Abstract
Male premature neonates are more susceptible than females to the development of bronchopulmonary dysplasia (BPD). The reasons underlying sexually dimorphic outcomes in premature neonates are not known. GDF15 (Growth and differentiation factor 15) is a secreted cytokine and plays a role in cell proliferation, apoptosis, and angiogenesis. In this study, we sought to elucidate the sex-specific expression of Gdf15 in the lung in vivo in neonatal hyperoxic lung injury and its regulation by Hif-1α, and to delineate the differences in GDF15 expression in male and female human umbilical venous endothelial cells in an in vitro model of oxygen toxicity. Following hyperoxia exposure (95% FiO2, PND (postnatal day 1-5: saccular stage of lung development), neonatal male mice (C57BL/6) show increased GDF15 and decreased HIF-1α expression compared to female mice. For the in vitro experiments, male and female HUVECs were exposed to room air condition (21% O2, 5% CO2) or in hyperoxia condition (95% O2, 5% CO2) for up to 72 h. Male HUVECs had greater expression of GDF15 mRNA and protein. To study the inter-relationship between GDF15 and HIF-1α, we measured the expression of GDF15 in H441 cells after HIF-1α knockdown using promoter dual luciferase reporter assay, which showed that HIF-1α and GDF15 expression are inversely related under normoxia and hyperoxia. The results indicate that sex differences exist in the expression and modulation of GDF15 by HIF-1α in neonatal hyperoxic injury both in vivo and in vitro. These differences could explain in part the mechanisms behind sex-specific differences in BPD.
Keywords: Hyperoxia, GDF15, HIF-1alpha, Bronchopulmonary Dysplasia, Sex-specific
Introduction
Bronchopulmonary dysplasia (BPD) is a debilitating lung disease with long-term consequences and is one of the most common causes for morbidity in premature neonates (Natarajan et al., 2012). It is characterized by arrest in lung development with severe impairment of alveolar septation and vascular development, leading to the development of pulmonary hypertension. It is well known that male premature neonates are more susceptible than females for the development of many prematurity related morbidities including BPD (Binet et al., 2012; Costeloe et al., 2000; Kraybill et al., 1989; O'Shea et al., 2012; Trembath and Laughon, 2012; Zysman-Colman et al., 2013), however, the underlying molecular mechanism(s) are not completely understood.
Sex-specific modulation of multiple biological processes (developmental, hormonal, physiological, inflammatory or vascular) may lead to the male bias in the development of BPD. Sex has an effect on prenatal lung development and on response to lung injury postnatally (Enomoto et al., 2012; Neriishi and Frank, 1984). Differences in intracellular signaling pathways may also be playing a major role in the sexually dimorphic responses that are seen in neonatal hyperoxic lung injury (Penaloza et al., 2009). Sex-specific differences in injury patterns have been observed both in vivo and in vitro models. Male neonatal mice (C57BL/6J) exposed to hyperoxia (PND1-5, 95% FiO2) show greater arrest in alveolarization and vascular development when compared to females (Lingappan et al., 2016). Male human umbilical venous endothelial cells have decreased survival, greater oxidative stress and impairment in angiogenesis compared to similarly exposed female cells (Zhang and Lingappan, 2017).
GDF15 is recognized as a stress-responsive cytokine and its levels are elevated in diseases such as acute respiratory distress syndrome, pulmonary hypertension and heart failure (Clark et al., 2013; Kempf and Wollert, 2013; Nickel et al., 2011). It is increased in response to oxidative stress (Han et al., 2008). Recently, we showed induction in GDF15 expression in pulmonary epithelial and endothelial cells (Tiwari et al., 2015). Hypoxia-inducible factor (HIF) is a master transcription factor, which modulates the expression of many oxygen sensitive genes including VEGF. HIF-1 expression is significantly decreased in the lungs of preterm lambs and neonatal rats exposed to hyperoxia (Grover et al., 2007; Hosford and Olson, 2003). In hypoxic human umbilical vein endothelia l cells, GDF15 pretreatment increases HIF-1α expression and nuclear translocation and knock-down of HIF-1α abolishes GDF15 mediated angiogenic effect and suppressed VEGF expression (Song et al., 2012). The interactions between HIF-1α and GDF15 under hyperoxic conditions have not been studied. The overall objective of this study was to elucidate the sex-specific expression of Gdf15 in the lung in vivo in neonatal hyperoxic lung injury and its regulation by HIF-1α, and to delineate the differences in GDF15 and HIF-1α expression in male and female human umbilical venous endothelial cells in an in vitro model of oxygen toxicity. We hypothesized that sex differences exist in the expression and modulation of GDF15 by HIF-1α in neonatal hyperoxic injury both in vivo and in vitro.
Methods
Animals
Timed pregnant C57BL/6J wild type (WT) mice were obtained from Charles River Laboratories (Wilmington). The sex in neonatal mouse pups was determined by both the anogenital distance and pigmentation in the anogenital region method (Wolterink-Donselaar et al., 2009). In younger mice, the sex was reconfirmed with PCR analysis for the Sry gene in genomic DNA obtained from mouse-tail clips as described before (Lingappan et al., 2016).
Mouse model of BPD
Mouse pups were divided into two groups: one group exposed to normoxia (21% O2) and the other group exposed to hyperoxia (95% O2), within 12 h of birth for 5 days (saccular stage of lung development) (Harijith et al., 2013; Lin et al., 2005). The saccular stage of lung development extends from 26-36 weeks in human preterm neonates. The pups were pooled from multiple litters before being randomly and equally redistributed to two groups, and in each cage the litter size was limited to six pups to control for the effects of litter size on nutrition and growth. The dams were rotated between air- and hyperoxia-exposed litters every 24 hours to prevent oxygen toxicity in the dams and to eliminate maternal effects between the groups. Mice were sacrificed on PND7, and PND21 (after recovery in room air) as most of postnatal lung development in mice is completed by this age (5, 19, 74). The control group was kept at room air for the same duration of time (PND7 and PND21).
Cell culture and hyperoxia treatment
Male and Female human umbilical endothelial cells were obtained from Lonza and maintained in EGM-plus medium (CC-5036, Lonza) with SingleQuots (CC-4542, Lonza) at 37°C in 5% CO2. Male and Female HUVECs were used from passages 3 - 6 to ensure their endothelial characteristics. H441 cells were obtained from ATCC and maintained in 1640 medium (Cat#11875093, ThermoFisher) with 10% FBS at 37°C in 5% CO2. 1×105 cells were seeded in a 6 mm dish. 24 h later, these cells were incubated at 37°C in room air (37% O2, 5% CO2) or hyperoxia (95% O2, 5% CO2) for 24, 48 and 72 h.
HIF-1α inhibition
HIF-1α siRNA (SASI_Hs02_00332063, SASI_Hs01_00122699) and negative Control siRNA (4457289) were purchased from Sigma-Aldrich and used for knocking down HIF-1α in H441 cells. Chetomin (Hif-1α inhibitor) was purchased from Santa Cruz and was used at a concentration of 150 nm or 300 nm to inhibit HIF-1α function in H441 cells.
Luciferase reporter assay
GDF15 promoter (-1000bp – 0bp) was cloned using PCR, then inserted in PGL3-basic plasmid to create GDF15-PGL3 plasmid. Primers used in gene clone were listed in Table. 1. GDF15-PGL3 plasmid (2ug/well in a 6-well plate) and pRL-TK (0.5 ug/well in a 6-well plate; Promega Corp., Madison, WI) plasmid were co-transfected with Hif-1α siRNA or negative control siRNA in H441 cells using lipofectamine 2000 (ThermoFisher). Forty-eight hours after transfection, cells were harvested and used for dual-luciferase reporter assay (Promega Corp., Madison, WI). Extracts were assayed for firefly and the Renilla Luc activities using a dual Luc kit (Promega Corp., Madison, WI). Fire flyLuc activity was normalized to that of Renilla. The ratio of Firefly and Renilla was determined using a microplate reader (M3 SpectraMax)
Table 1. Primer List.
| Primer | Forward | Reverse |
|---|---|---|
| b-actin (mice) | GATCTGGCACCACACCTTCT | GGGGTGTTGAAGGTCTCAAA |
| b-actin (human) | CATCGAGCACGGCATCGTCA | TAGCACAGCCTGGATAGCAAC |
| GDF15 (human) | CCAAAGACTGCCACTGCATA | GAATCGGGTGTCTCAGGAAC |
| GDF15 (mice) | CGAGTGTCCCCACCTGTATC | TAAGAACCACCGGGGTGTAG |
| Hif1a (mice) | ATTCTCCAAGCCCTCCAAGT | TCATCAGTGGTGGCAGTTGT |
| Hif1a (human) | GATGTAATGCTCCCCTCACC | CTTGATTGAGTGCAGGGTCA |
| GDF15-Luc | ACACGGTACCTAGAGACAGGGTT TCTCCA | GTGTAGATCTGACCAGATGCTG CCGGA |
Quantitative PCR
Total RNA was extracted from the lung tissues using TRIzol-chloroform and then treated with DNase I (Invitrogen). cDNA was prepared by using the using iScript Advanced cDNA Synthesis Kit (Bio-Rad). Quantitative PCR was performed using the QuantStudio 7 Flex real-time PCR detection system (ThermoFIsher) and SYBR Green. The thermal cycling conditions used were as follows: one cycle at 95 °C for 1 min, 40 cycles at 95 °C for 15 s, and one cycle at 60 °C for 15 s. The primers used in real-time PCR test were listed in Table. 1. Relative mRNA levels were calculated using the 2−ΔΔCT method and normalized by β-actin in the same sample.
Western Immunoblotting
Protein was isolated using RIPA buffer (ThermoFisher) containing protease mixture inhibitors (ThermoFisher). Proteins were separated by 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a PVDF membrane using a mini-PROTEAN tetra cell system (Bio-Rad). The following primary antibodies were used: rabbit anti-GDF15 (1:1000, Cell Signaling), rabbit anti-HIF1α (1:1000, Cell Signaling) and rabbit anti-β-actin (1:5000, Cell Signaling Technology, Beverly, MA). ECL plus western blotting substrate was used for visualizing protein bands. The protein bands were normalized by β-actin (used as a loading control) on the same gel.
ELISA
Human GDF15 ELISA kit (ab155432, Abcam) was purchased from Abcam to determine the GDF15 concentration in the cell culture medium following the protocol. Briefly, 100-μl of cell culture medium from each sample was transferred into appropriate well. After 2.5 hours incubation at room temperature with gentle shaking, biotinylated GDF15 antibody was added to the wells for 1 hour at room temperature. After incubation with Streptavidin solution for 45 mins at room temperature, TMB substrate reagent was added to each well for 30 mins at room temperature. Stop solution was added to the appropriate wells and the signal measured. Final GDF15 concentration was calculated using a standard curve generated by standard samples.
Statistical analysis
GraphPad version 7 was used for the analysis of our data. Data is expressed as means ± SE. Data were analyzed by two-way ANOVA to test for the independent effects of sex and hyperoxia and to look for any interaction. Multiple-comparison testing (Bonferroni) was performed if statistical significance (P < 0.05) was noted by ANOVA.
Results
Hyperoxia increased Gdf15 expression in both male and female neonatal mice
Male and female WT (C57BL6) neonatal mice were exposed to hyperoxia from PND1-5 (95% FiO2) during the saccular stage of lung development and control mice were maintained in room air conditions. We measured the expression of Gdf15 mRNA (Figure 1A and 1C) on PND7 and 21 and protein (Figure 1B) on PND7 in the lungs. There was a significant increase in Gdf15 mRNA expression in the lungs after hyperoxia exposure in both male (P<0.0001) and female mice (P=0.02) on PND7. Both sex (P=0.0065) and hyperoxia treatment (P<0.0001) were significant in 2-way ANOVA analysis and the interaction term was also significant (P=0.0052). Gdf15 mRNA was greater in the lungs of neonatal male mice compared to similarly exposed female mice. By PND21, there were no differences between room air controls and hyperoxia-exposed animals in either sex. GDF15 protein expression on PND7 showed an increase in the lungs of hyperoxia-exposed animals compared to room air controls in both sexes (P=0.03), but no sex differences were noted.
Figure 1. Pulmonary Gdf15 and Hif-1α expression in male and female neonatal mice after postnatal hyperoxia (PND 1-5, 95% FiO2) exposure.
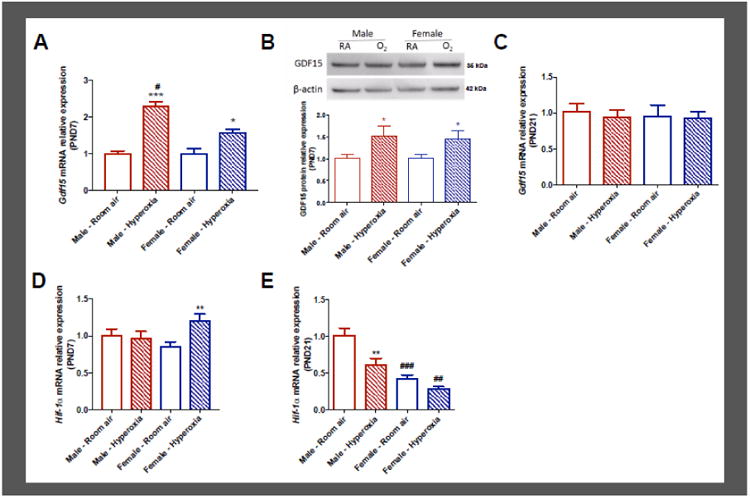
GDF15 mRNA (A) and protein expression and densitometry analysis (B) at postnatal day 7. GDF15 mRNA (C) expression at postnatal day 21. Hif-1α mRNA expression in mice lung tissue at postnatal day 7 (D) and postnatal day 21 (E). Fold change in mRNA expression over male room air values are shown. Values are expressed as mean ± SEM. N=5-6 for each group in each test. Significant differences between room air and hyperoxia within genotype are indicated by * P < 0.05 and ***P < 0.001. Significant differences between male and female mice are indicated by #P <0.05. RA: Room Air, O2: Hyperoxia
HIF-1α expression was increased in female mice at PND7 and decreased in male mice at PND21 after hyperoxia exposure
We measured the expression of HIF-1α mRNA on PND7 (Figure 1D) and PND21 (Figure 1E) in the lungs in vivo. In the acute phase (PND7), there was a significant increase in HIF-1α expression in the female lungs (P=0.0079) after hyperoxia exposure compared to female room air controls, while males showed no increase. Sex was not significant, while treatment (P=0.04) and the interaction term (0.02) were significant by 2-way ANOVA analysis. After recovery in room air, on PND21, there was decreased HIF-1α expression in the male but not in the female lungs compared to the room air controls. Interestingly, females had lesser HIF-1α expression in the lungs both at room air and after exposure to hyperoxia. 2- way ANOVA analysis was significant for treatment (P=0.001) and sex (P<0.001), but the interaction term was not significant.
Male HUVEC s have greater expression of GDF15 mRNA and protein after hyperoxia exposure in vitro
To assess sex-specific differences GDF15 expression in vitro, we exposed human umbilical venous endothelial cells of either sex to hyperoxia and measured mRNA (Figure 2A) and protein (Figure 2B) levels. There was an increase in GDF15 mRNA expression following hyperoxia exposure at 24 and 48 h in both male and female HUVECs compared to cells maintained in normoxia (P<0.001). At 48 h, this increase was larger in male compared to female neonatal mice. We measured GDF15 protein concentration using ELISA in the cell culture supernatants, as it is a secreted protein. At 24 h, male HUVECs had significantly increased GDF15 protein levels compared to cells in normoxia and compared to hyperoxia-exposed female HUVECs. At 48 hours, female HUVECs displayed increased GDF15 protein expression, and male cells continued to have sustained increase in GDF15 protein levels. We also measured HIF-1α protein levels in male and female HUVECs after 48 h of hyperoxia or room air exposure. There was a significant decrease in HIF-1α protein expression in both male and female HUVECs exposed to hyperoxia compared to cells maintained in room air (Figure 2C and 2D).
Figure 2. Effect of hyperoxia on GDF15 and HIF-1α expression in male and female HUVECs.

GDF15 mRNA (A) and protein expression (B) in male and female HUVECs exposed to normoxic or hyperoxic conditions. HIF-1α protein expression and densitometry ananlysis in male (C) and female (D) HUVECs after hyperoxia exposure (48h) compared to cells maintained in room air. Values are expressed as mean ± SEM. N = 6 for each group in each test. Significant differences from baseline are indicated by ** P < 0.01 and *** P< 0.001. Significant differences between male and female HUVECs are indicated by ###P <0.001.
HIF-1α knockdown or inhibition increases GDF15 expression in H441 cells
To study the interaction between GDF15 and HIF-1α, we measured the expression of GDF15 in H441 cells after HIF-1α knockdown in H441 cells. H441 cell line was derived from a human lung adenocarcinoma and is representative of the human distal lung epithelium. To achieve silencing of HIF-1α, we performed siRNA transfection of H441 cells using two HIF-1α siRNAs, and used cells transfected with siRNA with a scrambled sequence as controls. Successful decrease in gene expression was achieved following siRNA transfection as shown in Figure 3A. Following successful siRNA transfection, GDF15 mRNA expression was increased in these lung cells (Figure 3B). We also verified these results by using a HIF-1α specific inhibitor, Chetomin (CAS 1403-36-7) at 150 and 300 nM. It disrupts HIF binding to its transcriptional co-activator p300. Similar to the results with siRNA mediated gene silencing inhibition of HIF-1α transcriptional activity also led to increased GDF15 mRNA expression in these cells (Figure 3C). To further confirm whether HIF-1α knockdown modulates GDF15 expression, we transfected H441 cells with GDF-15-Luc reporter construct bearing the promoter region o the GDF15 gene. These cells were then subjected to siRNA-mediated knockdown of HIF-1α. Results from the luciferase reporter assay experiment are shown in Figure 3D. Similar to the results with the qRT-PCR, knockdown of HIF-1α led to increased GDF15 promoter activity. Thus, we were able to show that knockdown or inhibition of HIF-1α leads to augmentation of GDF15 expression.
Figure 3. Effect of HIF-1α knockdown on GDF15 expression in H441 cells.
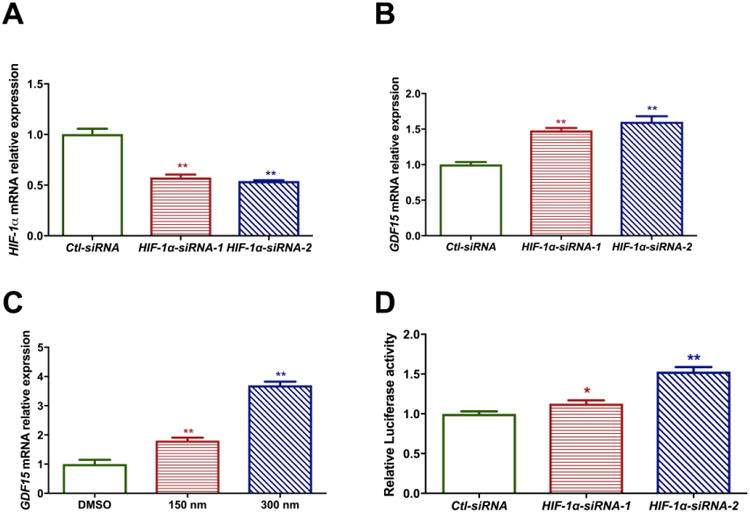
HIF-1α (A) and GDF15 (B) mRNA expression in H441 cells after HIF-1α knock down using siRNA. siRNA transfection was done using two separate siRNAs against HIF-1α. (C) GDF15 mRNA expression in H441 cells after HIF-1α inhibition using Chetomin. (D) Relative Luciferase activity of GDF15 promoter after HIF-1α knock down using siRNA. Values are expressed as mean ± SEM. N ≥ 4 for each group in each test. Significant differences from baseline are indicated by * P < 0.05 and ** P < 0.01.
GDF15 and HIF-1α expression in H441 cells exposed to hyperoxia
H441 cells showed increased expression of GDF15 mRNA at 48 and 72 h after hyperoxia exposure compared to cells in normoxia (Figure 4A). There was also a similar increase in GDF15 protein levels measured by ELISA in the cell culture supernatant after exposure to hyperoxia at 24, 48 and 72 h (Figure 4B). HIF-1α expression declined after hyperoxia exposure with a significant decrease seen at 72 hours after hyperoxia exposure (Figure 4C). Thus, hyperoxia exposure had opposing effects on GDF15 and HIF-1α expression in H441 cells.
Figure 4. Effect of hyperoxia on GDF15 and HIF-1α expression in H441 cells.

GDF15 mRNA (A) GDF15 protein concentration (B) and HIF-1α mRNA (C) expression in H441 cells exposed to hyperoxia or normoxic conditions. Values are expressed as mean ± SEM. N = 6 for each group in each test. Significant differences from baseline are indicated by ** P < 0.01.
Discussion
BPD is associated with significant morbidity and mortality in premature infants. Exposure to high concentrations of oxygen (hyperoxia) contributes to its development of by increasing oxidative stress in the developing lung (Saugstad, 2003). Multiple studies have revealed a male disadvantage towards the development of BPD in premature neonates, but the underlying molecular mechanisms are not well understood. The major finding of this study is that GDF15 was upregulated by hyperoxia in a sex-specific manner both in vivo and in vitro. We further highlight the sex-specific differences in the expression of GDF15 and its modulation by HIF-1α. Both in vivo and in vitro conditions, males showed increased expression of GDF15 upon exposure to hyperoxia. HIF-1α was upregulated in females in the acute phase and was downregulated in males in the late phase in vivo. Loss of function of HIF-1α leads to increased expression of GDF15 in H441 cells and there was an inverse relationship between GDF15 and HIF-1α expression in H441 cells and HUVECs upon exposure to hyperoxia. To the best of our knowledge, this is the first study showing the sex-specific differences in GDF15 induction in vivo in a model of neonatal hyperoxic lung injury and its modulation by HIF-1α in vitro in a model of pulmonary oxygen toxicity.
GDF15 is a member of the TGF- β superfamily and is expressed in many cell types and organs (Corre et al., 2013). It is a secreted pleiotropic cytokine and is a part of the stress response pathway after cellular injury. It is also considered as a biomarker and is indicative of prognosis in cancer, congestive cardiac failure and acute respiratory distress syndrome (Lok et al., 2013; Wang et al., 2013). Whether increased serum concentrations indicate ongoing cellular injury or represent a protective response to biologic stress is still an open question, and the answer might depend on the organ and cellular microenvironment. Published data from our laboratory and others show that GDF15 is a part of the gene expression signature of oxidative stress (Han et al., 2008; Tiwari et al., 2015). GDF15 is expressed and induced in response to hypoxia in human pulmonary vascular endothelial cells (Nickel et al., 2011). It is also induced in animal models of lung injury in both adult and neonatal mice (Bhattacharya et al., 2012; Lingappan et al., 2014; McGrath-Morrow et al., 2014; Zimmers et al., 2005). We have previously shown that GDF15 is induced in pulmonary epithelial (BEAS-2B) and endothelial (HPMEC) cells upon exposure to hyperoxia (Tiwari et al., 2015).
In this study, we report increased Gdf15 expression at the both the mRNA and protein levels at PND7 in male and female mice. The transcriptional response was higher in males. There were no differences noted between male and female mice in GDF15 protein expression in the lung homogenates. This could have been due to that fact that determination of protein levels in lung homogenates may not have captured the protein levels secreted into the systemic circulation.
Pulmonary angiogenesis is critical for alveolarization and inhibition of the former has an adverse impact on the latter (Jakkula et al., 2000; Thebaud, 2005). We have shown that following postnatal hyperoxia (PND 1-5) exposure, angiogenesis was impaired (lesser vessel number and decreased expression for PECAM1/CD31 and VEGFR2) in male neonatal mice compared to females. HIF is a transcription factor that functions as an oxygen sensor and modulates gene expression in response to hypoxia including induction of pro-angiogenic genes such as VEGF (vascular endothelial growth factor). HIF-1 is a heterodimer composed of HIF-1α and the aryl hydrocarbon nuclear translocator (ARNT), also known as HIF-1β. When activated, it accumulates and translocates to the nucleus and binds to the hypoxia response elements of different target genes to increase transcription Prolyl 4-hydorxylases are enzymes, which hydroxylate proline residues of HIF-1 and target them for polyubiquitination and proteosomal degradation mediated by pVHL protein (Maes et al., 2012). Blockade of prolyl hydroxylase stabilizes HIF, and leads to increases in VEGF, PEC AM-1 levels and angiogenesis in human microvascular endothelial cells exposed to hyperoxia (Asikainen et al., 2005). In our study, HIF-1α mRNA levels were increased in female neonatal mice exposed to hyperoxia at PND7 and were decreased in male mice at PND21 which was consistent with our previous findings of decreased lung vascular development in male mice at the same time point (Lingappan et al., 2016). HIF-1α was also decreased upon exposure to hyperoxia under in vitro conditions (Figures 2 and 4).
For the study of endothelial cell physiology, human umbilical vein endothelial cells provide a robust in vitro model (Cines et al., 1998). GDF15 has been found to have pro- or anti-angiogenic effects based on the cellular microenvironment/stressor (Song et al., 2012; Whitson et al., 2013). HUVECs exposed to hyperoxia showed a robust induction in GDF15 mRNA and protein levels and this was higher in male cells. We have shown previously that male HUVECs have higher oxidative stress under hyperoxic conditions (Zhang and Lingappan, 2017) and the increase in GDF15 levels in male HUVECs may be reflective of this phenomenon. HIF-1α protein levels were decreased in both male and female HUVECs upon exposure to hyperoxia. We have previously shown that after exposure to hyperoxia, male HUVECs have decreased tube formation capacity compared to similarly exposed female HUVECs (Zhang and Lingappan, 2017). Increased anti-angiogenic gene expression in male mice have been reported by other investigators (Keenaghan et al., 2013). Thus, in the hyperoxia model, GDF15 though induced, may not have a strong pro-angiogenic effect but maybe reflective of the levels of oxidative stress in the cells.
To study the interaction between HIF-1α and GDF15 under basal or normoxic conditions, we used the H441 cell line, which is representative of the human distal lung epithelium. We were able to show that both using siRNA mediated gene knockdown and inhibition of transcription that there was an inverse relationship between HIF-1α expression/function and GDF15 expression in this cell line. This was further confirmed using a dual luciferase reporter assay. These results were similar to the expression patterns seen in when H441 cells were exposed to hyperoxia, which elicited a decrease in HIF-1α, but an increase in GDF15 expression.
In conclusion, we show that there is sex-specific expression of Gdf15 and Hif-1α in the lung in vivo in neonatal hyperoxic lung injury and in male and female human umbilical venous endothelial cells in an in vitro model of oxygen toxicity. The increase in GDF15 expression may be a reflection of the levels of oxidative stress in the cellular environment and does not have a pro-angiogenic effect in the hyperoxia model. Also, HIF-1α and GDF15 expression were inversely related under normoxic and hyperoxic conditions in H441 cells. These results highlight sex-specific differences in the modulation of molecular pathways in hyperoxic lung injury both in vivo and in vitro, which may correlate with sexual dimorphism in the incidence of BPD.
Highlights.
Sex-specific differences exist in the GDF15 expression in hyperoxic conditions
Males show increased GDF15, decreased HIF-1α expression upon hyperoxia exposure
HIF-1α and GDF15 expression are inversely related under normoxia and hyperoxia
Acknowledgments
Funding Support: This work was supported in part by the National Institutes of Health [K08-HL-127103 to K.L. and the American Lung Association grant RG-418067 to KL. We would like to thank Dr. Bhagavatula Moorthy for critically reviewing this manuscript and for his mentorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Günzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA. 2005;102:10212–10217. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, Pryhuber GS. Genome-Wide Transcriptional Profiling Reveals Connective Tissue Mast Cell Accumulation in Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2012;186:349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B Canadian Neonatal Network™. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol. 2012;29:159–166. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Clark BJ, Bull TM, Benson AB, Stream AR, Macht M, Gaydos J, Meadows C, Burnham EL, Moss M the ARDS Network Investigators. Growth differentiation factor-15 and prognosis in acute respiratory distress syndrome: a retrospective cohort study. Crit Care. 2013;17:R92. doi: 10.1186/cc12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, Hébraud B, Bourin P. Concise Review: Growth Differentiation Factor 15 in Pathology: A Clinical Role? Stem Cells Transl Med. 2013;2:946–952. doi: 10.5966/sctm.2013-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics. 2000;106:659–671. doi: 10.1542/peds.106.4.659. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Gosal K, Cubells E, Escobar J, Vento M, Jankov RP, Belik J. Sex-dependent changes in the pulmonary vasoconstriction potential of newborn rats following short-term oxygen exposure. Pediatr Res. 2012;72:468–478. doi: 10.1038/pr.2012.120. [DOI] [PubMed] [Google Scholar]
- Grover TR, Asikainen TM, Kinsella JP, Abman SH, White CW. Hypoxia-inducible factors HIF-1 and HIF-2 are decreased in an experimental model of severe respiratory distress syndrome in preterm lambs. AJP: Lung Cellular and Molecular Physiology. 2007;292:L1345–L1351. doi: 10.1152/ajplung.00372.2006. [DOI] [PubMed] [Google Scholar]
- Han ES, Muller FL, Pérez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, Epstein CJ, Roberts LJ, Van Remmen H, Richardson A. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, Gorshkova I, Huang LS, Mohan V, Garzon S, Kanteti P, Reddy SP, Raj JU, Natarajan V. Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins. The American Journal of Pathology. 2013;183:1169–1182. doi: 10.1016/j.ajpath.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L161–8. doi: 10.1152/ajplung.00285.2002. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–7. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Keenaghan M, Cai CL, Kumar D, Valencia GB, Rao M, Aranda JV, Beharry KD. Response of vascular endothelial growth factor and angiogenesis-related genes to stepwise increases in inspired oxygen in neonatal rat lungs. Pediatr Res. 2013;73:630–638. doi: 10.1038/pr.2013.21. [DOI] [PubMed] [Google Scholar]
- Kempf T, Wollert KC. Risk stratification in critically ill patients: GDF-15 scores in adult respiratory distress syndrome. Crit Care. 2013;17:173. doi: 10.1186/cc12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill EN, Runyan DK, Bose CL, Khan JH. Risk factors for chronic lung disease in infants with birth weights of 751 to 1000 grams. J Pediatr. 1989;115:115–120. doi: 10.1016/s0022-3476(89)80345-2. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res. 2005;58:22–29. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- Lingappan K, Jiang W, Wang L, Couroucli XI, Moorthy B. Analysis of the transcriptome in hyperoxic lung injury and sex-specific alterations in gene expression. PLoS ONE. 2014;9:e101581. doi: 10.1371/journal.pone.0101581.t004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. AJP: Lung Cellular and Molecular Physiology. 2016 doi: 10.1152/ajplung.00047.2016. ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok DJ, Klip IT, Lok SI, Bruggink-André de la Porte PW, Badings E, van Wijngaarden J, Voors AA, de Boer RA, van Veldhuisen DJ, van der Meer P. Incremental prognostic power of novel biomarkers (growth-differentiation factor-15, high-sensitivity C-reactive protein, galectin-3, and high-sensitivity troponin-T) in patients with advanced chronic heart failure. Am J Cardiol. 2013;112:831–837. doi: 10.1016/j.amjcard.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8:358–366. doi: 10.1038/nrrheum.2012.36. [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow SA, Lauer T, Collaco JM, Lopez A, Malhotra D, Alekseyev YO, Neptune E, Wise R, Biswal S. Transcriptional responses of neonatal mouse lung to hyperoxia by Nrf2 status. Cytokine. 2014;65:4–9. doi: 10.1016/j.cyto.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, Laptook AR, Bell EF, Stoll BJ, Newman N, Hale EC, Bara R, Walsh MC. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88:509–515. doi: 10.1016/j.earlhumdev.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neriishi K, Frank L. Castration prolongs tolerance of young male rats to pulmonary O2 toxicity. Am J Physiol. 1984;247:R475–81. doi: 10.1152/ajpregu.1984.247.3.R475. [DOI] [PubMed] [Google Scholar]
- Nickel N, Jonigk D, Kempf T, Bockmeyer CL, Maegel L, Rische J, Laenger F, Lehmann U, Sauer C, Greer M, Welte T, Hoeper MM, Golpon HA. GDF-15 is abundantly expressed in plexiform lesions in patients with pulmonary arterial hypertension and affects proliferation and apoptosis of pulmonary endothelial cells. Respir Res. 2011;12:62. doi: 10.1186/1465-9921-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JE, Davis PG, Doyle LW Victorian Infant Collaborative Study Group. Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res. 2012;71:210–214. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- Penaloza C, Estevez B, Orlanski S, Sikorska M, Walker R, Smith C, Smith B, Lockshin RA, Zakeri Z. Sex of the cell dictates its response: differential gene expression and sensitivity to cell death inducing stress in male and female cells. FASEB J. 2009;23:1869–1879. doi: 10.1096/fj.08-119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- Song H, Yin D, Liu Z. GDF-15 promotes angiogenesis through modulating p53/HIF-1α signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep. 2012;39:4017–4022. doi: 10.1007/s11033-011-1182-7. [DOI] [PubMed] [Google Scholar]
- Thebaud B. Vascular Endothelial Growth Factor Gene Therapy Increases Survival, Promotes Lung Angiogenesis, and Prevents Alveolar Damage in Hyperoxia-Induced Lung Injury: Evidence That Angiogenesis Participates in Alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- Tiwari KK, Moorthy B, Lingappan K. Role of GDF15 (growth and differentiation factor 15) in pulmonary oxygen toxicity. Toxicol In Vitro. 2015;29:1369–1376. doi: 10.1016/j.tiv.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembath A, Laughon MM. Predictors of Bronchopulmonary Dysplasia. Clin Perinatol. 2012;39:585–601. doi: 10.1016/j.clp.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Baek SJ, Eling TE. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem Pharmacol. 2013;85:597–606. doi: 10.1016/j.bcp.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson RJ, Lucia MS, Lambert JR. Growth differentiation factor-15 (GDF-15) suppresses in vitro angiogenesis through a novel interaction with connective tissue growth factor (CCN2) J Cell Biochem. 2013;114:1424–1433. doi: 10.1002/jcb.24484. [DOI] [PubMed] [Google Scholar]
- Wolterink-Donselaar IG, Meerding JM, Fernandes C. A method for gender determination in newborn dark pigmented mice. Lab Anim (NY) 2009;38:35–38. doi: 10.1038/laban0109-35. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lingappan K. Differential sex-specific effects of oxygen toxicity in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2017;486:431–437. doi: 10.1016/j.bbrc.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock. 2005;23:543–548. [PubMed] [Google Scholar]
- Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health. 2013;18:86–90. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]


