Abstract
The human apurinic/apyrimidinic endonuclease 1 (APE1) is a pleiotropic nuclear protein with roles in DNA base excision repair pathway as well as in regulation of transcription. Recently, the presence of extracellular plasma APE1 was reported in endotoxemic rats. However, the biological significance and the extracellular function of APE1 remain unclear. In this study, we found that monocytes secrete APE1 upon inflammatory challenges. Challenging the monocytic cells with extracellular APE1 resulted in the increased expression and secretion of the pro-inflammatory cytokine IL-6. Additionally, the extracellular APE1 treatment activated the transcription factor NF-κB, followed by its increased occupancy at the IL-6 promoter, resulting in the induction of IL-6 expression. APE1-induced IL-6 further served to elicit autocrine and paracrine cellular responses. Moreover, the extracellular IL-6 promoted the secretion of APE1, thus indicating a functional feedforward loop in this pathway. Furthermore, we show that APE1 is secreted through extracellular vesicles formation via endosomal sorting complex required for transport (ESCRT)-dependent pathway. Together, our study demonstrates a novel role of extracellular APE1 in IL-6-dependent cellular responses.
Keywords: Apurinic/apyrimidinic endonuclease 1 (APE1), Secretion, Lipopolysaccharide (LPS), Interleukin 6 (IL-6), NF-kappa B (NF-κB)
Introduction
Apurinic/apyrimidinic endonuclease 1 (APE1) is a multifunctional protein, originally identified as an enzyme essential in the DNA base excision repair (BER) pathway [1]. Besides its classical function in DNA damage repair, APE1 also participates in transcriptional regulation, either as a cofactor in a transactivator complex, or as a redox signaling factor by stimulating the DNA-binding activity of other transcription factors [2, 3]. In agreement with its nuclear functions, the nuclear localization of APE1 has also been established [1]. However, the nucleo-cytoplasmic shuttling of APE1 has also been documented, particularly in metabolically-active or proliferative states [4]. Subsequently, an extra-nuclear role of the cytoplasmic APE1 in regulating oxidative stress and inflammatory responses was also reported [5]. Interestingly, a recent report demonstrated the secretion of APE1 into the extracellular medium in cultured HEK293 and tumor cells [6]. Concurrently, another report identified the presence of plasma APE1 in lipopolysaccharide (LPS)-induced endotoxemic rats [7]. While these reports support the idea that APE1 can be secreted from cells, the biological relevance of APE1 secretion into the plasma remains elusive. Moreover, the extracellular function(s) of the secretory APE1 remains largely unknown.
Mammalian cells secrete a variety of cellular proteins. Among the pool of secretory proteins, several members lack a leader sequence, and are thus not secreted through the classical endoplasmic reticulum (ER)-Golgi apparatus-mediated secretion [8]. These leader-less proteins follow a non-classical mode of secretion, and the pathways have been resolved in recent years [9]. Interestingly, certain chromatin proteins [10-12] feature in the list of leader-less secretory proteins that have been identified so far [8, 13], despite the fact that some of these proteins are established nucleus-specific factors essential to transcription and maintenance of the DNA structure. Subsequently, several reports also showed a cytokine-like activity of these secretory nuclear proteins [12, 14].
In this study, we used normal human donor derived fresh monocytes as well as human monocyte cell line THP-1 to show that APE1 is secreted by monocytes upon inflammatory challenges. This secretion follows an extracellular vesicles-mediated non-classical route. Additionally, we discovered that extracellular APE1 induces the production and secretion of the pro-inflammatory cytokine, IL-6 in monocytic cells through the transactivation of nuclear factor-κB (NF-κB). Furthermore, we identified a feedforward loop between IL-6 and APE1 secretion. Collectively, our results demonstrate a de novo role of extracellular APE1 in IL-6 mediated cellular responses.
Methods
Isolation of monocytes, B-cells and T-cells from human peripheral blood
Peripheral blood was collected from healthy donors using a University of Nebraska Medical Center Institutional Review Board-approved protocol. Using the density gradient-based technique with Lymphoprep solution (Stem Cell Technologies), mononuclear cells were isolated from the whole blood. Monocytes and B cells were isolated from peripheral blood mononuclear cells by immune-magnetic negative selection with the Monocyte Isolation Kit II and the B Cell Isolation Kit II (Miltenyi Biotech), respectively, using the manufacturer's protocol. T cells were isolated using positive selection with CD3 Micro beads (Miltenyi Biotech). Purity of cell fractions was confirmed using flow cytometry (FACS; BD LSR II).
Cell culture, plasmid constructs and transduction
Human monocyte cell line THP-1 and murine macrophage-like cell line RAW264.7 were kindly provided by Dr. Sutapa Ray and Dr. Kaustubh Datta (University of Nebraska Medical Center, USA), respectively. Human Telomerase Reverse Transcriptase (hTERT) immortalized BJ fibroblast cells (BJ-hTERT) have been described previously [15]. Human Colon cancer HCT116 (ATCC #CCL-247) and HCT116 cells stably expressing APE1-shRNA were grown in McCoy's 5A medium (Gibco) under normoxic or hypoxic (1% O2) as described previously [16]. THP-1 cells were cultured in RPMI 1640 medium (Gibco) and RAW264.7 and BJ-hTERT cells were cultured in Dulbecco's Modified Eagle's Medium (Gibco). Media were supplemented with 10% fetal calf serum (Sigma) and 1% Penicillin-streptomycin solution (Gibco). Lipopolysaccharide from Escherechia coli 026:B6 (LPS; Sigma, L2654), Tumor necrosis factor-α (TNF-α; ProSpec), Brefeldin A (Sigma), Interleukin-6 (IL-6; ProSpec), bovine serum albumin (BSA; Sigma), recombinant APE1, GST-APE1, 8-Oxoguanine DNA Glycosylase (OGG1) and GST were used at specific doses or for different time points as indicated in individual figures. Generation and purification of recombinant proteins were done as described previously [17]. Retrovirus expression plasmids encoding wild-type (WT) VPS4-GFP or its mutants VPS4K173Q-GFP and VPS4E228Q have been described previously [18]. HEK293T cells were transfected with expression plasmids for WT or mutant VPS4 and with expression plasmids encoding other virus packaging proteins. Forty eight hours after transfection, supernatant containing the viral particles were used to infect RAW264.7 cells.
Precipitation of extracellular proteins
Cells were incubated in reduced serum containing Opti-MEM culture medium (Gibco) and cell culture supernatants were collected upon treatment as mentioned in individual figures. Precipitation of extracellular proteins from cell culture supernatants was done as described previously [6].
Western Blotting and antibodies
Whole cell lysates or cell culture supernatants were resolved on polyacrylamide gels and transferred to nylon membranes for blotting. Various primary antibodies (Ab) used were mouse monoclonal APE1 (Novus Biologicals; # NB100-116), rabbit polyclonal phospho (Ser536) p65 (H-164, Santa Cruz Biotechnology), goat polyclonal p65 (C-20, Santa Cruz), mouse monoclonal phospho (Ser473) AKT (E-7, Santa Cruz), rabbit polyclonal HSC70 (Santa Cruz), goat polyclonal α-tubulin (N-13, Santa Cruz), mouse monoclonal β-actin (17D10, Santa Cruz), Human Rab-5b (Santa Cruz sc598), GAPDH (Santa Cruz sc32233), TSG101 (Santa Cruz sc6037), CD63 (Santa Cruz sc15363). Immunoblot signals were detected using Super Signal West pico chemiluminiscent substrate (Thermo Scientific) after treating with HRP-conjugated secondary Ab (GE Healthcare).
Extracellular Vesicles preparation
Extracellular Vesicles (EV) were prepared from the cell culture supernatant of the THP-1 human monocyte cell line using a modified ultracentrifugation and filtration protocol as described previously [19]. Briefly, THP-1 cells at 60% confluency were washed with PBS and cultured in RPMI 1640 media containing 10% EV-free FBS (System Biosciences), 1% glutamine and 5% penicillin streptomycin (Gibco). After 24 hrs, the cell culture supernatant was centrifuged for 10 min at 300 × g, 20 min at 2000 × g and then filtered (0.2 μm) to remove cell debris and micro-vesicles larger than 200 nm. Subsequently, EV were collected by ultracentrifugation at 100,000 × g for 16 hrs. in a 70.1 Ti rotor (Beckman Coulter). For EV isolation from RAW264.7, cells transfected with Rab5b siRNA or transduced with adenovirus expressing GFP-tagged VPS4. We used the Total EV Isolation reagent (Thermo Fisher) in accordance with the manufacturer's protocol. EV pellet was resuspended in 100 μl PBS and stored at -80°C. The amount of EV was estimated by measuring protein content with the Bradford assay (Bio-Rad).
HCT116 and HCT116APE1-ShRNA cells were cultured for 24 hrs; thereafter, media was replaced with McCoy's 5A medium supplemented with 10% EV-depleted FBS and cultured under normoxic (20% O2) or hypoxic (1% O2) conditions for 72 hrs. Subsequently, conditioned media was harvested and EV were isolated by a precipitation method using commercially available ExoQuick reagent (Thermo Fisher Scientific) according to the manufacturer's protocol. Briefly, conditioned media was incubated overnight with ExoQuick reagent, centrifuged at 10,000 rpm for 2 hrs and the pellet was washed once with PBS, and pelleted EV were lysed with RIPA buffer.
Electron microscopy
Electron microscopy imaging of EV was performed as previously described [20] with some modifications. Briefly, EV were fixed for 1 hr in a mixture of 2% paraformaldehyde, 2% glutaraldehyde in 0.1 M Sorensen's phosphate buffer. After fixation, the EV sample was layered on a formvar-coated grid and air-dried for 2 min. Grids were stained with a vanadium based negative stain (Nanoprobes) and air-dried for an additional 2 min. Samples were observed at 80 kV with a Tecnai G2 Transmission Electron Microscope (FEI Company).
Nanoparticle tracking analysis (NTA)
Extracellular vesicles were analyzed using a NanoSight LM10 [21] (NanoSight Ltd, Navato, CA). A red laser beam is used to illuminate the particles in the sample and their movement under Brownian motion was captured for 1 min. The recorded video was analyzed using the NTA software which applies the two-dimensional Stokes-Einstein equation to calculate the concentrations and size distribution of the nanoparticles.
Treatment of monocyte cell line with GW4869
THP-1 cells were treated with GW4869 (Sigma) as previously described [19]. To inhibit EV secretion, 1.2 × 106 THP-1 cells were plated in 100 mm petri dishes and pre-treated with 10 μM GW4869 for 2 hrs prior to treatment with 15 ng/ml LPS. Treated culture media containing EV-free FBS was collected and processed for EV isolation as previously mentioned. GW4869 was dissolved in DMSO (Sigma) into a 5 mM stock solution with a final DMSO concentration of 0.005%. THP-1 cells were treated with 0.005% DMSO with or without LPS for 24 hrs to negate the potential toxic effects of DMSO.
Cytokine ELISA
Detection of a set of twelve inflammatory cytokines (IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, TNF-α and IFN-γ) was done using multi-analyte ELISArray kit (Qiagen) according to the manufacturer's protocol.
RNA isolation, semi-quantitative PCR and Quantitative Real-Time (qRT) PCR
RNA isolation and qRT-PCR were done as described previously [22]. Semi-quantitative PCR was performed on the thermocycler (Biometra) using Taq polymerase (Invitrogen) according to the manufacturer's protocol. Primer sequences are provided below:
human IL-6(F)5′-TGACCCAACCACAAATGC-3′; human IL-6(R) 5′-CGAGCTCTGAAACAAAGGAT-3′; human TNF-α(F) 5′-CAGAGGGAAGAGTTCCCCAG-3′;human TNF-α(R) 5′-CCTTGGTCTGGAGACG-3′; human IL-8(F) 5′-CACCGGAAGGAACCATCTCACT-3′; human IL-8(R)5′-TCAGCCCTCTTCAAAAACTTCTCC-3′;human SOCS3(F) 5′-CAAGGACGGAGACTTCGATT-3′; human SOCS3(R) 5′-GACTGGGTCTTGACGCTGA-3′; human Ribosomal s9(F) 5′-AAGGCCGCCCGGGAACTGCTGAC-3′; human Ribosomal s9(R) 5′-ACCACCTGCTTGCGGACCCTGATA-3′; human APE1(F) 5′-TGTGTGGAGACCTCAATGTG-3′;human APE1(R) 5′-GTAGGCATAGGGTGTGTTGG-3′; mouse IL-6(F) 5′- CCACTTCACAAGTCGGAGGCTTA-3′; mouse IL-6(R) 5′-GCAAGTGCATCATCGTTGTTCATAC-3′; mouse TNF-α(F) 5′-CCCTCACACTCAGATCATCTTCT-3′; mouse TNF-α(R) 5′-GCTACGACGTGGGCTACAG-3′; mouse β-actin(F) 5′-AGGTGTGCACCTTTTATTGGTCTCAA-3′; mouse β-actin(R) 5′-TGTATGAAGGTTTGGTCTCCCT-3′
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was done as described previously [23] with 5 μg of Ab specific for phospho (Ser536) p65 or 5 μg of IgG control (Santa Cruz). PCR amplification of immunoprecipitated chromatin was done using primers listed below: human IL-6 promoter region(F) 5′-TAGAGCTTCTCTTTCGTTCCCGGT-3′; human IL-6 promoter region(R) 5′-TGTGTCTTGCGATGCTAAAGGACG-3′
Flow cytometry assay
To examine the cell-surface adherence of recombinant APE1 (rAPE1), cells were incubated with rAPE1 (1 μg/ml) for 20 mins at 37 °C. After washing the excess protein, cells were incubated with FITC conjugated anti-APE1 Ab (Novus Biologicals) for 30 mins at 4 °C. Cells were then subjected to fluorescence activated cell sorter analysis (FACS; BD LSR II).
Immunofluorescence
For immunofluorescence, THP-1cells, grown on coverslips, were fixed with 4% Formaldehyde (Sigma) and permeabilized with 0.2% Triton X-100 (Sigma) [24]. After blocking with 1% goat serum (Invitrogen), cells were incubated with mouse monoclonal APE1 Ab (Thermo Fisher Scientific) and rabbit Rab5b followed by anti-mouse Ab conjugated with alexa fluor 594 (Life Technologies) and FITC-conjugated rabbit IgG. Nuclei were stained with 4, 6′-diamidine-2-phenylindole (DAPI, Vector Laboratories). For detection of extracellular APE1, cells were initially treated with 1 μg/ml rAPE1 in culture medium for 1 hr at 37 °C and fixed with 4% Formaldehyde. Following this, cells were incubated with FITC-conjugated anti-APE1 Ab (Thermo Scientific). Cell membranes were stained with wheat germ agglutinin-alexa fluor 594 conjugate (Thermo Fisher Scientific, W11262) and nuclei were stained with DAPI. Slides were observed under a fluorescence microscope (Zeiss LSM 510).
Isolation of cytoplasmic, nuclear and chromatin fractions
Cells were lysed in cytosol extraction buffer (Tris-HCl pH8 10mM, Sucrose 0.34mM, CaCl2 3mM, MgCl2 2mM, EDTA 0.1mM, DTT 1mM, Nonidet P-40 0.1%, Protease inhibitor cocktail). After centrifugation, the supernatant was collected as cytoplasmic fraction. The pellet was then dissolved in nuclear extraction buffer (HEPES pH 7.9 20mM, EDTA 3mM, Glycerol 10%, Potassium acetate 10mM, Magnesium chloride 1.5mM, DTT 1mM, Nonidet P-40 0.5%, Protease inhibitor cocktail) and collected as nuclear fraction.
Cell viability assay
Cell viability was assessed using trypan blue reagent with Countess automated cell counter (Invitrogen) according to the manufacturer's protocol.
Immunopurification of FLAG tagged proteins
HEK293 cells, cultured in Dulbecco's Modified Eagle's Medium (Gibco) supplemented with 10% fetal calf serum (Sigma) and 1% Penicillin-streptomycin solution (Gibco), were transfected with empty pFLAG-CMV-5.1 vector or WT-APE1-pFLAG-CMV-5.1 plasmid [22] using Lipofectamine 2000 (Invitrogen) according to manufacturer's protocol. Immunoprecipitation of FLAG-tagged proteins was done using Anti-FLAG M2 affinity gel (Sigma) followed by elution with FLAG peptide (Sigma) according to the manufacturer's protocol.
Results
APE1 is secreted by monocytes upon inflammatory challenges
The finding by Park et al., showing the presence of plasma APE1 upon lipopolysaccharide (LPS)-induced endotoxemia in rats, provided the evidence of APE1 secretion upon a typical inflammatory response in an in vivo system [7]. To determine the cellular source of this plasma APE1, we stimulated peripheral blood mononuclear cells, (PBMCs) isolated from blood samples of normal donors, with LPS. Immunoblot analyses revealed the release of APE1 in the culture media upon LPS stimulation in a dose dependent manner (Fig. 1A). Following this, we isolated the monocytes, B-, and T-lymphocytes from the PBMCs, and stimulated them with LPS in the same manner. The results showed a significant induction (>2.5-fold) of APE1 secretion from the monocytes (Fig. 1B). Although a moderate induction (∼1.5-fold) of APE1 secretion was also observed in the B cells, the T cells remained unaffected (Fig. 1B). Similarly, LPS treatment also induced APE1 secretion from the human monocyte cell line THP-1 as well as murine macrophage cell line RAW264.7 (Fig. 1C and 1D). Intact cell viability, as judged by trypan blue exclusion assays (Fig. 1E), and the absence of GAPDH, tubulin or HSC70 release (Fig. 1A-C), indicated that the LPS-induced APE1 release was not due to cell death. Moreover, the levels of a well-known secretory protein TSG101 [18] remains unchanged upon LPS stimulation (Fig.1C). Furthermore, the APE1 mRNA levels were unaffected by LPS, indicating that the enhanced APE1 release is unlikely to be due to increased transcription upon LPS treatment (Fig. 1F). Since LPS elicits its effects mainly through the production of reactive oxygen species, we examined whether APE1 secretion occurs in monocytes in response to an exogenous oxidizing agent, such as glucose oxide. The result showed a considerable increase of APE1 secretion from human monocyte cell line THP-1 upon exposure to glucose oxidase (Fig. 1G). Additionally, stimulation of THP-1 and RAW264.7 cells with TNF-α, a pro-inflammatory cytokine, also induced the APE1 release (Fig. 1H and 1I). Thus, these data demonstrate that APE1 is a secretory protein, and is effectively released from monocytes upon inflammatory challenges. We also examined the secretion of APE1 from other types of cells such as non-tumorigenic fibroblast or epithelial cells as well as epithelial-derived cancer cell lines. We found a significant level of secretion of APE1 from multiple types of cancer cell lines (supplementary Fig. S1).
Fig. 1. APE1 is secreted from monocytes upon inflammatory challenges.

(A) APE1 is secreted from peripheral blood mononuclear cells (PBMCs) isolated from normal donor upon dose dependent LPS treatment. 106 cells were incubated in Opti-MEM medium for 3 hrs with LPS as indicated. Whole cell lysates and media precipitates were prepared followed by Western blot (WB) with antibodies as indicated. (B) APE1 is primarily secreted from monocytes upon LPS stimulation. 106 cells from monocyte, B-cell or T-cell enriched pool were incubated in Opti-MEM medium for 3 hrs with LPS as indicated. WB was done as described in (A). Densitometric values of band intensity were plotted and normalized to no LPS treated condition for each experiment. The values represent three independent experiments (average ± SD). ** indicates p value <0.005. n.s. indicates non-significant p-value. (C & D) APE1 is secreted from THP-1 cells (C) and RAW264.7 cells (D) upon LPS treatment. 106 cells were incubated in Opti-MEM medium with 0 or 100 ng/ml of LPS for 3 hrs as indicated. Whole cell lysates and media precipitates were prepared followed by WB with antibodies as indicated. (E) LPS does not affect THP-1 cell viability. 106 cells were incubated in Opti-MEM medium with 0, 50, 100 or 200 ng/ml of LPS for 3 hrs. Cell viability was measured by trypan blue exclusion assay. Numbers of viable cells were summarized. The values represent three independent experiments (average ± SD). (F) LPS treatment does not stimulate APE1 expression. THP-1 cells were incubated with 0, 50, 100 or 200 ng/ml of LPS for 3 hrs. Total RNA was isolated and reverse transcribed. cDNAs were subjected to qRT-PCR using primers for APE1 or ribosomal s9 (ribo s9). Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). n.s. indicates non-significant p-value. (G) APE1 is secreted from THP-1 cells upon glucose oxidase treatment. 106 cells were incubated in Opti-MEM medium for 3 hrs with glucose oxidase as indicated. WB was done as described in (C). (H& I) APE1 is secreted from THP-1 (H) and RAW264.7 (I) cells upon TNF-α treatment. 106 cells were incubated in Opti-MEM medium with 0, or 10 ng/ml of TNF-α for 3 hrs. WB was done as described in (C).
Extracellular APE1 induces production and secretion of the pro-inflammatory cytokine IL-6
A few non-classically secreted proteins such as HMGB-1 and YB-1 induce inflammatory responses by the immune cells [25, 26]. In this context, we studied the inflammatory response of monocytic cells upon treatment with extracellular recombinant APE1 (rAPE1). Initially, we challenged THP-1 cells with rAPE1, and examined the levels of a set of pro-inflammatory cytokines in the culture medium. The cytokine array analysis with a set of antibodies against twelve pro-inflammatory cytokines revealed a significant induction of IL-6 secretion from the cells upon rAPE1 treatment (Fig. 2A, left panel). However, unlike LPS, rAPE1 did not induce the secretion of other cytokines like IL-8 and TNF-α (Fig. 2A, middle and right panel). This led us to examine the effect of rAPE1 on the endogenous IL-6 level. Real-time (RT)-PCR analyses revealed the rAPE1-mediated induction of IL-6 mRNA in PBMCs from four normal volunteers (donor 1 to 4) (Fig. 2B). Indeed, rAPE1-treated THP-1 and RAW264.7 cells also showed increased IL-6 mRNA expression in a dose, as well as time-dependent manner (Fig. 2C-F). The specificity of rAPE1 for IL-6 induction was further validated by measuring the levels of two other cytokines, TNF-α and IL-8. RT-PCR analyses showed that rAPE1 had no influence on the expression profiles of these two cytokines (Supplementary Fig. S2). To eliminate the possibility of LPS contaminants from bacterial source, we performed several control experiments. As illustrated by the figures, both the immuno-purified (IP) APE1 from mammalian cells (Fig. 2G), and another source of rAPE1 (recombinant GST-tagged APE1) similarly induced the IL-6 expression (Fig 2H). The N-terminal 30 amino acids deleted APE1 mutant also induced IL-6 expression albeit to a much lesser extent, suggesting that the N-terminal domain of secreted APE1 plays a role for inducing IL-6-mediated inflammatory response. However, recombinant GST (rGST), or recombinant 8-Oxoguanine DNA Glycosylase (rOGG1, another BER protein), or purified bovine serum albumin (BSA), could not produce the similar effect on IL-6 induction, eliminating the possibility of IL-6 induction by any contaminating LPS or other proteins (Fig. 2H). Importantly, the rAPE1-driven IL-6 expression was gradually suppressed by pre-incubation of rAPE1 with increasing amount of anti-APE1 antibody (Ab) (Fig. 2I). To further validate our results, we examined whether conditioned media of cells expressing APE1 can induce IL-6 expression in THP-1. THP-1 cells were grown with conditioned media collected from colon cancer cells HCT116 expressing WT APE1 and isogenic cells stably expressing APE1-shRNA. As LPS itself is a potent IL-6 inducer, we cultured HCT116 cells under hypoxic condition which was shown to induce vesicles secretion [27, 28]. Total RNA was isolated from THP-1 cells and IL-6 mRNA expression was measured by real-time RT-PCR. We found that conditioned medium of APE1 expressing cells, but not APE1 downregulated cells, significantly stimulated IL-6 mRNA levels in THP-1 cells (Fig. 2J).
Fig. 2. Extracellular APE1 induces IL-6.
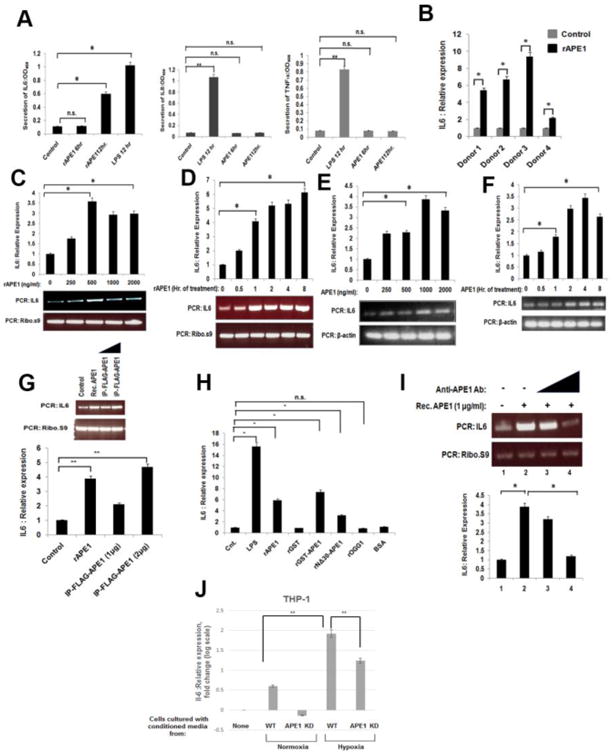
(A) Extracellular APE1 specifically induces IL-6 secretion. THP-1 cells were treated with LPS (100 ng/ml) for 12 hrs or recombinant APE1 (rAPE1; 500 ng/ml) for 6 or 12 hrs. Culture supernatants were processed for detection of a set of cytokines. Secretion of IL-6, IL-8 and TNF-αwas measured at OD450. The values represent three independent experiments (average ±SD). * indicates p value <0.05. n.s. indicates non-significant p-value. (B) Extracellular APE1 induces IL-6 expression from PBMCs isolated from normal donors (N=4). 106 cells were incubated with 0 or 1 μg/ml rAPE1 for 3 hrs. Total RNA was isolated and reverse transcribed. cDNAs were subjected to quantitative Real-Time (qRT) PCR using primers for IL-6 or ribosomal s9 (ribo s9). Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). * indicates p value <0.05. (C -F) Extracellular APE1 induces IL-6 expression from THP-1 and RAW264.7 cells in a dose and time dependent manner. Cells were treated with rAPE1 for 3 hrs as indicated (C &E) or with 1 μg/ml of rAPE1 for times as indicated (D&F). q-RT PCR, normalization and plotting were done as described in (B). The values represent three independent experiments (average ± SD). Representative semi quantitative PCR gel picture was shown. * indicates p value <0.05. (G) Different sources of extracellular APE1 induce IL-6 expression. THP-1 cells were treated with rAPE1 (1 μg/ml) or immunopurified FLAG-tagged APE1 (IP-FLAG-APE1) (1 or 2 μg/ml) for 3 hr. q-RT PCR, normalization and plotting were done as described in (B). The values represent three independent experiments (average ± SD). Representative PCR gel picture was shown. ** indicates p value <0.005. (H) IL-6 induction is specific to extracellular APE1. THP-1 cells were treated with 1 μg/ml of proteins as indicated. q-RT PCR, normalization and plotting were done as described in (B). The values represent three independent experiments (average ± SD). * indicates p value <0.05. n.s. indicates non-significant p-value. (I) Anti-APE1 antibody neutralizes the rAPE1-mediated induction of IL-6 expression. THP-1 cells were treated with 0 or 0.5 ug/ml or 1 μg/ml of rAPE1 in absence or presence of increasing concentrations (2 ug/ml and 4 ug/ml) of anti-APE1 antibody. Total RNA was isolated and reverse transcribed. cDNAs were subjected to semiquantitative PCR using primers for IL-6 or ribo s9. Representative PCR gel picture was shown. * indicates p value <0.005. (J) HCT116 expressing WT APE1 and isogenic cells stably expressing APE1-shRNA were grown in normoxic and hypoxic (1% O2) condition. Conditioned media of these cells were used to grow THP-1 cells. Total RNA was isolated and IL-6 mRNA level was measured by q-RT PCR in THP-1 cells. ** indicates p value <0.005.
Extracellular APE1 activates NF-κB to induce IL-6 expression
To test whether extracellular APE1 can associate with the THP-1 or RAW264.7 cell surface, we incubated the cells with rAPE1. Following incubation, the excess rAPE1 was washed off and the cells were treated with FITC-tagged anti-APE1 Ab. Flow cytometry analyses revealed the presence of APE1 on the surfaces of these cells (Fig. 3A and 3B). Additionally, microscopic analyses also revealed the co-localization of rAPE1 on primary monocyte cell membranes (Fig. 3C), indicating a possible downstream signal cascade initiated via cell surface-binding of extracellular APE1.
Fig. 3. Extracellular APE1 associates with monocyte cell surfaces and activates NF-kB.
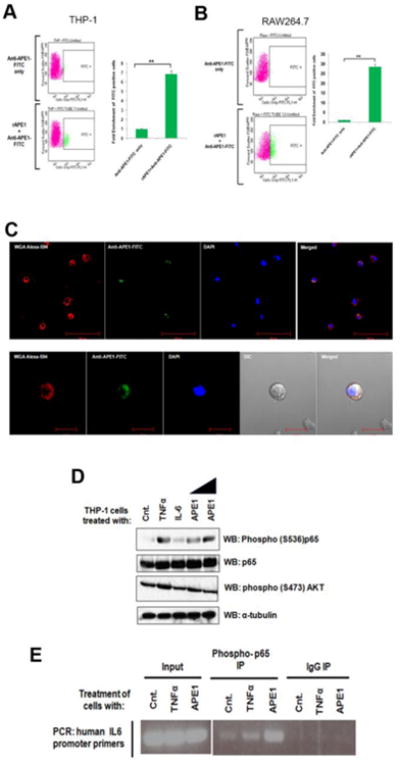
(A & B) Extracellular APE1 associates with THP-1 and RAW264.7 cell surface. Cells were treated with 1 μg/ml of rAPE1. Treated cells were incubated with anti-APE1-FITC Ab. FITC positive cells were sorted and calculated by FACS analysis. ** indicates p value <0.005. (C) Extracellular APE1 colocalizes with primary monocyte cell membrane. Primary monocytes were treated with 1 μg/ml of rAPE1 followed by immunostaining with anti-APE1-FITC Ab. Cell membrane was stained with wheat germ agglutinin-alexa fluor 594 conjugate and nuclei were stained with DAPI. Cells were visualized under a fluorescence microscope and representative images are shown. (D) Extracellular APE1 induces phosphorylation of NF-κB subunit p65. THP-1 cells were treated with TNF-α for 30 min (20 ng/ml), IL-6 for 30 min (20 ng/ml) or rAPE1 for 60 min (0.5 or 1 μg/ml). Whole cell lysates were prepared followed by WB with antibodies as indicated. (E) Extracellular APE1 induces recruitment of NF-κB (phospho-p65) on IL6 promoter. THP-1 cells were treated with TNF-α (20 ng/ml) or rAPE1 (1 μg/ml). ChIP assay was done using antibodies as indicated. Precipitated DNA was PCR amplified using primers encompassing IL6 promoter region.
The NF-κB and AKT signaling pathways are primarily responsible for the induction of IL-6 transcription as inflammatory response to agents like TNF-α [29]. Therefore, we investigated the effect of rAPE1 on the signal transduction mediated by NF-κB or AKT. Initially, immunoblot analyses revealed that, similar to TNF-α, addition of rAPE1 in the THP-1 culture medium induces phosphorylation of the NF-κB subunit p65 (Fig. 3D). Unaltered total p65 levels confirmed the specificity of this phosphorylation. However, no change in the AKT phosphorylation eliminated its role in the rAPE1-mediated IL-6 trans-regulation. Next, we also examined the occupancy of activated NF-κB on the endogenous IL-6 promoter. ChIP analyses revealed an increased occupancy of phospho-p65 on the IL-6 promoter upon stimulation with rAPE1 (Fig. 3E). Collectively, these data suggest that extracellular APE1 can associate with the monocyte cell surface, and initiate a distinct signaling cascade to activate NF-κB, resulting in IL-6 induction.
Extracellular APE1 influences IL-6 activity in both autocrine and paracrine fashions
The inflammatory response is driven by the release of inflammation mediators and a variety of cytokines, including IL-6. IL-6, in turn, serves as a ligand for the activation of the GP130-STAT3 signaling pathway [30]. The suppressor of cytokine signaling (SOCS) protein family, a family of intracellular inhibitors of cytokine signaling, is a target of the IL-6-GP130-STAT3 signaling pathway [31]. To test the secretory APE1's mode of action, we examined the expression of SOCS3. Initially, we treated THP-1 cells with IL-6 or rAPE1. Similar to the IL-6-mediated effect, a significant increase in the SOCS3 mRNA was observed in the rAPE1-treated cells (Fig. 4A). This data indicates that IL-6 is secreted from the THP-1 cells upon rAPE1 treatment, and induces the SOCS3 expression in autocrine manner. Next, we overlaid rAPE1-pretreated THP-1 cells with skin fibroblast BJ cells. Intriguingly, the co-culture resulted in a marked increase in SOCS3 expression from the BJ cells (Fig. 4B). Similarly, SOCS3 expression was also increased in THP-1 cells co-cultured with rAPE1-treated BJ cells (Fig. 4C), suggesting a paracrine mode of IL-6 function as well. Together, these data clearly demonstrate that extracellular APE1 stimulates IL-6 secretion, and this pool of IL-6 functions both in autocrine and paracrine manners.
Fig. 4. Extracellular APE1 influences IL-6-dependent target gene expression in autocrine and paracrine fashion.
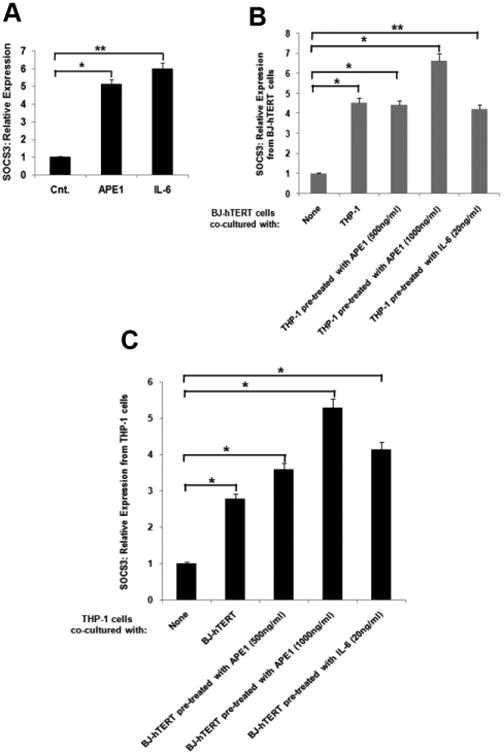
(A) Extracellular APE1 induces IL-6 target gene SOCS3 in an autocrine manner. THP-1 cells were treated with IL-6 (20 ng/ml) or rAPE1 (1 μg/ml). Total RNA was isolated and reverse transcribed. cDNAs were subjected to qRT-PCR using primers for SOCS3 or ribosomal s9 (ribo s9). Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). ** indicates p value <0.005. * indicates p value <0.05. (B) Extracellular APE1 induces IL-6 target gene SOCS3 in a paracrine manner. THP-1 cells were pre-treated with IL-6 (20 ng/ml) or rAPE1 (0.5 or 1 μg/ml) and co-cultured with hTERT immortalized BJ cells (BJ-hTERT) for 16 hrs. Total RNA was isolated from BJ cells and reverse transcribed. q-RT PCR, normalization and plotting were done as described in (A). The values represent three independent experiments (average ± SD). ** indicates p value <0.005. * indicates p value <0.05. (C) Extracellular APE1 induces IL-6 target gene SOCS3 in a paracrine manner from THP-1 cells. BJ-hTERT cells were pre-treated with IL-6 (20 ng/ml) or rAPE1 (0.5 or 1 μg/ml) and co-cultured with THP-1 for 16 hrs. Total RNA was isolated from THP-1 cells and reverse transcribed. q-RT PCR, normalization and plotting were done as described in (A). The values represent three independent experiments (average ± SD). * indicates p value <0.05.
Secretory APE1 precedes cytoplasmic accumulation and operates a positive feedforward loop with IL-6
The secretion of any nuclear protein requires nucleocytoplasmic translocation prior to exocytosis [12]. To investigate the cytoplasmic localization pattern of APE1 upon induction of its secretion, we treated THP-1 cells with LPS and isolated their cytoplasmic and chromatin fractions. The data revealed a dose-dependent cytoplasmic accumulation of APE1 upon LPS treatment with concomitant decrease in chromatin APE1 levels (Fig. 5A). While we observed treatment of LPS induced secretion of APE1 into media, we could not observe corresponding decrease in APE1 level in whole cell extracts (Fig. 5A). Additionally, confocal microscopic data showed that while APE1 is predominantly present in the nuclei of the untreated cells (Fig. 5B, left panel), LPS stimulates the translocation of APE1 from the nucleus to the cytoplasm (Fig. 5B, right panel). Interestingly, earlier studies have shown that treatment with IL-6 induces the cytoplasmic localization of APE1 [32]. This raises the possibility that secretory APE1 and IL-6 work in a positive feedforward loop. Indeed, we too observed that the addition of IL-6 in the culture medium elevates the cytoplasmic pool of APE1, with decrease in the nuclear pool of APE1 in THP-1 cells (Fig. 5C). Following this, treatment of both THP-1 and RAW264.7 cells with IL-6 induced APE1 secretion (Fig. 5D and 5E). Therefore, these results suggest that secretory APE1 stimulates IL-6 production, and subsequent secretion of IL-6 positively influences APE1 secretion. Importantly, these observations indicate an operative positive feedforward loop in this secretion phenomenon.
Fig. 5. Secretion of APE1 and IL6 works in a positive feed forward loop.
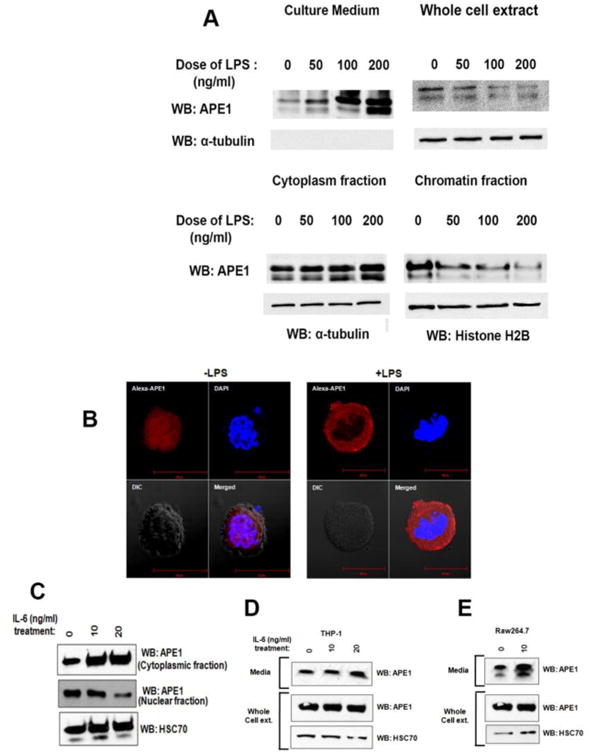
(A) LPS treatment induces cytoplasmic accumulation of APE1. THP-1 cells were treated with LPS as indicated. APE1 level in the medium was measured. Whole cell extract, cytoplasmic and chromatin fractions were isolated followed by WB with antibodies as indicated. (B) LPS treatment induces nucleo-cytoplasmic transport of APE1. THP-1 cells were treated with 0 or 100 ng/ml LPS. Immunostaining was done using Ab against APE1, and nuclei were stained with DAPI. Cells were visualized under a fluorescence microscope and representative images are shown. (C) IL-6 treatment induces nuclear to cytoplasmic accumulation of APE1. THP-1 cells were treated with IL-6 as indicated. Cytoplasmic and nuclear fractions were isolated followed by WB with antibodies as indicated. (D) IL-6 treatment induces APE1 secretion from THP-1 cells. 106 cells were treated with IL-6 as indicated. Whole cell lysates and media precipitates were prepared followed by WB with antibodies as indicated. (E) IL-6 treatment induces APE1 secretion from RAW264.7 cells. 106 cells were treated with IL-6 as indicated. Whole cell lysates and media precipitates were prepared followed by WB with antibodies as indicated.
APE1 is secreted through vesicle formation via endosomal sorting complex required for transport (ESCRT)-dependent pathway
To understand the mechanism by which APE1 is secreted from THP-1 cells, we adopted several biochemical and biophysical approaches. First, pre-incubation of THP-1 cells with Brefeldin A, an agent that interferes with the classical ER-Golgi apparatus-mediated secretion, was found not to reduce the release of APE1 (Fig. 6A), indicating an alternative pathway for APE1 secretion. Moreover, instead of reducing, Brefeldin A increased the APE1 secretion (Fig. 2A). This has been reported for other proteins that follow alternative modes of secretion [12, 33]. Cells release protein, lipids and RNA into the extracellular environment through multiple types of membrane vesicles of endosomal and plasma membrane origin, being known as Extracellular Vesicles (EV) [34]. EV found to be released by B-lymphocytes, monocytes and dendritic cells [34, 35] [20]. We examined whether APE1 is secreted form THP-1 cells through vesicle formation. We isolated EV from conditioned culture supernatants of THP-1 cells treated with or without LPS by high speed ultracentrifugation and filtration methods as described in methods. Electron microscopy revealed the presence of vesicles in the isolated fraction (Fig. 6B). Nanoparticle tracking analysis (NTA) allows determination of the size distribution of isolated extracellular vesicles based on the Brownian motion of vesicles in suspension [21]. Complementary to electron microscopy, NTA data further confirmed the presence of secreted vesicles from both untreated or LPS-treated THP-1 cells (Fig. 6C). The particle size distribution for THP-1 derived EV was within 30-200 nm; [34, 35]. We also confirmed the presence of CD63, a tetraspanins membrane protein (Fig. S3) and TSG101 (Tumor Susceptibility Gene 101), normally present in isolated vesicles [36-38] [39], biochemically by Western blot analysis (Fig. 6E). After biophysical and biochemical characterization of the purified EV, we examined the presence of APE1 in isolated EV by immunoblotting. Western blot analyses showed significant increase of the APE1 levels in the secreted EV but not in whole cell extract of LPS-treated THP-1 cells (Fig. 6D & 6E). Furthermore, treatment with GW4869, an inhibitor of EV secretion, also reduced the level of both TSG101 and APE1 in isolated EV from LPS-treated THP-1 cells but not in whole cell extracts (Fig. 6E). Hypoxic condition was shown to induce vesicles secretion. We found that hypoxia induced APE1 secretion through EV in HCT116 cells expressing WT APE1 (Fig. 2F, right panel). Moreover, we also confirmed that downregulation of APE1 in HCT116shRNA-APE1 cells significantly reduced the amount of APE1 in EV secreted into the media under both normoxia and hypoxia conditions (Fig. 2F, right panel). Together, these data suggest that APE1 is secreted through EV formation and LPS treatment significantly enhanced its secretion. [34].
Fig. 6. . APE1 is secreted by a non-classical pathway through vesicle formation.
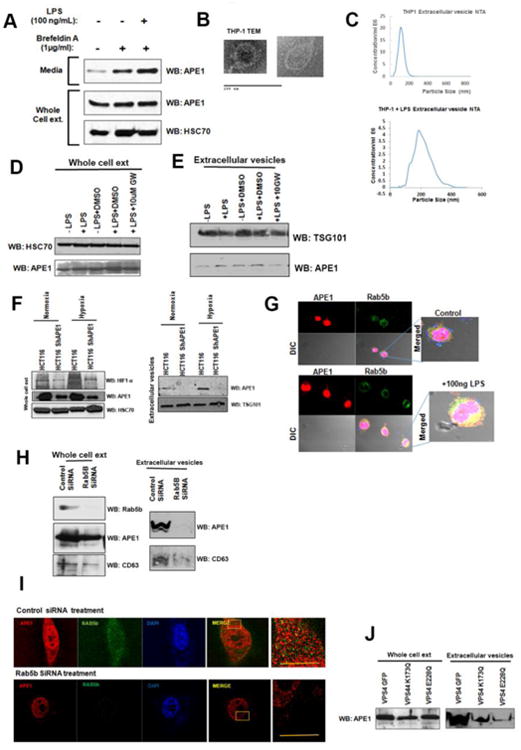
(A) Secretion of APE1 is not inhibited by Brefeldin A. THP-1 cells were pre-incubated in Opti-MEM medium with 1 μg/ml Brefeldin A for 1 hr followed by 100 ng/ml LPS treatment for 3 hrs. Whole cell lysates and media precipitates were prepared followed by WB with antibodies as indicated. (B) Electron microscope images of isolated extracellular vesicles. Vesicles from LPS treated culture media were enriched by high speed sequential centrifugation steps followed by filtration and plated in slides for imaging by electron microscopy. (C) Determination of the size of the isolated vesicles. Vesicles isolated from supernatant of THP-1 cells were analyzed using a NanoSight equation to calculate the size distribution of the particles. Representative figure is shown. (D & E) LPS induces APE1 secretion via EV from THP-1 cells. THP-1 cells grown in special serum (extracellular vesicle free) containing medium were treated with LPS (15 ng/ml) for 12 hrs and cell culture supernatants were collected. Vesicles were enriched by high speed sequential centrifugation steps followed by filtration as described in methods. The resultant pellet was dissolved in Laemmli buffer and analyzed for the presence of APE1 by Western blot analysis. (D) Western blot analysis of whole cell extract demonstrating no change of APE1 level with drug treatment. (E)LPS-stimulated APE1 level in EV and this was significantly inhibited by addition of 10 μM GW4869 to THP-1 cultured cells vs. LPS or DMSO treated cells. TSG101 was used as an extracellular vesicle marker. (F) Hypoxia induces APE1 level in EV from HCT116 cells and APE1 downregulation reduces its level in EV. HCT116 and HCT116-APE1shRNA cells were cultured under normoxic or hypoxic (1% O2) conditions. Whole cell extracts from cell pellet (left panel) and EV from culture supernatant (right panel) were isolated and immunoblotted with anti-APE1, anti-HIF1-α, anti-HSC70, anti-TSG101. (G) Colocalization of APE1 and Rab5b in THP-1 cells upon LPS stimulation. THP-1 cells grown on cover slip were treated with 100 ng/ml LPS for 2 hrs and then immuno-stained with mouse APE1 Ab and rabbit Rab5b Abs. Cells werevisualized under a confocal microscope and representative images and their overlap are shown.(H) Rab5b knockdown affects APE1 secretion. (Left panel), RAW264.7 cells were transfectedwith control and Rab5b-SiRNA to knockdown Rab5b. Rab5b and APE1 levels were examinedby Western blot analysis. (Right panel) EV isolated from media demonstrates reduced APE1level upon Rab5b knockdown. (I) Rab5b knockdown affects co-localization of APE1 with Rab5b.Super resolution Structured Illumination microscopy demonstrates significant reduction in APE1co-localization with Rab5b upon knockdown. (J) ESCRT-associated VPS4 function is requiredfor APE1 secretion via exosome. RAW264.7 cells were infected with retro virus expressing WTVPS4 or dominant negative mutants of VPS4 (K173Q and E228Q). After 48 hours of infection,cells were treated with LPS (100 ng/ml for 2 hours) and EVs were isolated from the culturesupernatant. EV extracts were immunoblotted with anti-APE1 antibody.
Cell membrane associated Ras-related protein, Rab5b, is known to be involved in endosomal sorting and EV biogenesis [40, 41]. Our confocal analysis revealed an increased co-localization of Rab5b with APE1 after LPS-treatment (Fig. 6G). To test the direct involvement of Rab5b in APE1 secretion via EV, we downregulated Rab5b levels in RAW264.7 cells using Rab5b-specific siRNA (Fig. 6H). We observed that silencing of Rab5b significantly reduced the EV secretion as shown by reduced levels of CD63 as well as APE1 in isolated EV (Fig. 6H). A decrease in APE1 level in whole cell extract was also observed in Rab5b downregulated cells. Furthermore, we also utilized super-resolution (100 nm) Structured Illumination Microscopy (SIM) and found that LPS-stimulation enhances the co-localization of Rab5b and APE1 in control siRNA transfected cells. Downregulation of Rab5b levels by siRNA treatment significantly reduced the co-localization of APE1 with Rab5b (Fig. 6I). Thus both immunofluorescence and biochemical analyses suggest the role of Rab5b in APE1 secretion through EV.
ESCRT complexes, consisting of an array of membrane associating protein complexes, coordinate the process of multi vesicular bodies (MVB) biogenesis and transport [42-44]. Knocking down ESCRT components inhibits the EV biogenesis or production, suggesting that EV derived from MVBs are generated by the ESCRT machinery [45, 46]. The identification of some ESCRT proteins, such as TSG101 and VPS4 (Vacuolar protein sorting 4), in EV further supports the role of ESCRT complex in EV biogenesis [39, 47]. We examined the role of ESCRT complex in APE1 secretion via EV by targeting ESCRT associated protein VPS4. VPS4 is an ATPase associated with ESCRT and involved in disassembly and return of ESCRT complexes from MVB membranes to the cytoplasm, ensuring continuity of the process [47, 48]. To address the role of VPS4 in APE1 secretion, RAW264.7 cells were infected with retrovirus expressing dominant negative (dn) VPS4K173Q-GFP or VPS4E228Q mutants [18]. Western blot analysis showed that ectopic dnVPS4 mutants reduced APE1 levels in EV isolated from the medium (Fig. 6J). These data confirmed the role of ESCRT complex in APE1 secretion throughEV. Together, our data indicates that APE1 secretion occurs through EV via an ESCRT-dependent pathway.
Discussion
Over the last 20 years, growing evidences clearly indicate that APE1 has multiple nuclear and cytoplasmic functions [2, 3]. While recent evidences indicate that APE1 can be secreted into the plasma [6, 7], the physiological or pathological role of extracellular APE1 has not been documented yet. In this study, for the first time, we show that the oxidative DNA damage repair protein APE1 can be actively released from monocytes upon inflammatory challenges. Moreover, B-lymphocytes also showed the secretion of APE1 upon stimulation with LPS. Interestingly, unlike monocytes and THP-1 cells, in the B- and T-cell studies, full-length APE1 and a truncated APE1 band was observed. In this respect, the presence of two APE1 isoforms supports previous finding of intracellular proteolytic cleavage of the N-terminal domain of APE1 in some cell types and in tumor tissues [49]. Indeed, previous studies showed that the APE1 N-terminal domain (1-33 aa) is cleaved in human pro-myelocytic leukemia cells line HL60 [50]. Moreover, it has also been shown that APE1 is targeted and cleaved at Lys 31 by granzyme A, a highly abundant serine protease found in cytotoxic granules of T-lymphocytes [51].
Although our data show that stimulation of monocytes with LPS significantly induced APE1 secretion, however, we observed a basal secretion of APE1 from unstimulated cells. Of note, in our experimental system, we incubated the cells in reduced serum containing Opti-MEM medium to minimize contaminations from serum proteins. However, several reports showed that secretion of leader-less proteins (for e.g. IL1-β and HIV-tat) are inversely proportional to the amount of the serum in the medium [52, 53]. In our experimental conditions, too, the use of reduced serum in the medium might influence the level of basic secretion of APE1 from unchallenged cells. Thus, we believe that the basal secretion of APE1 could be due to serum deprivation-mediated cellular stress. Nonetheless, we have provided evidences that upon inflammatory challenges primarily monocytes and B cells significantly induced secretion of APE1 and APE1 is secreted through extracellular vesicle formation via endosomal sorting complex required for transport (ESCRT)-dependent pathway. We also established that secretory APE1 acts as a mediator of the cellular response by inducing IL-6 production and secretion. Thus, our results illuminate a new perspective that secretory APE1 has an extracellular role under inflammatory conditions.
Our data show that not only the immune cells but also other cell types such as epithelial derived cancer cells secretes APE1. Thus under certain conditions such as inflammatory response or tumorigenic condition induce secretion of APE1 from cells. Consistent with this idea, elevated levels of serum APE1 and its auto-antibody, have been found to be present in lung cancer patients' [54, 55] The presence of the extracellular APE1, therefore, questions the biological significance of this secretion. Moreover, a recent study has shown that extracellularly secreted APE1/Ref-1 triggers apoptosis in triple-negative breast cancer cells [24]. Previous literatures have documented the secretory behavior and cytokine-like activities of several nuclear proteins [10, 12, 14, 56]. For example, HMGB1 is a nuclear protein involved in the maintenance of the nucleosome structure, DNA repair, replication, and regulation of transcription. However, it is also released from activated monocytes, and damaged or necrotic cells. Secretory HMGB1 can act as a cytokine, initiating various inflammatory responses like stimulation of multiple cytokines, chemo-attraction of several cell types for tissue regeneration and inflammation, induction of vascular adhesion molecules, and bactericidal activities [14, 26]. YB-1, a protein involved in transcription, DNA repair, and RNA metabolism is the latest addition to this group of secretory proteins, mediating local and systemic inflammation [26]. Previous reports have shown a role for the intracellular APE1 in inflammatory responses [57]. For example, nuclear APE1 is involved in regulating the expression of several cytokines, including TNF-α, IL-6 and IL-8 [32]. This study demonstrated that APE1-specific siRNA significantly inhibited DNA binding activity of AP-1 and NF-κB transcription factors in the regulation IL-6/8 secretion in bone marrow stromal cells (BMSCs), These results implicated that Ref-1 function of N-terminal domain of APE1 plays a regulatory role in IL-6/8 production by BMSCs. However, in this study, we discovered that the extracellular APE1 (released from monocytes upon LPS treatment) also induces the production and secretion of IL-6. Moreover, we found that the N-terminal domain of APE1 also plays a role in inducing IL-6 presumably through its Ref-1 functions. Notably, while our study could find a significant effect of extracellular APE1 on the IL-6 regulation, rAPE1 treatment showed no effect on the regulation of a subset of other pro-inflammatory cytokines including TNF-α and IL-8. However, multiple lines of evidence confirmed that the induction of IL-6 is mediated by the extracellular APE1. First, rAPE1-mediated IL-6 stimulation could not be replicated by another BER protein, OGG1. Second, pre-treatment with the APE1 Ab reduced the effect of rAPE1 on the IL-6 production. Third, detailed analyses revealed that rAPE1 could induce the expression of IL-6 by activating the NF-κB-mediated transcription. Fourth, APE1 treatment induced the IL-6 target gene, SOCS3, through the autocrine and paracrine natures of the IL-6 activity. Importantly, we have provided evidence that conditioned medium of APE1 expressing cells but not APE1 downregulated cells has the ability to induce IL-6 mRNA levels in THP-1 cells. Additionally, we have provided evidence for the adherence of rAPE1 on the monocyte cell surfaces. IL-6 is involved in acute-phase responses. Whether the secretory APE1 is an induced outcome of the acute-phase responses, and related to the IL-6 level, are yet to be elucidated. However, the general observation for IL-6 induction in various acute-phase responses [58], together with the recent identification of plasma APE1 in endotoxemia-induced acute-phase response (14) hint at a possible association. On the other hand, APE1, by acting as an intracellular redox factor, has been reported to inhibit inflammatory responses. To note, IL-6 can also act as an anti-inflammatory cytokine, depending on the physiological context [29]. An alternative possibility that extracellular APE1 specifically induces IL-6 production as a mediator of anti-inflammatory response needs further investigation. Further studies would also focus on particular cell surface receptor(s) and downstream signal transduction pathway(s) associated with extracellular APE1 in the regulation of inflammation. Of note, several cell surface receptors (TLR2, TLR4 and receptor for advanced glycation end product; RAGE) for secretory HMGB1 [59] and Notch-3 for secretory YB-1 [60] have been reported to initiate signaling cascade leading to inflammatory regulation. As TLR4, TLR2 and RAGE receptors are also known to be involved in LPS-mediated stimulation of IL-6 production during inflammation, it is likely that secretory APE1 binds to RAGE receptors on monocytes or B-cells to induce IL-6 induction and secretion. Indeed, a recent study have shown that APE1 can be released from tumor cells and can bind to RAGE receptor [24]. Further studies are needed to confirm whether RAGE or novel receptor(s) in monocytes are involved in binding with secretory APE1.
We have provided several lines of evidences that strongly suggest that APE1 is secreted through EV via ESCRT-dependent pathway. EV together with their protein and RNA cargo enable an intriguing form of cell-cell communication in paracrine and endocrine fashions [34, 35]. [20, 34]. [35]. EV including Exosome and micovesicles characteristically carry specific proteins markers of the endosomal pathways such as TSG101, Alix and proteins involved in vesicle formation such as tetraspanins, CD8, CD63 etc. [37, 38, 61]. Presence of APE1 along with EV marker TSG101 and CD63 in purified EV from THP-1 suggested that APE1 can be secreted through EV formation and LPS treatment stimulated its secretion. EV are secreted by cell through endocytosis MVBs followed by the fusion of MVBs with the plasma membrane [34, 35]. ESCRT pathway, consisting of multiple complexes (ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III), is a key mediator of MVB biogenesis [42-44]. Several recent studies have established that knocking down ESCRT components inhibits MVB production or release [45, 46]. The identification of some ESCRT proteins such as Alix, TSG101 and VPS4, in EV by proteomic analyses further supports a role of ESCRT complex in exosome biogenesis and release [39, 61]. AAA-ATPase VPS4 is an essential component for ESCRT-III complex [47, 48]. Binding of VPS4 to ESCRT-III complex and ATP hydrolysis play a key role in membrane abscission, and disassembly and return of ESCRT complexes from MVB membranes to the cytoplasm [47, 48]. Consistent with this, our data showed that ectopic expression of dominant negative K173QVPS4 (defective in ATP hydrolysis, [48] significantly reduced MVB biogenesis which concomitantly reduced APE1 level in EV, confirming the role of ESCRT complex in APE1 secretion through EV. However, we cannot eliminate the possibility that APE1 can be secreted through both EV formation and shedding microvesicles at plasma membrane. Further studies are necessary to confirm this.
Cellular APE1 stimulates the IL-6 expression through redox regulation of NF-κB and AP1 [32]. Furthermore, IL-6 treatment was reported to elevate the cytoplasmic translocation of APE1 [32]. Indeed, in our experimental set up, we observed APE1 accumulation in the cytoplasm, followed by its secretion from monocyte cells upon IL-6 treatment. Based on these observations, we propose a feedforward loop depicting a cycle of APE1 and IL-6 secretion complemented by each other. The physiological relevance of this feedforward cycle will be interesting to investigate in the future. Notably, we found that the secreted IL-6 (upon rAPE1 treatment) acts on cultured monocyte cells in an autocrine manner, inducing the expression of the target gene SOCS3. Moreover, IL-6 showed a paracrine fashion of SOCS3 induction in a rAPE1-treated co-culture of fibroblasts and monocyte cells. Based on our observations, we hypothesize that inflammatory challenges might set the stage for the promotion of co-regulated APE1/IL-6 secretion in physiological responses. EV were found to be released by B lymphocytes and dendritic cells [20]. Furthermore, the secretion of APE1 from different monocyte lineage cells like histiocytes, dendritic cells, Kupffer cells is beyond our experimental observations.
The altered expressions of nuclear proteins and their secretions have been linked with various diseases. For example, the non-classical secretion of HMGB1 has been associated with immunologic disorders like sepsis, pneumonia, and endotoxemia [14, 25]. Simultaneously, targeted therapies against the extracellular HMGB1 showed promising results in disease remedy [14, 25, 59, 62, 63]. DNA repair enzyme OGG1 was found to be involved in inflammation [64]. In this vein, the altered expression as well as the sub-cellular localization of APE1 has long been associated with cancer, neurodegenerative, cardiovascular, and other diseases [65]. Indeed, auto-antibodies against APE1 have been identified in systemic lupus erythematous patients [66]. Recently, the identification of serum APE1 and its auto-antibody, and its differential presence among normal controls and lung cancer patients further indicate APE1 as an autoimmune antigen [54, 55]. Our finding of the secretory APE1 from monocytes upon inflammatory challenges, and its role as a regulator of IL-6 mediated cellular responses marks this protein as a target for therapeutic intervention. Overall, this study implicates that the extracellular APE1 may have an important pathogenic role in many diseases that are linked to inflammation. Further studies will provide avenues for elucidating the molecular mechanisms and targeting the extracellular APE1 in systemic inflammatory and autoimmune diseases.
Supplementary Material
Non-tumorigenic BEAS-2B, HEK293T and MEF and colon cancer HCT116, lung adenocarcinoma A549 and breast cancer MCF7 cells were incubated in Opti-MEM medium for 3 hrs. Whole cell lysates and media precipitates were prepared followed by Western blot (WB)with APE1 antibody.
(A & B) IL-8 expression is not affected by a time and dose dependent treatment of rAPE1 from THP-1 cells. Cells were treated with rAPE1 as indicated. cDNAs were subjected to qRT-PCR using primers for IL-8 or ribo s9. Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). Representative semiquantitative PCR gel picture was shown. n.s. indicates non-significant p-value. (C-F) TNF-α expression is not affected by a dose and time dependent treatment of rAPE1 from THP-1 cells (C&D) or RAW264.7 cells (E&F). cDNAs were subjected to qRT-PCR using primers for TNF-α or ribo s9. Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). Representative semi quantitative PCR gel picture was shown. n.s. indicates non-significant p value.
THP-1 cells grown in special serum (extracellular vesicles free) containing medium were treated with LPS (15 ng/ml) for 12 hrs and cell culture supernatants were collected. Vesicles were enriched by high speed sequential centrifugation steps followed by filtration as described in methods. The resultant pellet was dissolved in Laemmli buffer and analyzed for the presence of APE1, CD63 by Western blot analysis.
Highlights.
DNA damage repair protein APE1 is secreted from monocytes upon inflammatory challenges.
Extracellular APE1 induces IL-6 gene expression.
APE1 is secreted through extracellular vesicles formation.
Acknowledgments
This study was supported by NIH RO1 CA148941 to KKB.
Footnotes
Author contributions: SN designed, performed and analyzed all the experiments, and wrote the paper. AS and HS performed the experiments shown in figure 1. SRC, MK and PB performed some experiments shown in Fig. 2, 5 and 6. SJ conceived and coordinated the study, and analyzed all the experiments. HB provided reagents and analyzed the data. KB conceived and coordinated the study, and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interests: The authors declare no conflict of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li M, Wilson DM., 3rd Human apurinic/apyrimidinic endonuclease 1. Antioxid Redox Signal. 2014;20(4):678–707. doi: 10.1089/ars.2013.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhakat KK, Mantha AK, Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid Redox Signal. 2009;11(3):621–38. doi: 10.1089/ars.2008.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11(3):601–20. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7(3-4):367–84. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 5.Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9(7):717–25. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- 6.Choi S, Lee YR, Park MS, Joo HK, Cho EJ, Kim HS, Kim CS, Park JB, Irani K, Jeon BH. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem Biophys Res Commun. 2013;435(3):403–7. doi: 10.1016/j.bbrc.2013.04.101. [DOI] [PubMed] [Google Scholar]
- 7.Park MS, Lee YR, Choi S, Joo HK, Cho EJ, Kim CS, Park JB, Jo EK, Jeon BH. Identification of plasma APE1/Ref-1 in lipopolysaccharide-induced endotoxemic rats: implication of serological biomarker for an endotoxemia. Biochem Biophys Res Commun. 2013;435(4):621–6. doi: 10.1016/j.bbrc.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Prudovsky I, Tarantini F, Landriscina M, Neivandt D, Soldi R, Kirov A, Small D, Kathir KM, Rajalingam D, Kumar TK. Secretion without Golgi. J Cell Biochem. 2008;103(5):1327–43. doi: 10.1002/jcb.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10(2):148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 10.Maizel A, Tassetto M, Filhol O, Cochet C, Prochiantz A, Joliot A. Engrailed homeoprotein secretion is a regulated process. Development. 2002;129(15):3545–53. doi: 10.1242/dev.129.15.3545. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobilitygroup protein-1 by pituicytes. Surgery. 1999;126(2):389–92. [PubMed] [Google Scholar]
- 12.Frye BC, Halfter S, Djudjaj S, Muehlenberg P, Weber S, Raffetseder U, En-Nia A, Knott H, Baron JM, Dooley S, Bernhagen J, Mertens PR. Y-box protein-1 is actively secreted through a non-classical pathway and acts as an extracellular mitogen. EMBO Rep. 2009;10(7):783–9. doi: 10.1038/embor.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malhotra V. Unconventional protein secretion: an evolving mechanism. EMBO J. 2013;32(12):1660–4. doi: 10.1038/emboj.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Wang H, Tracey KJ. HMG-1 rediscovered as a cytokine. Shock. 2001;15(4):247–53. doi: 10.1097/00024382-200115040-00001. [DOI] [PubMed] [Google Scholar]
- 15.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 16.Roychoudhury S, Nath S, Song H, Hegde ML, Bellot LJ, Mantha AK, Sengupta S, Ray S, Natarajan A, Bhakat KK. Human Apurinic/Apyrimidinic Endonuclease (APE1) Is Acetylated at DNA Damage Sites in Chromatin, and Acetylation Modulates Its DNA Repair Activity. Mol Cell Biol. 2017;37(6) doi: 10.1128/MCB.00401-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantha AK, Oezguen N, Bhakat KK, Izumi T, Braun W, Mitra S. Unusual role of a cysteine residue in substrate binding and activity of human AP-endonuclease 1. J Mol Biol. 2008;379(1):28–37. doi: 10.1016/j.jmb.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu C, Ortega-Cava CF, Winograd P, Stanton MJ, Reddi AL, Dodge I, Arya R, Dimri M, Clubb RJ, Naramura M, Wagner KU, Band V, Band H. Endosomal-sorting complexes required for transport (ESCRT) pathway-dependent endosomal traffic regulates the localization of active Src at focal adhesions. Proc Natl Acad Sci U S A. 2010;107(37):16107–12. doi: 10.1073/pnas.1009471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, Fan GC. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015;1852(11):2362–71. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87(1):146–50. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta S, Mantha AK, Mitra S, Bhakat KK. Human AP endonuclease (APE1/Ref-1) and its acetylation regulate YB-1-p300 recruitment and RNA polymerase II loading in the drug-induced activation of multidrug resistance gene MDR1. Oncogene. 2011;30(4):482–93. doi: 10.1038/onc.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta S, Mitra S, Bhakat KK. Dual regulatory roles of human AP-endonuclease (APE1/Ref-1) in CDKN1A/p21 expression. PLoS One. 2013;8(7):e68467. doi: 10.1371/journal.pone.0068467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YR, Kim KM, Jeon BH, Choi S. Extracellularly secreted APE1/Ref-1 triggers apoptosis in triple-negative breast cancer cells via RAGE binding, which is mediated through acetylation. Oncotarget. 2015;6(27):23383–98. doi: 10.18632/oncotarget.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34(6):1503–12. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- 26.Hanssen L, Alidousty C, Djudjaj S, Frye BC, Rauen T, Boor P, Mertens PR, van Roeyen CR, Tacke F, Heymann F, Tittel AP, Koch A, Floege J, Ostendorf T, Raffetseder U. YB-1 is an early and central mediator of bacterial and sterile inflammation in vivo. J Immunol. 2013;191(5):2604–13. doi: 10.4049/jimmunol.1300416. [DOI] [PubMed] [Google Scholar]
- 27.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288(48):34343–51. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 30.Horvath CM. The Jak-STAT pathway stimulated by interleukin 6. Sci STKE. 2004;2004(260):tr9. doi: 10.1126/stke.2602004tr9. [DOI] [PubMed] [Google Scholar]
- 31.Ray S, Ju X, Sun H, Finnerty CC, Herndon DN, Brasier AR. The IL-6 trans-signaling-STAT3 pathway mediates ECM and cellular proliferation in fibroblasts from hypertrophic scar. J Invest Dermatol. 2013;133(5):1212–20. doi: 10.1038/jid.2012.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie JY, Li MX, Xiang DB, Mou JH, Qing Y, Zeng LL, Yang ZZ, Guan W, Wang D. Elevated expression of APE1/Ref-1 and its regulation on IL-6 and IL-8 in bone marrow stromal cells of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(5):385–93. doi: 10.3816/CLML.2010.n.072. [DOI] [PubMed] [Google Scholar]
- 33.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3(10):995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11–5. doi: 10.1016/j.biocel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi E, Brambilla D, Venturi G, Lozupone F, Santinami M, Huber V, Maio M, Rivoltini L, Fais S. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–72. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 38.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 39.Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1(3):248–58. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 40.Wilson DB, Wilson MP. Identification and subcellular localization of human rab5b, a new member of the ras-related superfamily of GTPases. J Clin Invest. 1992;89(3):996–1005. doi: 10.1172/JCI115683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirota Y, Kuronita T, Fujita H, Tanaka Y. A role for Rab5 activity in the biogenesis of endosomal and lysosomal compartments. Biochem Biophys Res Commun. 2007;364(1):40–7. doi: 10.1016/j.bbrc.2007.09.089. [DOI] [PubMed] [Google Scholar]
- 42.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–9. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16(6):317–26. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106(2):145–55. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 45.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–65. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 46.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109(11):4146–51. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17(11):2982–93. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuchell-Brereton MD, Skalicky JJ, Kieffer C, Karren MA, Ghaffarian S, Sundquist WI. ESCRT-III recognition by VPS4 ATPases. Nature. 2007;449(7163):740–4. doi: 10.1038/nature06172. [DOI] [PubMed] [Google Scholar]
- 49.Bhakat KK, Sengupta S, Adeniyi VF, Roychoudhury S, Nath S, Bellot LJ, Feng D, Mantha AK, Sinha M, Qiu S, Luxon BA. Regulation of limited N-terminal proteolysis of APE1 in tumor via acetylation and its role in cell proliferation. Oncotarget. 2016;7(16):22590–604. doi: 10.18632/oncotarget.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida A, Urasaki Y, Waltham M, Bergman AC, Pourquier P, Rothwell DG, Inuzuka M, Weinstein JN, Ueda T, Appella E, Hickson ID, Pommier Y. Human apurinic/apyrimidinic endonuclease (Ape1) and its N-terminal truncated form (AN34) are involved in DNA fragmentation during apoptosis. J Biol Chem. 2003;278(39):37768–76. doi: 10.1074/jbc.M304914200. [DOI] [PubMed] [Google Scholar]
- 51.Fan Z, Beresford PJ, Zhang D, Xu Z, Novina CD, Yoshida A, Pommier Y, Lieberman J. Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme A. Nat Immunol. 2003;4(2):145–53. doi: 10.1038/ni885. [DOI] [PubMed] [Google Scholar]
- 52.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9(5):1503–10. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11(12):1421–31. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, He L, Dai N, Guan W, Shan J, Yang X, Zhong Z, Qing Y, Jin F, Chen C, Yang Y, Wang H, Baugh L, Tell G, Wilson DM, 3rd, Li M, Wang D. Serum APE1 as a predictive marker for platinum-based chemotherapy of non-small cell lung cancer patients. Oncotarget. 2016;7(47):77482–77494. doi: 10.18632/oncotarget.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai N, Cao XJ, Li MX, Qing Y, Liao L, Lu XF, Zhang SH, Li Z, Yang YX, Wang D. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer. PLoS One. 2013;8(3):e58001. doi: 10.1371/journal.pone.0058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115(9):1755–64. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeon BH, Irani K. APE1/Ref-1: versatility in progress. Antioxid Redox Signal. 2009;11(3):571–4. doi: 10.1089/ars.2008.2223. [DOI] [PubMed] [Google Scholar]
- 58.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo ZS, Liu Z, Bartlett DL, Tang D, Lotze MT. Life after death: targeting high mobility group box 1 in emergent cancer therapies. Am J Cancer Res. 2013;3(1):1–20. [PMC free article] [PubMed] [Google Scholar]
- 60.Brandt S, Raffetseder U, Djudjaj S, Schreiter A, Kadereit B, Michele M, Pabst M, Zhu C, Mertens PR. Cold shock Y-box protein-1 participates in signaling circuits with auto-regulatory activities. Eur J Cell Biol. 2012;91(6-7):464–71. doi: 10.1016/j.ejcb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 62.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141(3):347–57. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members' review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20(16):4337–40. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ba X, Aguilera-Aguirre L, Rashid QT, Bacsi A, Radak Z, Sur S, Hosoki K, Hegde ML, Boldogh I. The role of 8-oxoguanine DNA glycosylase-1 in inflammation. Int J Mol Sci. 2014;15(9):16975–97. doi: 10.3390/ijms150916975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK. APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med. 2014;46:e106. doi: 10.1038/emm.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katsumata Y, Kawaguchi Y, Baba S, Hattori S, Tahara K, Ito K, Iwasaki T, Yamaguchi N, Oyama M, Kozuka-Hata H, Hattori H, Nagata K, Yamanaka H, Hara M. Identification of three new autoantibodies associated with systemic lupus erythematosus using two proteomic approaches. Mol Cell Proteomics. 2011;10(6):M110 005330. doi: 10.1074/mcp.M110.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-tumorigenic BEAS-2B, HEK293T and MEF and colon cancer HCT116, lung adenocarcinoma A549 and breast cancer MCF7 cells were incubated in Opti-MEM medium for 3 hrs. Whole cell lysates and media precipitates were prepared followed by Western blot (WB)with APE1 antibody.
(A & B) IL-8 expression is not affected by a time and dose dependent treatment of rAPE1 from THP-1 cells. Cells were treated with rAPE1 as indicated. cDNAs were subjected to qRT-PCR using primers for IL-8 or ribo s9. Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). Representative semiquantitative PCR gel picture was shown. n.s. indicates non-significant p-value. (C-F) TNF-α expression is not affected by a dose and time dependent treatment of rAPE1 from THP-1 cells (C&D) or RAW264.7 cells (E&F). cDNAs were subjected to qRT-PCR using primers for TNF-α or ribo s9. Relative expression values were normalized to the ribo s9 transcript levels. The values represent three independent experiments (average ± SD). Representative semi quantitative PCR gel picture was shown. n.s. indicates non-significant p value.
THP-1 cells grown in special serum (extracellular vesicles free) containing medium were treated with LPS (15 ng/ml) for 12 hrs and cell culture supernatants were collected. Vesicles were enriched by high speed sequential centrifugation steps followed by filtration as described in methods. The resultant pellet was dissolved in Laemmli buffer and analyzed for the presence of APE1, CD63 by Western blot analysis.


