Abstract
Background
Thirty percent of children with food allergies have multiple simultaneous allergies, however, the features of these multiple allergies are not well characterized serologically or clinically.
Objective
We comprehensively evaluated 60 multi-allergic patients by measuring serum IgE to key allergen components, evaluating clinical histories and medication use, performing skin tests, and conducting double-blind, placebo-controlled food challenges (DBPCFCs).
Methods
Sixty participants with multiple food allergies were characterized by clinical history, DBPCFCs, total IgE, sIgE, and component resolved diagnostics (IgE and IgG4) data. The food allergens tested were almond, egg, milk, sesame, peanut, pecan, walnut, hazelnut, cashew, pistachio, soy and wheat.
Results
Our data demonstrate that of the reactions observed during a graded DBPCFC, gastrointestinal reactions occurred more often in boys than in girls, as well as in individuals with high levels of IgE to 2S albumins from cashew, walnut and hazelnut. Certain food allergies often occurred concomitantly in individuals (i.e., cashew/pistachio and walnut/pecan/hazelnut). IgE testing to components further corroborated serological relationships between and among these clustered food allergies.
Conclusions
Associations of certain food allergies was shown by DBPCFC outcomes as well as by correlations in IgE reactivity to structurally related food allergen components. Each of these criteria independently demonstrated a significant association between allergies to cashew and pistachio, as well as among allergies to walnut, pecan and hazelnut.
Keywords: Multifood allergy, component-resolved testing, IgE, Double-blind placebo-controlled food challenge
INTRODUCTION
It has been estimated that about 8% of children suffer from food allergy and of those, 30% are allergic to multiple foods.1,2 However, little information is available regarding the clinical and serological characteristics associated with multiple food allergies, and such information could advance our understanding of phenotypes and endotypes, respectively, of food allergy.3 Clinically, it has been well recognized that certain food allergies occur concurrently. For example, studies have indicated that about 92% of patients with cow’s milk allergy are also allergic to goat’s milk,4 and individuals with shrimp allergy are often allergic to other shellfish. Among tree nuts, allergies to pistachios have been found more commonly in individuals with cashew nut allergy5 and individuals with walnut allergy are often allergic to pecan nuts.6 Studies by Goetz et al7 and Masthoff et al8 found that there was little cross-reactivity between proteins found in peanuts and tree nuts suggesting that concurrent sensitization between these foods is likely due to independent sensitization to both peanuts and tree nuts. Clinical associations between allergens are more commonly seen among allergens that are closely related botanically like tree nuts7; however, links between allergens from very different taxonomical groups have also been observed.9 Many foods that are closely related share homologous proteins and sensitization to one food is therefore likely to result in positive tests or clinical reactivity to related foods. Additionally, sensitization to proteins that are particularly highly conserved can result in associated allergies to foods that are not closely related. Therefore, we set out to test if individuals with multifood allergies have clinically-relevant food allergies to proteins belonging to similar food categories and/or to similar protein classes.
Understanding the patterns of cross-reactivity between and among foods may aid the clinician in evaluating the risk of food reactions as well as reactions to multiple foods. Moreover, the application of food allergy diagnostics, using knowledge of relevant protein relationships, along with clinical history, could enable more precise allergen avoidance strategies to avoid unnecessarily eliminating entire food groups from the diet. Also, recent data indicates that multifood-allergic patients carry a higher risk for developing asthma and other co-morbid conditions compared to single food allergies, which further underscores the need to test relationships of cross reactivity in multifood-allergy patients.10
There has been great interest in developing novel in vitro tests that can more accurately predict food allergies. One of these approaches is based on component-resolved diagnostics (CRD) in which native or recombinant allergens are used to test IgE sensitivity to individual allergen proteins.11 At the present time, CRD tests are mainly performed in research settings. However, FDA-approved milk, egg, peanut and tree nut CRD tests are available for clinical use in the USA.
In this study, our goal was to comprehensively evaluate the characteristics of patients with multiple food allergies. We focused especially on the association of multifood allergies with the phenotype (for our study, clinical symptoms during a double-blind, placebo-controlled food challenge (DBPCFC) and co-morbidities) and endotype (for our study, the component IgE levels) of the participants. We analyzed the baseline data of a study in which the participants were screened and eligible only if they had a high likelihood of reactions to more than one food allergen in separate DBPCFCs. Accordingly, the study was not aimed at evaluating the ability of new diagnostic tools to predict negative vs. positive reactions to DBPCFCs. Instead, we focused on testing whether there were associations between the extent and type of food challenge reactions and relatedness of allergen proteins. To our knowledge, this is the first study that systematically investigates multi-allergic participants with the aim of identifying associations among food challenge outcomes, component testing, and levels of whole allergen specific IgE.
METHODS
The protocol for this study was reviewed and approved by the Institutional Review Board of Stanford University.
Study population
Sixty pediatric participants allergic to multiple foods were included in this study. Their demographic characteristics are summarized in Table 1. Information about participant selection (screening criteria, DBPCFC, skin prick test (SPT), food flours) can be found in this article’s Online Repository.
Table 1.
Demographics
| Number participants | 60 |
| Female [n, %] | 30 (50%) |
| Age (years) [median, range] | 8 (4 – 15) |
| With atopic dermatitis [%] | 46 (77%) |
| With allergic rhinitis [%] | 44 (73%) |
| With asthma [n, %] | 30 (50%) |
| DBPCFCs performed | 311 |
| Positive DBPCFCs [n, %] | 273 (88%) |
| Number of DBPCFCs performed for participant (one per food) [median, range] | 5 (2 – 8) |
DBPCFC, double-blind placebo-controlled food challenge
Antibody measurements
Total IgE and allergen-specific IgE (sIgE) and IgG4 antibody concentrations were determined using the ImmunoCAP 250 assay (Thermo Fisher Scientific, Portage, MI). Antibodies to the following foods and allergen components were measured: peanut (Ara h 1, Ara h 2, Ara h 3, Ara h 8, Ara h 9), hazelnut (Cor a 1, Cor a 8, Cor a 9, Cor a 14), walnut (Jug r 1, Jug r 3), cashew (Ana o 3), egg white (Gal d 1, Gal d2, Gal d 3), cow’s milk (Bos d 4, Bos d 5, Bos d 8), soy (Gly m 4, Gly m 5), wheat (Tri a 14, Tri a 19) and Bet v 1 (a Birch component).
Statistical analysis
Differences between nonparametric unpaired variables were assessed using a two-sided Mann Whitney U test. P-values were adjusted for multiple comparisons using the approach by Benjamini and Hochberg12 to control the false discovery rate (FDR) and the corrected values were noted as q-values.
To characterize the multi-allergic character of the participants, for each allergen combination, the number of participants that were allergic against both allergens in DBPCFC tests was determined. To take the different counts of allergic participants for the various allergens into account, the number of co-allergic participants was examined using the Jaccard similarity coefficient (Jaccard index).13 This coefficient measures the similarity between sample sets, in our case each set was the list of participants that were allergic against one of the allergens. The Jaccard similarity coefficient is calculated by dividing the size of the intersection of the sample sets by the size of the union between the sample sets. The result is a value between 0 and 1, with 1 being a perfect overlap between the sample sets and 0 meaning that the sample sets don’t overlap at all. To determine the pairs of allergens against which participants were more often concurrently allergic than by random chance, a resampling permutation approach was applied.
Keeping the count of DBPCFC positive participants per allergens the same as present in the study, we permuted the DBPCFC positive cases so that they get randomly assigned to the 60 participants. Each time we calculated for each allergen combination the Jaccard similarity coefficient and recorded the results. This way, for each allergen combination we estimated the null distribution for the case if participants would be randomly co-allergic against them. Afterwards, for each allergen combination the count of permutations that resulted in a greater or equal Jaccard similarity coefficient than our original study data was determined and divided by 1000 (the number of permutations). These results were the p-values which we adjusted for multiple comparisons.12
To evaluate the association between the IgE responses against different components, hierarchical clustering of IgE values against the components across all 60 participants was done using 1 minus the absolute Spearman’s rank correlation as distance measure and complete linkage as agglomeration method. To assess the uncertainty of the clusters in the hierarchical clustering procedure, the pvclust R package14 was used. This function uses a multiscale bootstrap resampling approach to assign a p-value to each cluster. au (Approximately Unbiased) p-values, which are computed by multiscale bootstrap resampling, and bp (Bootstrap Probability) values were calculated and presented as percentages. The authors described that the au p-value is a better approximation to unbiased p-value. Clusters with q-values (FDR adjusted p-values) < 0.05 are highlighted by a red rectangle in the cluster dendrogram.
RESULTS
Multifood allergen testing by DBPCFC
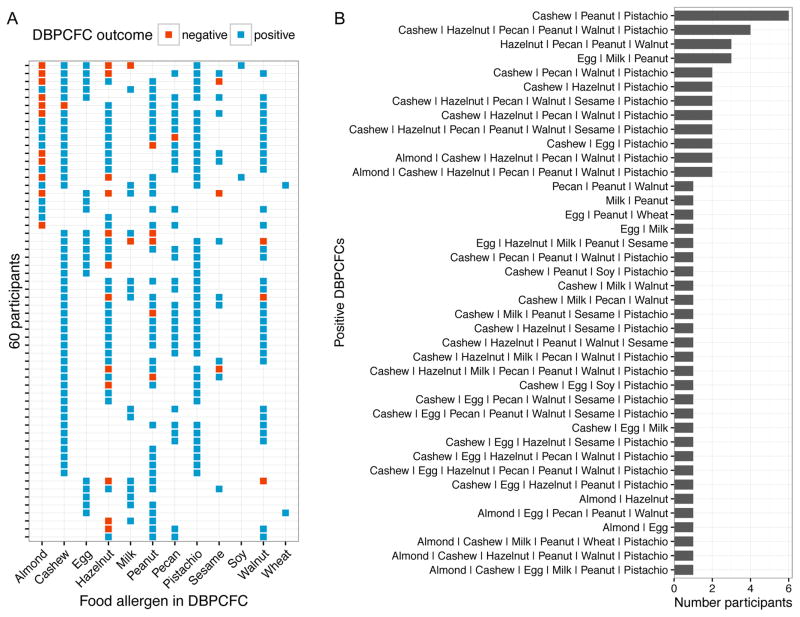
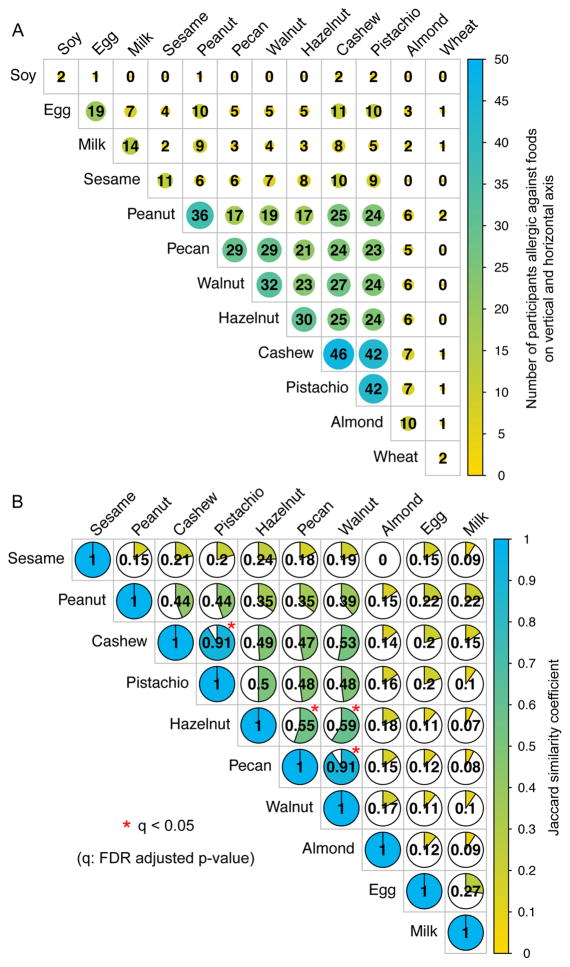
The study examined a total of 12 different food allergens and each participant underwent a DBPCFC for between 2 and 8 foods (Fig 1A). Overall 311 DBPCFCs were performed and the details of the food allergens, DBPCFC outcomes, as well as the specific IgE and SPT results for positive DBPCFCs are presented in Table 2. The number of positive DBPCFCs per allergen ranged from 46 (cashew) to 2 (soy and wheat) (Table 2, diagonal in Fig 2A). Participants were allergic (positive DBPCFC) to between 2 and 7 of the tested foods (Fig 1A). Combinations of foods to which participants were allergic varied widely (Fig 1B). Six participants were allergic to the exact same food allergen combination of cashew, peanut, and pistachio; however, most combinations were unique to participants. Twenty-eight participants (47%) were allergic to a unique combination of food allergens. Fig 2A presents the number of participants being co-allergic to defined pairs of allergens. The number of participants who were allergic to a pair of food allergens does depend on the number of occurrences of the single allergies in this data set (eg, more than 4 times the number of participants were allergic to pistachio than to almond). To adjust for this, Fig 2B presents a pairwise plot between the allergens, showing the Jaccard similarity coefficient. The greater this coefficient is, the greater is the similarity between the lists of participants that were shown to be allergic to either of the two allergens. We used a resampling permutation test to identify the allergen pairs that occurred with a greater Jaccard similarity coefficient than by chance in our data set. These significant allergen pairs (q < 0.05) are represented by red stars in Fig 2B.
Fig. 1.
FA and DBPCFC outcome combinations. A, Overview of DBPCFC outcomes in the study population. B, Number of participants that had positive DBPCFCs against different foods.
Table 2.
Participant’s food allergy and outcome overview
| Food allergen | Cashew | Pistachio | Hazelnut | Peanut | Walnut | Pecan | Almond | Egg | Milk | Sesame | Soy | Wheat |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Number participants undergoing DBPCFC | 47 | 42 | 42 | 41 | 35 | 30 | 21 | 19 | 16 | 14 | 2 | 2 |
|
| ||||||||||||
| Positive DBPCFCs | ||||||||||||
| n | 46 | 42 | 30 | 36 | 32 | 29 | 10 | 19 | 14 | 11 | 2 | 2 |
| % | 98% | 100% | 71% | 88% | 91% | 97% | 48% | 100% | 88% | 79% | 100% | 100% |
|
| ||||||||||||
| Of positive DBPCFCs: | ||||||||||||
|
| ||||||||||||
| Cumulative tolerated dose in DBPCFC (mg) | ||||||||||||
| median | 2.5 | 0 | 5 | 5 | 0 | 5 | 5 | 0 | 2.5 | 5 | 75 | 187.5 |
| 1st – 3rd quartile | 0 – 25 | 0 – 5 | 0 – 75 | 0 – 25 | 0 – 5 | 0 – 5 | 0 – 58 | 0 – 25 | 0 – 25 | 2.5 – 15 | 75 | 94 – 281 |
| range | 0 – 275 | 0 – 175 | 0 – 375 | 0 – 375 | 0 – 75 | 0 – 375 | 0 – 75 | 0 – 75 | 0 – 175 | 0 – 375 | 75 | 0 – 375 |
|
| ||||||||||||
| Skin reaction in DBPCFC | ||||||||||||
| n | 42 | 40 | 28 | 32 | 30 | 27 | 10 | 16 | 13 | 11 | 2 | 2 |
| % | 91% | 95% | 93% | 89% | 94% | 93% | 100% | 84% | 93% | 100% | 100% | 100% |
|
| ||||||||||||
| GI reaction in DBPCFC | ||||||||||||
| n | 28 | 16 | 6 | 9 | 11 | 12 | 0 | 7 | 2 | 5 | 0 | 0 |
| % | 61% | 38% | 20% | 25% | 34% | 41% | 37% | 14% | 45% | |||
|
| ||||||||||||
| Respiratory reaction in DBPCFC | ||||||||||||
| n | 5 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 |
| % | 11% | 2% | 7% | 6% | 3% | 3% | 10% | 5% | 14% | |||
|
| ||||||||||||
| Specific IgE (KU/L) | ||||||||||||
| median | 10.5 | 13 | 19.7 | 57.6 | 36 | 13.4 | 5.2 | 6.1 | 3.1 | 16.3 | 36.5 | 67.2 |
| range | 0.4 – 100 | 0.4 – 100 [1 NA] | 0.1 – 91.6 | 0.3 – 100 | 1.1 – 100 | 0.4 – 91.8 | 0.4 – 100 | 1.8 – 84 | 0.4 – 100 | 0.1 – 100 | 1 – 71.9 | 37.3 – 97.1 |
|
| ||||||||||||
| Skin prick test wheal diameter (mm) | ||||||||||||
| median | 15 | 12.5 | 9.5 | 16 | 12.5 | 8.5 | 9.5 | 11.5 | 15.5 | 16.5 | 7.3 | 13.3 |
| range | 6.5 – 32 [1 NA] | 3.5 – 31 [8 NA] | 3.5 – 27 | 7 – 40 | 5 – 24.5 | 4.5 – 20.5 [4 NA] | 6 – 25 | 7 – 36.5 | 4.5 – 40 | 6 – 27.5 | 7 – 7.5 | 10.5 – 16 |
|
| ||||||||||||
| Epis used | ||||||||||||
| n | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
DBPCFC, double-blind placebo-controlled food challenge; GI, gastrointestinal
Fig. 2.
Concurrent occurrences of food allergies. A, Number of participants who were allergic against a specific food (diagonal) and any pairwise combination of two food allergens (intersection of column and row foods). B, Pie charts and numbers showing the Jaccard similarity coefficient. This similarity measure represents the overlap of participants that were allergic against the two food allergens adjusted for the number of participants being allergic against each of the food allergens separately. A coefficient of 1 means perfect overlap of participants, 0 means no participant is allergic against both of the two food allergens. Soy and wheat were excluded because only 2 participants were allergic against each of them.
Participants were significantly frequently co-allergic to pecan and walnut, as reported before based on a strong correlation of their sIgE and SPT values.6,15 In our set of multi-allergic participants, all participants that were allergic to pecan (29 participants) were also allergic to walnut and only 3 of 32 walnut allergic participants (9%) tolerated pecan. Goetz et al7 showed a strong serological cross-reactivity not only between walnut and pecan but also with hazelnut. Our data reflects this observation, as we identified significant (q < 0.05) co-occurrences of allergies to hazelnut and pecan or walnut (Fig 2B). Even though they were significant in our analysis, the Jaccard similarity coefficients were much lower for the hazelnut-pecan pair (0.55) and hazelnut-walnut pair (0.59) than for a co-occurrence of pecan and walnut allergies (Jaccard similarity coefficient: 0.91). From the 39 participants who were allergic to walnut, pecan and/or hazelnut, 21 (54%) were allergic to all three allergens. Eight had a positive DBPCFC against walnut and pecan, 2 against walnut and hazelnut while only 1 participant for walnut and 7 for hazelnut were solely allergic to these allergens, respectively.
All of our 42 pistachio allergic participants were DBPCFC positive against cashew. This co-occurrence of pistachio and cashew allergies is in line with previous findings.5,6,15–17 The reason for the small proportion of DBPCFCs being negative was that a challenge was only performed if an allergy to a specific food was suspected based on SPT and IgE data screening (see DBPCFC methods section for details). Even though there were few negative DBPCFCs in this data set, we analyzed the overlap between all available DBPCFC outcomes (positive or negative) of each allergen pair for the participants. Fig E1 in the Online Repository shows the percentage of pairwise DBPCFC outcomes in which the participants tested positive for both allergens, negative for both allergens or positive for one allergen and negative for the other.
Organ system involvement with reaction and comorbid conditions
For each positive DBPCFC, the reacting organ system was recorded as per Bock’s criteria.18 The organ involved most frequently was the skin, followed by gastrointestinal (GI) and respiratory (93%, 36%, and 6% of positive DBPCFCs, respectively). The respective frequencies of a skin (p = 0.94) and respiratory reaction (p = 0.74) were similar across food allergens (Table 2), while the probability of a GI reaction significantly varied among allergens (p < 0.01). After adjusting for age and allergen, the sex of the participant was associated with the probability of having a GI reaction (p < 0.01); specifically a higher rate of GI reactions occurred in boys (42% of all positive DBPCFCs in boys; 24 of 30 boys (80%) had at least 1 GI reaction) than in girls (27% of all positive DBPCFCs in girls; 16 of 30 girls (53%) had at least 1 GI reaction). Sex was also found to be significant for the frequency of having a skin reaction (p < 0.01), but with a lower rate of skin reaction in boys (87% of all positive DBPCFCs in boys; every boy had at least one skin reaction) than girls (99% of all positive DBPCFCs in girls; every girl had at least one skin reaction). The occurrence of a respiratory reaction did not vary significantly between male and female participants (p = 0.1). More than half of the participants had at least one atopic comorbid condition (asthma, atopic dermatitis or allergic rhinitis) (Table 1). None of these conditions was significantly associated with a specific organ system showing an allergic reaction during the DBPCFCs.
IgE levels for whole allergens and components
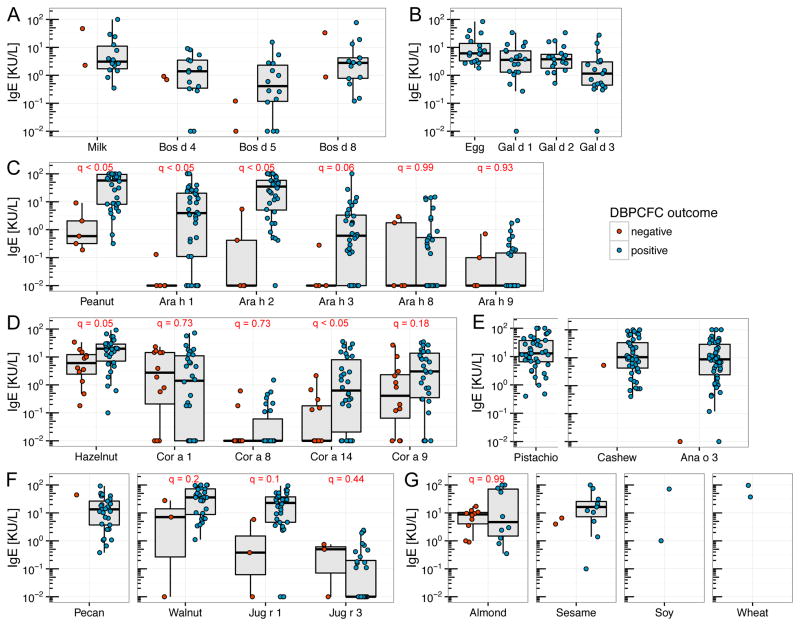
We measured serum IgE levels for all 12 whole allergens and the components of 9 allergens (overview in Table E1 in the Online Repository). Components for 8 of the studied food allergens and birch were included. Fig 3 shows the IgE levels for whole food allergens and components for participants who were either DBPCFC positive or negative against the allergen or the source allergen of the respective component. For peanut (Fig 3C), Ara h 1, Ara h 2 and the whole allergen showed significantly (q < 0.05) different IgE levels between the 2 groups. The hazelnut DBPCFC positive group showed significantly greater IgE values against Cor a 14 (q < 0.05) than the negative group. The other hazelnut components as well as the whole allergen were not significantly different between the groups (Fig 2D). Notably, for all the components, some participants with negative DBPCFCs showed greater IgE levels than some participants with positive DBPCFCs. Walnut and its components, as well as almond, showed no significant difference in IgE levels between the positive and negative DBPCFC groups. For milk, egg, pistachio, cashew, pecan, sesame, soy and wheat, no (or a too small a number of) DBPCFC negative participants prevented a statistical analysis.
Fig. 3.
Whole food allergen and component IgE levels stratified by DBPCFC outcomes. A, Milk and its components; B, Egg and its components; C, Peanut and its components; D, Hazelnut and its components; E, Pistachio, Cashew and its component; F, Pecan, Walnut and its components; G, Almond, Sesame, Soy, Wheat. Soy and wheat components were not plotted because only two values were obtained. (q: FDR adjusted p-value)
In addition to IgE responses, available IgG4 levels are shown in Fig E2 in the Online Repository. None of the IgG4 levels were significantly different between the participants that were DBPCFC positive or negative.
IgE correlation analyses
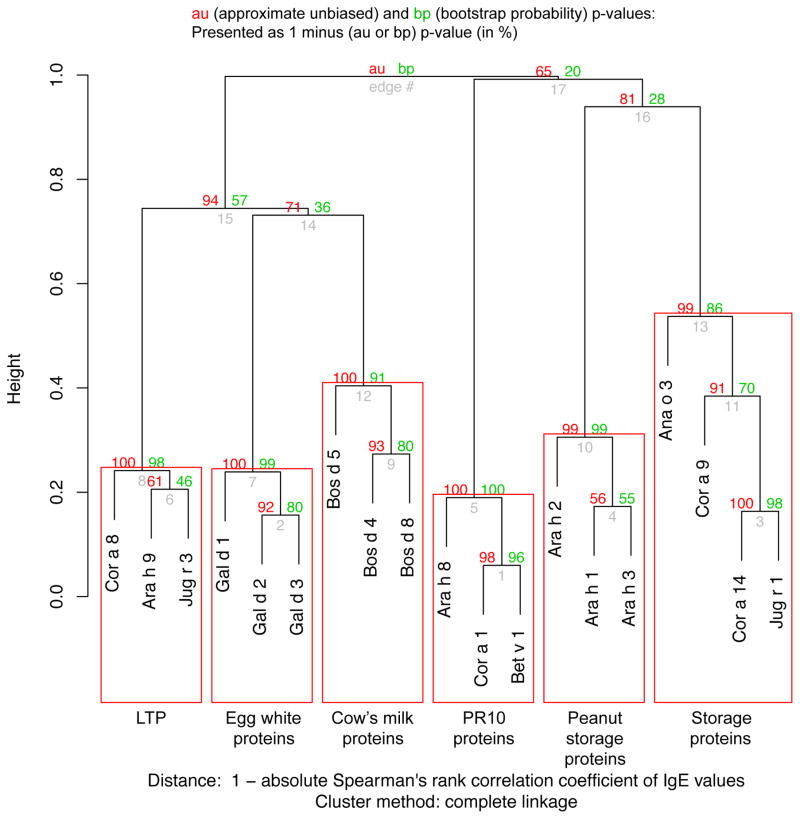
To evaluate the association between the IgE responses against different components across our multi-allergic participants, the Spearman’s correlation matrix was calculated. By using a multiscale bootstrap resampling approach on the hierarchical clustering of these correlation data (R package pvclust14), we identified significant clusters (Fig 4). The IgE levels against the components built 6 significant clusters that can be characterized by the function/family of the proteins. One cluster consisted of all PR-10 proteins in this analysis (Cor a 1, Bet v 1, Ara h 8) which share significant structural similarity.19,20 Another cluster was formed by 3 of the LTP components (Cor a 8, Ara h 9, and Jug r 3). Jug r 1, Cor a 14, and Ana o 3, all being 2S albumin storage proteins, formed another significant cluster with Cor a 9 (an 11S globulin), despite a lack of structural homology among members of these protein families. The three peanut storage proteins Ara h 1, Ara h 2 and Ara h 3 clustered significantly together. The final two significant clusters were built out of the three egg white proteins (Gal d 1, Gal d2, Gal d 3) and the cow’s milk proteins (Bos d 4, Bos d 5, Bos d 8), respectively.
Fig. 4.
Hierarchical clustering of the component IgE indicates a clustering of components by protein family or origin. Significant clusters (au p < 0.05) are shown by red rectangles. After FDR adjusting the p-value, all rectangles are also significant at q < 0.05 (numbers not in plot). Soy and wheat components were excluded in this analysis because of too few positive observations.
To study the relationship between the IgE levels to the components and to the whole allergen extracts, Spearman’s rank correlation coefficients are shown in Fig E3 of the Online Repository. All measured IgE levels were included, even when no DBPCFC was performed for the respective allergen. Significant correlations (q < 0.05) which show an absolute Spearman’s rank correlation of greater than 0.5 were marked with a colored background. Looking at the pairs of components and their sources (red bordered boxes in Fig E3 and listed in Table E1 in the Online Repository), the correlations reflect the trends of components that were different between the groups of DBPCFC positive and negative participants (Fig 3). Ara h 1, Ara h 2, and Ara h 3 were significantly positively correlated with peanut sIgE levels. Also Jug r 1 IgE levels were significantly positively correlated with walnut sIgE levels, as well as Ana o 3 with cashew, and Cor a 9 and Cor a 14 with hazelnut sIgE levels. Focusing on the pairs between components and food allergens that are not their source allergen (outside of the red boxes in Fig E3), several strong significant correlations were detected. IgE levels to Ana o 3, a cashew component, correlated not only to cashew sIgE levels (Spearman’s rho = 0.86) but also as strongly positively with pistachio sIgE levels (Spearman’s rho = 0.85). It has previously been shown that cashew and pistachio sIgE levels are correlated5,6,15–17 and we can see from Fig 2B that in our cohort, a significant percent of patients were allergic to both pistachio and cashew. Jug r 1 from walnut is another example of strong correlation (Spearman’s rho = 0.89) with sIgE levels to another species, in this case pecan. Pecan and walnut allergies are known to occur together6,7,15 and our study shows this connection by IgE levels as well as DBPCFC outcomes (Fig 2B), thereby enhancing our understanding of the possible molecular underpinnings of the co-existence of these allergies (i.e. protein sequence homology). Fig 2B also shows a significant co-occurrence of pecan or walnut allergy with hazelnut allergy. This association can also be seen by significant correlations of pecan as well as walnut sIgE levels with Cor a 14 and Cor a 9 from hazelnut. Furthermore, hazelnut sIgE levels were significantly correlated to Jug r 1, even though less strongly (Spearman’s rho = 0.59) than to pecan (0.84) or walnut (0.89) sIgE.
Allergen component IgE levels and DBPCFC reactions
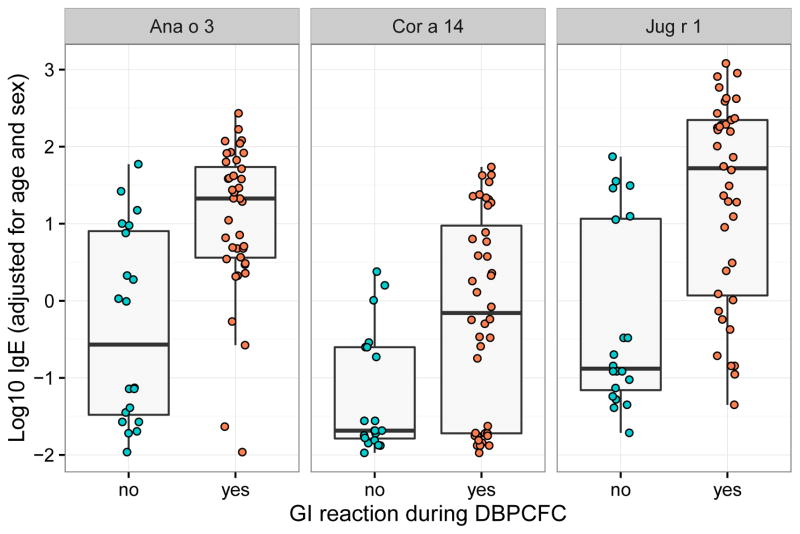
After adjusting the data for sex and age, we performed a logistic regression to analyze the contribution of the IgE level for each component to the chance of having a GI reaction in any positive DBPCFC. High IgE levels against Ana o 3 (cashew), Jug r 1 (walnut) or Cor a 14 (hazelnut) were significantly (q < 0.05) associated with the chance of participants exhibiting a GI reaction in a DBPCFC (Fig 5). All three components are 2S albumin storage proteins. In models analyzing sIgE values against whole cashew, walnut or hazelnut, respectively, rather than their 2S albumins, these sIgE values were not significantly associated with having a GI reaction or not. Since almost every positive DBPCFC resulted in a skin reaction and nearly none in a respiratory reaction, a similar analysis for these reactions was not possible.
Fig. 5.
After adjusting the component IgE levels for age and sex, Ana o 3, Cor a 14 and Jug r 1 levels were significantly different between participants showing a GI reaction during any DBPCFC and those who didn’t. q-value (FDR adjusted p-value) < 0.05 for all three components.
DISCUSSION
To our knowledge, the study of multifood-allergic children presented here is the largest and most comprehensive of its kind to date. Cashew, pistachio, peanut, walnut and hazelnut caused the majority of reactions, but extensive variation was seen in individual challenge patterns. Strong associations, both serologically and clinically, were seen for cashew and pistachio as well as for walnut, pecan and to some extent hazelnut. GI reactions were more frequently seen in boys than in girls, as well as in individuals with high IgE levels to 2S albumins from cashew, walnut and/or hazelnut.
Our study population represents a selected group of multifood-allergic children, who were at high risk of reacting to a variety of food allergens. A strength of our study is that all participants were DBPCFC challenged to all possible offending foods (determined by skin and blood testing) in order to verify their food hypersensitivity clinically. They were, on average, challenged with five food items.
We observed serological associations between cashew and pistachio as well as among walnut, pecan and hazelnut, suggestive of cross-reactivity within these groups of nuts. Consistent with these observations, our data demonstrate, to our knowledge for the first time, the association of positive DBPCFC outcomes for these groups of nuts (Fig. 2). Significant associations of positive DBPCFC outcomes, reflected in high, significant Jaccard similarity coefficients, were seen between cashew and pistachio as well as among hazelnut, pecan and/or walnut (Fig. 2B). This extensive clinical association was not seen, for example, between peanut and pecan. Savvatianos and coworkers17 have earlier described that extensive cross-reactivity occurs at the clinical level between the two Anacardiaceae nuts (cashew and pistachio). This was based on open nut challenges and we can now verify that this also can be observed through DPBCFCs. Our data confirm that individuals diagnosed with cashew allergies should also avoid pistachio and vice versa. The same likely applies to walnut, pecan and possibly hazelnut, for which we could show serological relationships as well as an increased probability of co-existing allergies by DBPCFC.
This association analysis was based on proven allergy by positive DBPCFCs. A DBPCFC was only performed if the screening by SPT and IgE level suggested a possible allergy. So, for the foods for which no DBPCFC was performed, we assume that it would be a negative DBPCFC. With this assumption, we might miss a few positive DBPCFCs but we don’t think that this weakens our findings because these possible additional positive DBPCFCs would not reduce the number of co-occurrence between allergies that we found.
The male/female ratio in our study population was 1:1. Boys showed a higher chance than girls of having a GI reaction during DBPCFCs, while girls showed a greater probability than boys of having a skin reaction. Sex differences in food allergy during childhood were previously reported with boys appearing more likely than girls to develop food allergies.21–26 To our knowledge, the association between sex and the type of reaction has not before been explicitly reported. However, eosinophilic esophagitis (EoE), an allergic/immune condition affecting the esophagus, has been shown to be three to four times more common among males than females.27 The results in our study population, which was comparatively small for this kind of analysis, point towards a trend in sex differences in reactions to food challenges that should be studied in larger cohorts in the future.
In line with previous findings28–32, the comparison of IgE levels among peanut allergic and non-allergic participants showed greater IgE responses to Ara h 1, Ara h 2 and whole peanut extract in peanut DBPCFC positive than DBPCFC negative children (Fig 3C). The IgE levels against hazelnut component Cor a 14 were also significantly different between hazelnut DBPCFC positive and negative participants (Fig 3D). Cor a 14 levels were previously shown to be useful in predicting the outcome of a DBPCFC against hazelnut.31,33
A cluster analysis of the IgE levels to the components over all 60 participants revealed several significant groupings (Fig 4) reflecting, as expected, both phylogenetically and/or structurally defined protein families and the food from which the proteins originate. Two clusters of LTP and PR-10 proteins, respectively, reflect structural homology, while two other clusters (egg white and cow’s milk proteins) reflect the component source origin. In the last two clusters, four storage proteins from different sources cluster separately from the three peanut storage proteins.
To study the quantitative relationship between IgE responses to individual components and whole food extracts, we analyzed the significant Spearman’s correlations across all 60 participants (Fig E3). The levels of IgE to Ara h 1, Ara h 2 and Ara h 3 were significantly positively correlated with the sIgE level against peanut, as well as Jug r 1 with walnut, Ana o 3 with cashew and Cor a 9 and Cor a 14 with hazelnut. Similarly, levels of IgE to Ana o 3 and pistachio were strongly correlated. It has previously been shown that cashew and pistachio sIgE levels are correlated.5,6,15–17 Also, Jug r 1 was strongly significantly correlated with pecan sIgE values. Pecan and walnut allergies are known to occur together.6,7,15 Hazelnut, pecan and walnut sIgE levels were significantly correlated with Cor a 14, Cor a 9 as well as Jug r 1 IgE levels. Cross-reactivity between walnut, pecan, and hazelnut by IgE enzyme-linked immunosorbent assay inhibition was previously shown.7 These IgE correlations are consistent with the clinical associations among these three allergens reported in this manuscript.
The Spearman’s correlation matrix between IgE levels against components and the whole allergen extracts (Fig E3) is not free of bias since the allergy status was not known for every participant and every food. Even though we found known patterns of co-occurring allergies, we cannot assume that this is a perfect reflection of the overall population of multi-allergic individuals. Furthermore, allergies that cannot be attributed as IgE-mediated will most likely not be revealed in this correlation analysis.
After adjusting for age and sex, Ana o 3, Jug r 1 and Cor a 14 IgE levels were significantly higher in participants that experienced GI reactions during any DBPCFC than in those who did not. These three components are all 2S albumin storage proteins. Members of this protein family are recognized as major food allergens of nuts and seeds.
Little is known about how sensitization occurs both for 2S albumins as well as for other allergens, however it was reported previously that 2S albumins are thought to sensitize directly via the gastrointestinal tract.34 Several 2S albumins have been shown to be highly resistant to heat and gastrointestinal digestion.34–36 Both stability and abundance of a food component are important factors that determine the amount of the protein that can reach the “effector system” and cause a reaction. While all three classes of seed storage proteins (2S albumins, 7S and 11S globulins) are highly abundant, 2S albumins are small and compact proteins containing intramolecular disulfide bonds acting to maintain a tight conformation, which may contribute to their demonstrated stability under conditions in the stomach that would rapidly denature or degrade many other proteins.37 While the data set is very complex and too small to allow a firm conclusion, the results indicate a trend that sensitization against 2S albumin proteins might serve to predict a higher probability of having a GI reaction.
This study provides evidence for the significant associations of certain food allergies, namely cashew and pistachio and walnut, pecan and hazelnut, as shown both by DBPCFC outcomes as well as by correlations in IgE reactivity to structurally related food allergen components. These findings thus help to elucidate the clinical and serological features of multi-allergic children. However, many open questions remain that in part reflect the design of our study, which attempted to minimize the risk associated with exposure of multiallergic individuals to allergenic food items and to reduce stress and discomfort to the study participants and their families. For example, because DBPCFCs were only performed with foods for which there was strong clinical evidence for a suspected allergy, the low number of DBPCFCs that were negative prevented certain analyses, such as a systematic study of the sensitivity and specificity of whole food extract or component IgE levels for predicting allergies. In summary, our study enhances understanding of the clinical relevance of the molecular connections among various allergen protein groups, and that this may aid in diagnosing and managing patients with food allergies.
Supplementary Material
1. What is already known about this topic?
It is estimated that 6–8% of children suffer from food allergy and, of these, 30% are multifood-allergic. Multifood-allergic children frequently suffer from nut allergies and can exhibit varied reactions to their food allergens.
2. What does this article add to our knowledge?
This study of multifood-allergic children is the largest and most comprehensive of its kind, providing a systematic analysis of associations among different food allergies based on clinical (DBPCFC) as well as serological (IgE levels) data.
How does this study impact current management guidelines?
Cashew/pistachio and walnut/pecan/hazelnut allergies show significant clinical associations, suggesting that this be considered when giving avoidance advice.
Acknowledgments
This work is supported by: NIH U19 AADCRC-IOF, U19 AI104209, Sean N Parker Center for Allergy and Asthma Research at Stanford University
Abbreviations used
- au
Approximately unbiased
- CRD
Component-resolved diagnostics
- DBPCFC
Double-blind, placebo-controlled food challenge
- FDR
False discovery rate
- GI
Gastrointestinal
- LTP
Lipid transfer protein
- sIgE
Specific immunoglobulin E
- SPT
Skin prick test
Footnotes
Conflict of interest
Drs. Borres, Lindholm and Jones are employees of Thermo Fisher Scientific, the manufacturer of the diagnostic equipment for the allergen component Ig levels.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta ARS, Elizabeth E. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. J Allergy Clin Immunol. 2013:132. doi: 10.1016/j.jaci.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bird JA. Approach to evaluation and management of a patient with multiple food allergies. Allergy Asthma Proc. 2016;37:86–91. doi: 10.2500/aap.2016.37.3924. [DOI] [PubMed] [Google Scholar]
- 4.Bellioni-Businco B, Paganelli R, Lucenti P, Giampietro PG, Perborn H, Businco L. Allergenicity of goat’s milk in children with cow’s milk allergy. J Allergy Clin Immunol. 1999;103:1191–4. doi: 10.1016/s0091-6749(99)70198-3. [DOI] [PubMed] [Google Scholar]
- 5.Rancé F, Bidat E, Bourrier T, Sabouraud D. Cashew allergy: Observations of 42 children without associated peanut allergy. Allergy Eur J Allergy Clin Immunol. 2003;58:1311–4. doi: 10.1046/j.1398-9995.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–51. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Goetz DW, Whisman Ba, Goetz AD. Cross-reactivity among edible nuts: double immunodiffusion, crossed immunoelectrophoresis, and human specific IgE serologic surveys. Ann Allergy, Asthma Immunol. 2005;95:45–52. doi: 10.1016/S1081-1206(10)61187-8. [DOI] [PubMed] [Google Scholar]
- 8.Masthoff LJ, van Hoffen E, Mattsson L, Lidholm J, Andersson K, Zuidmeer-Jongejan L, et al. Peanut allergy is common among hazelnut-sensitized subjects but is not primarily the result of IgE cross-reactivity. Allergy. 2015;70:265–74. doi: 10.1111/all.12554. [DOI] [PubMed] [Google Scholar]
- 9.García BE, Lizaso MT. Cross-reactivity syndromes in food allergy. J Investig Allergol Clin Immunol. 2011;21:162–70. [PubMed] [Google Scholar]
- 10.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treudler R, Simon JC. Overview of component resolved diagnostics. Curr Allergy Asthma Rep. 2013;13:110–7. doi: 10.1007/s11882-012-0318-8. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y, Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 13.Jaccard P. The distribution of the flora in the alphine zone. New Phytol. 1912;XI:37–50. [Google Scholar]
- 14.Suzuki R, Shimodaira H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–2. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 15.Uotila R, Kukkonen AK, Pelkonen A, Mäkelä MJ. Cross-sensitization profiles of edible nuts in a birch-endemic area. Allergy. 2015;71:514–21. doi: 10.1111/all.12826. [DOI] [PubMed] [Google Scholar]
- 16.Noorbakhsh R, Mortazavi SA, Sankian M, Shahidi F, Tehrani M, Jabbari Azad F, et al. Pistachio Allergy-Prevalence and In vitro Cross-Reactivity with Other Nuts. Allergol Int. 2011;60:425–32. doi: 10.2332/allergolint.10-OA-0222. [DOI] [PubMed] [Google Scholar]
- 17.Savvatianos S, Konstantinopoulos AP, Borga A, Stavroulakis G, Lidholm J, Borres MP, et al. Sensitization to cashew nut 2S albumin, Ana o 3, is highly predictive of cashew and pistachio allergy in Greek children. J Allergy Clin Immunol. 2015;136:192–4. doi: 10.1016/j.jaci.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: A manual. J Allergy Clin Immunol. 1988;82:986–97. doi: 10.1016/0091-6749(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 19.Jahn-Schmid B, Radakovics A, Lüttkopf D, Scheurer S, Vieths S, Ebner C, et al. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J Allergy Clin Immunol. 2005;116:213–9. doi: 10.1016/j.jaci.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Bublin M, Breiteneder H. Cross-reactivity of peanut allergens. Curr Allergy Asthma Rep. 2014;14:426. doi: 10.1007/s11882-014-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: Results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. e14. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly C, Gangur V. Sex Disparity in Food Allergy: Evidence from the PubMed Database. J Allergy. 2009;2009:159845. doi: 10.1155/2009/159845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbes SJ, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: A 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 25.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. e5. doi: 10.1016/j.jaci.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 26.DunnGalvin A, Hourihane JOB, Frewer L, Knibb RC, Oude Elberink JNG, Klinge I. Incorporating a gender dimension in food allergy research: A review. Allergy Eur J Allergy Clin Immunol. 2006;61:1336–43. doi: 10.1111/j.1398-9995.2006.01181.x. [DOI] [PubMed] [Google Scholar]
- 27.Sperry SL, Woosley JT, Shaheen NJ, Dellon ES. Influence of race and gender on the presentation of eosinophilic esophagitis. Am J Gastroenterol. 2012;107:215–21. doi: 10.1038/ajg.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–5. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Klemans RJB, Broekman HCHP, Knol EF, Bruijnzeel-Koomen CAFM, Otten HG, Pasmans SGMA, et al. Ara h 2 is the best predictor for peanut allergy in adults. J Allergy Clin Immunol Pract. 2013;1:632–8. doi: 10.1016/j.jaip.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Borres MP, Sato S, Ebisawa M. IgE-related examination in food allergy with focus on allergen components. Chem Immunol Allergy. 2015;101:68–78. doi: 10.1159/000371675. [DOI] [PubMed] [Google Scholar]
- 31.Beyer K, Grabenhenrich L, Härtl M, Beder A, Kalb B, Ziegert M, et al. Predictive values of component-specific IgE for the outcome of peanut and hazelnut food challenges in children. Allergy Eur J Allergy Clin Immunol. 2015;70:90–8. doi: 10.1111/all.12530. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. e13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Masthoff LJN, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akkerdaas JH, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393–9. doi: 10.1016/j.jaci.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Moreno FJ, Clemente A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem J. 2008;2:16–28. doi: 10.2174/1874091X00802010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeifer S, Bublin M, Dubiela P, Hummel K, Wortmann J, Hofer G, et al. Cor a 14, the allergenic 2S albumin from hazelnut, is highly thermostable and resistant to gastrointestinal digestion. Mol Nutr Food Res. 2015;59:2077–86. doi: 10.1002/mnfr.201500071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann K, Schweimer K, Reese G, Randow S, Suhr M, Becker W-M, et al. Structure and stability of 2S albumin-type peanut allergens: implications for the severity of peanut allergic reactions. Biochem J. 2006;395:463–72. doi: 10.1042/BJ20051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppelman SJ, Hefle SL, Taylor SL, de Jong GAH. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: a comparative in vitro study and partial characterization of digestion-resistant peptide. Mol Nutr Food Res. 2010;54:1711–21. doi: 10.1002/mnfr.201000011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.