et al., 2007; Surjadi et al., 2011; Treloar, Hull, Dore, & Grebely, 2012; Zeremski et al., 2014). Among these studies, baseline average HCV knowledge scores were reported to range from 42–71% with lower scores generally reported in studies using more rigorous assessment tools. While heterogeneous assessment tools used across the studies do not permit direct comparison, knowledge amongst our sample appeared to be high relative to prior studies possibly reflecting cumulative population knowledge as HCV treatment efforts have scaled up over the years.
Caucasian ancestry was associated with greater HCV knowledge, with the second largest ancestral grouping amongst our study participants being comprised of individuals of Indigenous heritage. Within the social constructs that exist in our study setting, individuals of Caucasian ancestry may face fewer structural barriers thus enhancing their ability to acquire HCV knowledge. This finding also suggests that both assessment of HCV knowledge and provision of HCV education may benefit from attention to the cultural competence of providers.
Not surprisingly, previous HCV treatment was associated with increased HCV knowledge. Individuals with a history of HCV treatment would have had multiple HCV-specific healthcare encounters, accounting for their higher knowledge levels. Similarly, participants with a diagnosis of a mental health disorder would also have more frequent healthcare contact, which may have provided the opportunity for education surrounding HCV. Treatment with interferon based therapy is generally contraindicated in comorbid mental health disorders. Providers caring for this population may be more attuned to the advancements in interferon-sparing therapy for this reason, and thus may have discussed this option more readily with this group of participants. Frequency of healthcare contacts has also been associated with greater knowledge levels in previous studies (Dunn et al., 2013; Marshall et al., 2015; Treloar et al., 2011).
Based on prior studies, we had anticipated that HIV co-infection and OAT would be associated with HCV knowledge levels in our sample. However, this was not demonstrated in our analyses. The historically poor treatment response rates to interferon and ribavirin combination therapy, particularly in HIV co-infected individuals (Chung et al., 2004), may have resulted in HIV providers prioritizing other health concerns over HCV thus potentially explaining why those co-infected with HIV did not have an increased level of HCV knowledge despite more intense healthcare contact.
Daily crack smoking was associated with less willingness to undergo HCV treatment, as was DTES residence. The physiologic effects of crack cocaine intoxication may contribute to the association of daily crack cocaine smoking with lower HCV treatment willingness (Fischer et al., 2015). As well, given the silent nature of chronic HCV infection (Smith, Combellick, Jordan, & Hagan, 2015), competing health and socioeconomic priorities may supersede individual interest in HCV treatment among some individuals living in the DTES.
Consistent with previous studies (Norton et al., 2014; Surjadi et al., 2011; Zeremski et al., 2014), we found that a greater knowledge of HCV was associated with an increased willingness to undergo HCV therapy. These same studies have also demonstrated that educational interventions can increase participant knowledge levels as well as increase treatment willingness and subsequent follow up attendance at specialist clinics suggesting that knowledge does in fact play a role in willingness to undergo therapy.
HCV treatment willingness in populations similar to ours have been reported to be as high as 97% in the pre-DAA era (Surjadi et al., 2011). While greater HCV awareness amongst PWID may explain our participants’ high knowledge scores, this may also contribute to less willingness to initiate HCV treatment in our population due to the greater appreciation of treatment side effects from interferon containing regimens. This is supported by the finding that a significant proportion of our sample expressed concern regarding the risk of side effects from HCV therapy. Although specific knowledge questions in our final knowledge score did not address HCV treatment side effects, this finding suggests that the significantly improved side effect profile of the new interferon-sparing DAA regimens is likely an important knowledge gap to target in future educational interventions. Further, educational interventions targeting the paradigm shift in HCV treatment with respect to both improved tolerability and treatment success need to be prioritized in order to increase treatment enthusiasm amongst PWID.
Our study represents one of the largest cohort studies focused on assessing correlates of HCV knowledge and treatment willingness in PWID. The main limitation in our study was the lack of a validated HCV knowledge assessment tool. No single tool exists to assess an individual’s knowledge of HCV, making comparison of the findings of individual studies difficult. However, our findings identified similar factors and themes associated with a greater or lesser degree of HCV knowledge amongst PWID as seen in previous studies, suggesting that these various methods of knowledge assessment are in fact measuring similar constructs (Grau, Zhan, & Heimer, 2016; Marshall et al., 2015; Surjadi et al., 2011). A second limitation was that a large number of study participants answered “I don’t know” or declined to answer at least one of the knowledge questions. This group with missing or inadequate responses was significantly different from the final analytical sample in both socioeconomic factors and access to care. However, we note that sensitivity analyses did not significantly change our results. Thirdly, reporting bias in the self-reported data and the possibility of unidentified confounders, as occurs in all observational studies, were additional sources of limitations. Finally, non-random sampling methods used in our cohorts may limit the generalizability of our findings.
In summary, our study identified several socioeconomic and structural factors to be associated with both HCV knowledge and treatment willingness, suggesting that addressing the underlying structural inequality facing PWID is likely to represent an important aspect of HCV treatment rollout. As interferon sparing DAA-based therapies were introduced near the beginning of the study period, wide-spread community awareness of these new therapies was likely limited, as reflected in our findings of more modest knowledge pertaining to these treatments. Our findings suggest that it will be important for HCV programs to continue to focus on education, with an emphasis on the substantial improvement in side effect profiles and SVR rates of the current DAA treatments if expansion of HCV therapy amongst PWID is to be accomplished.
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. The study was supported by the US National Institutes of Health (VIDUS & ARYS: U01DA038886, ACCESS: R01DA021525). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine which supports Dr. Evan Wood, as well as a CIHR Foundation grant supporting Dr. Thomas Kerr (20R74326). Dr. Kora DeBeck is supported by a Michael Smith Foundation for Health Research (MSFHR)/St. Paul’s Hospital Foundation–Providence Health Care Career Scholar Award and a Canadian Institutes of Health Research (CIHR) New Investigator Award. Dr. Hayashi is supported by a CIHR New Investigator Award (MSH-141971). Dr. Milloy is supported by a CIHR New Investigator Award, an MSFHR Scholar Award and the US NIH (R01-DA0251525). His institution has received an unstructured gift from NG Biomed, Ltd., to support his research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Mark Hull has received honoraria for speaking engagements and advisory boards paid to his institution from Abbvie, BMS, Gilead, Janssen, Merck, Viiv.
All other co-authors have no conflict of interest to declare.
References
- Alavi M, Raffa JD, Deans GD, Lai C, Krajden M, Dore GJ, et al. Continued low uptake of treatment for hepatitis C virus infection in a large community-based cohort of inner city residents. Liver International. 2014;34(8):1198–1206. doi: 10.1111/liv.12370. [DOI] [PubMed] [Google Scholar]
- Cohen-Moreno R, Schiff M, Levitt S, Bar-Hamburger R, Strauss S, Neumark Y. Knowledge about Hepatitis-C among methadone maintenance treatment patients in Israel. Subst Use Misuse. 2010;45(1–2):58–76. doi: 10.3109/10826080902864894. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Saulsgiver KA, Patrick ME, Heil SH, Higgins ST, Sigmon SC. Characterizing and improving HIV and hepatitis knowledge among primary prescription opioid abusers. Drug and Alcohol Dependence. 2013;133(2) doi: 10.1016/j.drugalcdep.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Blanken P, Da Silveira D, Gallassi A, Goldner EM, Rehm J, et al. Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: A comprehensive narrative overview of English-language studies. International Journal of Drug Policy. 2015;26(4):352–363. doi: 10.1016/j.drugpo.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Grau LE, Zhan W, Heimer R. Prevention knowledge, risk behaviours and seroprevalence among nonurban injectors of southwest Connecticut. Drug and Alcohol Review. 2016:628–636. doi: 10.1111/dar.12396. August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Suwannawong P, Ti L, Kaplan K, Wood E, Kerr T. High rates of midazolam injection and associated harms in Bangkok. Thailand: 2012. pp. 944–952. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Heath KV, Yip B, Craib KJP, Shaughnessy MVO, Schechter MT, Montaner JSG. Improved Survival Among HIV-Infected Individuals Following Initiation of Antiretroviral Therapy. 1998;279(6) doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, Montaner JSG. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA: The Journal of the American Medical Association. 2001;286(20):2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. Journal of Viral Hepatitis. 2014;21(3):198–207. doi: 10.1111/jvh.12129. [DOI] [PubMed] [Google Scholar]
- Kim WR, Lake JR, Smith M, Skeans MA, Schladt P, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report. 2015 doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction. 2002;97(10):1289–1294. doi: 10.1046/j.1360-0443.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Lance CE, Butts MM, Michels LC. The sources of four commonly reported cutoff criteria: What did they really say? Organizational Research Methods. 2006;9(2):202–220. [Google Scholar]
- Marshall AD, Micallef M, Erratt A, Telenta J, Treloar C, Everingham H, et al. Liver disease knowledge and acceptability of non-invasive liver fibrosis assessment among people who inject drugs in the drug and alcohol setting: The LiveRLife Study. International Journal of Drug Policy. 2015;26(10):984–991. doi: 10.1016/j.drugpo.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Milloy MJ, Alexandra K, Thomas K, Evan A, Hasina S, Silvia G, et al. Improvements in HIV treatment outcomes among indigenous and non-indigenous people who use illicit drugs in a Canadian setting. Journal of the International AIDS Society. 2016;19(1):1–8. doi: 10.7448/IAS.19.1.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Rethinking Drinking. NIH Publication. 2015:1–16. [Google Scholar]
- Ng MH, Chou JY, Chang TJ, Lee PC, Shao WC, Lin TY, et al. High prevalence but low awareness of hepatitis C virus infection among heroin users who received methadone maintenance therapy in Taiwan. Addict Behav. 2013;38(4):2089–2093. doi: 10.1016/j.addbeh.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Norton BL, Voils CI, Timberlake SH, Hecker EJ, Goswami ND, Huffman KM, et al. Community-based HCV screening: knowledge and attitudes in a high risk urban population. BMC Infect Dis. 2014;14(1):74. doi: 10.1186/1471-2334-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar P, Corsi DJ, Cooper C. Distribution of Hepatitis C Risk Factors and HCV Treatment Outcomes among Central Canadian Aboriginal. Canadian Journal of Gastroenterology and Hepatology, 2016. 2016 doi: 10.1155/2016/8987976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SC, Thomas DL. Hepatitis C. In: Bennet JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th. Philadelphia: Elsevier Saunders; 2015. pp. 1920–1924. [Google Scholar]
- Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis c virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. Int J Drug Policy. 2015;26(10):911–921. doi: 10.1016/j.drugpo.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: Knowledge, perceived risk and willingness to receive treatment. Drug and Alcohol Dependence. 2001;61(3):211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Strauss SM, Astone-Twerell J, Munoz-Plaza CE, Des Jarlais DC, Gwadz M, Hagan H, et al. Drug treatment program patients’ hepatitis C virus (HCV) education needs and their use of available HCV education services. BMC Health Services Research. 2007;7:39. doi: 10.1186/1472-6963-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjadi M, Torruellas C, Ayala C, Yee HF, Khalili M. Formal patient education improves patient knowledge of hepatitis C in vulnerable populations. Digestive Diseases and Sciences. 2011;56(1):213–219. doi: 10.1007/s10620-010-1455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, Hull P, Bryant J, Hopwood M, Grebely J, Lavis Y. Factors associated with hepatitis C knowledge among a sample of treatment naive people who inject drugs. Drug and Alcohol Dependence. 2011;116(1–3):52–56. doi: 10.1016/j.drugalcdep.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Zeremski M, Dimova RB, Zavala E, Kritz S, Lin M, Smith BD, et al. Hepatitis C virus-related knowledge and willingness to receive treatment among patients on methadone maintenance. J Addict Med. 2014;8(4):249–257. doi: 10.1097/ADM.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Tepper M, Giulivi A. Current status of hepatitis C in Canada. Canadian Journal of Public Health. 2000;91(SUPPL 1) doi: 10.1007/BF03405100. [DOI] [PMC free article] [PubMed] [Google Scholar]


