Abstract
Background
The orthodontics industry has paid great attention to the aesthetics of orthodontic appliances, seeking to make them as invisible as possible. There are several advantages to clear aligner systems, including aesthetics, comfort, chairside time reduction, and the fact that they can be removed for meals and oral hygiene procedures.
Methods
Five patients were each given a series of F22 aligners, each to be worn for 14 days and nights, with the exception of meal and brushing times. Patients were instructed to clean each aligner using a prescribed strategy, and sections of the used aligners were observed under SEM. One grey-scale SEM image was saved per aligner in JPEG format with an 8-bit colour depth, and a total of 45 measurements on the grey scale (“Value” variable) were made. This dataset was analysed statistically via repeated measures ANOVA to determine the effect of each of the nine cleaning strategies in each of the five patients.
Results
A statistically significant difference in the efficacy of the cleaning strategies was detected. Specifically, rinsing with water alone was significantly less efficacious, and a combination of cationic detergent solution and ultrasonication was significantly more efficacious than the other methods (p < 0.05).
Conclusions
Of the nine cleaning strategies examined, only that involving 5 min of ultrasonication at 42 k Hz combined with a 0.3% germicidal cationic detergent was observed to be statistically effective at removing the bacterial biofilm from the surface of F22 aligners.
Background
A recent survey by YouGov estimated that 45% of adults are unsatisfied with their smile and that 20% would like to undergo orthodontic treatment to improve their tooth alignment and appearance (www.bos.org.uk/news/NOWYouGovSurvey). Hence, the orthodontics industry has paid great attention to the aesthetics of orthodontic appliances, seeking to make them as invisible as possible [1]. In this context, in 1997, Zia Chishti and Kelsey Wirth developed a barely noticeable aligner system, which they called Invisalign [2]. This system involves the use of a series of customised aligners made of transparent plastic; if worn for a minimum of 20 h per day and replaced every 2 weeks, this system can achieve dental movements of approximately 0.25–0.33 mm per tooth, or group of teeth, per aligner. Dental movement and malocclusion correction stages are planned using virtual planning software 3D (ClinCheck), based on CAD-CAM technology, which is also used to view the final result before treatment is begun [3].
There are several other advantages to clear aligner systems, including aesthetics, comfort, chairside time reduction, and the fact that they can be removed for meals and oral hygiene procedures [4]. However, like any orthodontic device, aligners do contribute to a worsening of oral health due to the accumulation of biofilm. Indeed, biofilm deposition on aligner surfaces has been clearly documented and its impact on oral health requires careful investigation [5, 6]. That being said, there have thus far been very few studies to analyse the impact of clear orthodontic aligners on the oral ecosystem, and their consequent influence on caries formation and decalcification. Nevertheless, it has been shown that adults in active treatment with clear aligners have better periodontal health than those undergoing fixed multibracket appliance treatment, who show worse plaque index, bleeding on probing and periodontal pocketing [7]. This is likely due to the fact that clear aligners can be removed and, therefore, cleaned more effectively to remove dental plaque—a complex community of microorganisms existing on the surface of the teeth in a polymeric matrix of bacterial origin. This biofilm is the cause of many oral diseases, and it has been estimated that roughly 60% of human infections can be ascribed to microbial biofilms [8].
Recent research has shown that a minimal dose of chlorhexidine (0.06%) has no beneficial effect on the oral health of aligner wearers [9], who must keep their appliances in place for 22 h per day in order to ensure good orthodontic outcomes (though it should be mentioned that many patients also keep their aligners on while eating and drinking). While they are being worn, aligners accumulate plaque and a bacterial biofilm forms around the teeth [10]. Aligners also limit the buffering, detergent and remineralising effects of saliva.
The biofilm on the enamel is not disturbed by the mechanical action of the lips, cheeks and tongue if an aligner is in place. In fact, one of the main characteristics of the biofilm is the cellular adhesion that occurs between microorganisms and non-exfoliative surfaces. The structure of the biofilm changes according to the bacterial species it is composed of [11–14], but the way in which it is organised protects against the action of chemical and pharmaceutical agents. Indeed, all infections closely linked to the development of the biofilm are highly resistant to non-invasive treatments (i.e. pharmacological) [12, 15, 16].
However, it has been demonstrated that oral bacteria can be destroyed by ultrasonication, via a mechanical phenomenon known as cavitation (bubble formation) [17]. Hence, in a recent study, published by the Journal of Clinical Orthodontics in 2013, Moshiri et al. [10] included this method of cleaning in a list of recommendations they drew up for orthodontists. They also included it in the suggested home hygiene protocol to be given to patients in order to ensure optimal outcomes in aligner therapy. In particular, they advised that patients should refrain from eating with their aligners in, remove any white deposits from their aligners, brush their teeth with a soft brush for two minutes, use dental floss and rinse with fluoride mouthwash in the evening, and always put clean aligners into a clean mouth. For aligner cleaning, they advised the use of either an ultrasonic bath or the Invisalign Cleaning System detergent. However, they cited no specific information regarding the efficacy of these methods.
Hence, in order to provide more information on the topic, we set out to conduct a comparative SEM (scanning electron microscopy) study of various appliance cleaning strategies in real-world patients being treated using F22 aligners [18, 19].
Methods
Five patients were scheduled for orthodontic treatment via clear aligners. Silicon impressions were taken of their upper and lower teeth using the dual-impression method. Class 4 plaster (Ortotypo, Lascod®) was used to make the casts, which were then scanned in the lab using an extraoral scanner (SMART, Open Technologies), to obtain STL files and 3D renderings of the patients’ dentition. These were then converted into resin models using a laser printer (EnvisionTEC ULTRA 3SP), which were in turn used as moulds for a series of nine F22 aligners [18, 19], formed out of thermoplastic polyurethane (TPU), after isolating them with Isofolan sheets.
Each aligner in the series was worn by the patient for 14 days, being removed only at meals and during oral hygiene procedures. Patients were instructed to clean the nine aligners using the following strategies, respectively:
Rinsing with water
Immersion in water in a sonic bath
Immersion in water in an ultrasonic bath
Immersion in water and anionic detergent
Immersion in water and anionic detergent in a sonic bath
Immersion in water and anionic detergent in an ultrasonic bath
Immersion in water and cationic detergent
Immersion in water and cationic detergent in a sonic bath
Immersion in water and cationic detergent in an ultrasonic bath
The sonic device used (TCS Fresh®) for aligners 2, 5 and 8 in the series features a bath in which to immerse the aligner and vibrates at a frequency 5800 Hz, while the ultrasonic device (iSonic F3900®) used for aligners 3, 6 and 9, though very similar in structure, vibrates at a frequency of 42,000 Hz. According to the manufacturer’s instructions, the anionic detergent used (TCS Fresh®) for aligners 4, 5 and 6 should not be used directly in the mouth or for more than 7 days. It is sold in powder form in 1.75-g packets, each to be dissolved in 100 ml of water. Similarly, direct contact with the cationic detergent (benzalkonium chloride, Caelo®) used with aligners 7, 8 and 9, sold in a 0.3% aqueous solution, should also be avoided, as it is toxic to the skin and upon ingestion, and can irritate the skin and eyes. Before replacing in the mouth, therefore, patients were instructed to thoroughly rinse their aligners under running water to remove any trace of detergent. Aligner cleaning times for each strategy were standardised at 5 min, and each disinfection procedure was performed by the patient at home twice a day.
After being worn for 14 days, the aligners were returned to the orthodontist for analysis. Specifically, a 6 × 6-mm section of each aligner was cut from the vestibular surface of the upper right first premolar area (the transition zone between anterior and posterior sectors). Each section was immediately conserved in glutaraldehyde, and the aligner sealed in a plastic bag; both the container of glutaraldehyde were labelled with the same ID (Fig. 1). The preserved aligner sections were dried and gilded at the University of Ferrara Microscopy Lab and then observed under SEM. The scanning electron microscope was focused on the centre of each sample, which was enlarged 10 thousand times. The resulting SEM images were saved in JPEG format on an 8-bit colour-depth grey scale. A histogram of the grey-scale attributes was created for each SEM image to provide a graphical representation of the number of pixels in the image for each grey on the scale.
Fig. 1.
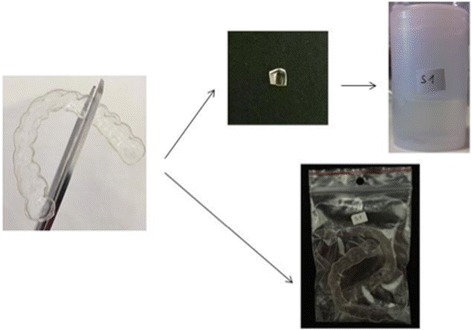
Processing a used aligner before sending to the lab
The dataset composed of the 45 observations, representing the measures on the grey scale (Value variable) of the nine different cleaning methods (one per aligner) used by the five patients, was then subjected to statistical analysis. Repeated-measures ANOVA was used to determine whether the Value variable was influenced by the different aligner/cleaning strategy combinations. The software R nlme [20] was used for ANOVA modelling, specifying “cleaning strategy” as a “within subject” variable, and the patient as a random factor. In order to identify statistically significant differences between aligner and cleaning strategy combinations, a post hoc analysis was performed using Multcomp software [21]. This analysis showed that the differences between the effects of distinct cleaning strategy pairs were statistically equal to zero.
Results
The SEM image analysis (Table 1) and statistical analysis summarising the Value measurements per aligner/cleaning strategy (Table 2) show that the cleaning strategy variable does have a statistically significant effect on the Value (p value zero), i.e. the “cleanliness” of the aligner. However, as shown in Table 3 and Fig. 2, only the first and ninth cleaning strategies (rinsing with water and immersion in water and cationic detergent in an ultrasonic bath, respectively) were significantly different from the others, the former being significantly less efficacious, and the latter being significantly more efficacious.
Table 1.
The mean grey values of each image of aligners cleaned by each of the nine cleaning strategies in each of the five patients, showing the mean calculated for each cleaning method
| Method 1 | Method 2 | Method 3 | Method 4 | Method 5 | Method 6 | Method 7 | Method 8 | Method 9 | |
|---|---|---|---|---|---|---|---|---|---|
| PT 1 | 143.618 | 126.714 | 111.379 | 112.007 | 109.033 | 124.756 | 132.793 | 117.481 | 81.492 |
| PT 2 | 154.730 | 115.470 | 111.380 | 109.226 | 109.219 | 132.481 | 131.457 | 114.486 | 83.033 |
| PT 3 | 140.032 | 107.871 | 112.168 | 140.546 | 133.622 | 107.512 | 125.045 | 129.604 | 84.454 |
| PT 4 | 163.922 | 114.175 | 131.064 | 108.467 | 145.483 | 138.692 | 117.787 | 119.598 | 80.219 |
| PT 5 | 147.972 | 122.249 | 134.454 | 130.679 | 141.776 | 134.050 | 108.033 | 116.978 | 73.534 |
| MEAN | 150.055 | 117.296 | 120.089 | 120.185 | 127.827 | 127.498 | 123.023 | 119.629 | 80.546 |
Table 2.
Summarises the values for each cleaning strategy: number of observations, mean grey value and standard deviation
| Method | Length | Mean | SD |
|---|---|---|---|
| 1 | 5 | 150.1 | 9.492 |
| 2 | 5 | 117.3 | 7.334 |
| 3 | 5 | 120.1 | 11.63 |
| 4 | 5 | 120.2 | 14.57 |
| 5 | 5 | 127.8 | 17.6 |
| 6 | 5 | 127.5 | 12.25 |
| 7 | 5 | 123 | 10.28 |
| 8 | 5 | 119.6 | 5.865 |
| 9 | 5 | 80.55 | 4.232 |
Table 3.
A p value of zero indicates that the ANOVA test used is significant and refutes the hypothesis that all methods tested are similar
| numDF | denDF | F value | p value | |
|---|---|---|---|---|
| (Intercept) | 1 | 32 | 5317 | 0 |
| Method | 8 | 32 | 13.13 | 0.00000003592 |
Fig. 2.
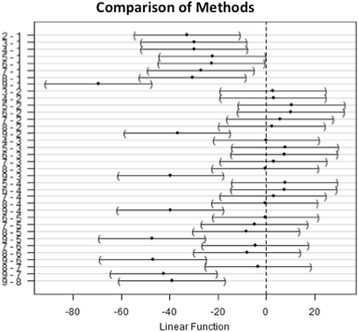
Post hoc analysis of cleaning strategy pairs
Discussion
All aligner cleaning strategies did reduce the bacterial biofilm on the surface of our aligner samples, with the exception of rinsing under tap-water alone. However, our data also show that the most efficacious strategy tested was cationic detergent combined with ultrasonication. Our comparative results also appear to confirm the literature reports that the only way to kill bacteria via low-intensity ultrasound is by combining it with a bactericidal agent [22, 23]. Indeed, we clearly show that a cationic detergent alone is not sufficient to eliminate the bacterial biofilm from the surface of aligners, despite its bactericidal properties, which have been demonstrated in other studies [24, 25]. Similarly, 5 min of 42,000 Hz ultrasound alone was not sufficient to remove the biofilm [26], and only a combination of the two methods proved useful in this regard according to our statistical analysis.
Indeed, even a brief glance at the SEM images for each of the aligners reveals evident bacterial colonisation of each, with the exception of those cleaned by the ninth strategy, which combined an ultrasonic bath with a cationic bactericide. These compare very favourably not only with those rinsed with water alone, which show visible layers of abundant bacteria organised into a biofilm, but also those cleaned using the other strategies (2–8), which show variable levels of bacterial proliferation.
As the SEM image of the unused aligner (Fig. 3) is much darker than that cleaned by the first method (Fig. 4)—rinsing with water alone—we decided to numerically quantify the visible biofilm on the aligner sections using grey-scale analysis, taking a mean of the levels of grey in the images of the aligners cleaned by each method. The cleaner aligners are darker in colour and show grey-scale levels closer to that of the unused aligner, i.e. 61.924, which appears totally dark. Bacterial proliferation on the surface of the aligner, on the other hand, makes them appear lighter in colour, and the multi-layer biofilm causes the mean level of grey to differ considerably from that of the clean TPU of the unused aligner. By statistically analysing this data, we were able to confirm that there was indeed a statistically significant relationship between the SEM grey-scale levels and the cleanliness of the aligner sections.
Fig. 3.
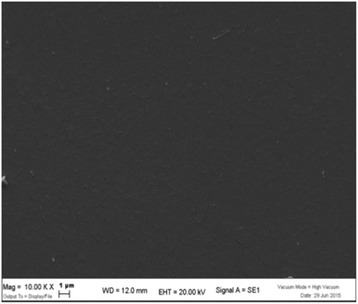
Unused aligner
Fig. 4.

Aligner cleaned by rinsing with water alone (method 1)
Careful SEM analysis of the aligner sections cleaned via the ninth method (Fig. 5), i.e. ultrasonic bath and cationic detergent, reveal interesting points regarding their surface characteristics. The first is that the TPU material itself bears clear striations, an indication of its hydrophilic status [25, 26], and its absorption of water. The second visible feature is related to the method of cleaning used; the surface of the aligner bears signs of pitting. This is likely an effect of ultrasonic cavitation and is visible thanks to the lack of bacterial proliferation. It demonstrates that the mechanical turbulence generated by the acoustic waves, the continual formation and rupture of tiny bubbles, releases sufficient energy to damage not only bacterial cell walls [27] but also the surface of thermoplastic polyurethane.
Fig. 5.
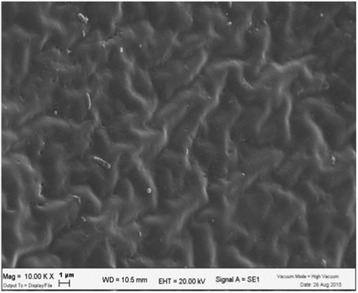
Aligner cleaned by method 9 shows clear signs of water absorption and ultrasonic cavitation
In order to directly compare these findings with those in the literature in any meaningful way, we would need to rely on studies conducted using the same methods for analysing the aligner surfaces and counting the bacterial colonies. However, there is great variability in the literature in terms of these factors, and in any case, our design prevented us from providing precise bacterial counts for comparison. Nevertheless, our findings generally confirm those of Torlak and Sert [28] and Mermillod-Blondin et al. [29] that the association between a germicidal detergent and ultrasonication is able to reduce the bacterial load on the surfaces analysed.
Conclusions
Of the nine cleaning strategies examined, only that involving 5 min of ultrasound treatment at 42 kHz combined with a 0.3% solution of the germicidal cationic detergent benzalkonium chloride was statistically observed to be effective at removing the bacterial biofilm from the surface of used F22 TPU aligners [18, 19] (p < 0.05).
Acknowledgments
Funding
There are no sponsors for this study. The research is funded by its own means of the researchers.
Authors’ contributions
Conception and design of study: SG; LL. Acquisition of data: MM, CF. Analysis and/or interpretation of data: FC, SGA, GS. Drafting the manuscript: CF, MM, LL. Revising the manuscript critically for important intellectual content: SG. Approval of the version of the manuscript to be published: GS, LL, MM, FC, SGA. Conception and design of study and acquisition of data: TO. All authors read and approved the final manuscript.
Ethics aproval and consent to participate
The protocol of the study was approved by the Director and Chairman of School of Orthodontics, University of Ferrara Prof., Giuseppe Siciliani.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luca Lombardo, Phone: +393496189796, Phone: +390532242776, Email: lulombardo@tiscali.it.
Marco Martini, Email: dottmarcomartini@gmail.com.
Francesca Cervinara, Email: cervinarafrancesca@gmail.com.
Giorgio Alfredo Spedicato, Email: giorgio.spedicato4@unibo.it.
Teresa Oliverio, Email: terek84@hotmail.it.
Giuseppe Siciliani, Email: giuseppe.siciliani@unife.it.
References
- 1.McDonald F, Cobourne M. Adult orthodontics: perils and pitfalls. Prog Orthod. 2007;8(2):308–13. [PubMed] [Google Scholar]
- 2.Wong BH. Invisalign A to Z. Am J Orthod Dentofacial Orthop. 2002;121(5):540–1. doi: 10.1067/mod.2002.123036. [DOI] [PubMed] [Google Scholar]
- 3.Malik OH, McMullin A, Waring DT. Invisible orthodontics part 1: Invisalign.. Dent Update. 2013;40(3):203-4, 207-10, 213-5. [DOI] [PubMed]
- 4.Guarneri, Lombardo, Gracco, Siciliani. Lo stato dell’arte del trattamento con allineatori. Dalla nascita ai sistemi più attuali. Ed. Martina. 2013.
- 5.Steinberg D, Eyal S. Initial biofilm formation of Streptococcus sobrinus on various orthodontics appliances. J Oral Rehabil. 2004;31(11):1041–5. doi: 10.1111/j.1365-2842.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 6.Eliades T, Eliades G, Athanasiou AE, Bradley TG. Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 2000;22(3):317–26. doi: 10.1093/ejo/22.3.317. [DOI] [PubMed] [Google Scholar]
- 7.Karkhanechi M, Chow D, Sipkin J, Sherman D, Boylan RJ, Norman RG, Craig RG, Cisneros GJ. Periodontal status of adult patients treated with fixed buccal appliances and removable aligners over one year of active orthodontic therapy. Angle Orthod. 2013;83(1):146–51. doi: 10.2319/031212-217.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh PD, Bradshaw DJ. Dental plaque as a biofilm. J Ind Microbiol. 1995;15(3):169–75. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer I, Braumann B.Source Department of Orthodontics, University of Cologne, Cologne, Germany. Halitosis, oral health and quality of life during treatment with Invisalign(®) and the effect of a low-dose chlorhexidine solution.. J Orofac Orthop. 2010 Nov;71(6):430-41 [DOI] [PubMed]
- 10.Moshiri M, Eckhart JE, Mcshane P, German D. Consequences of poor oral hygiene during clear aligner therapy. JCO. 2013;8(47):494–98. [PubMed] [Google Scholar]
- 11.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aparna MS, Yadav S. Biofilms: microbes and disease. Braz J Infect Dis. 2008;12:526–530. doi: 10.1590/S1413-86702008000600016. [DOI] [PubMed] [Google Scholar]
- 13.Hornemann JA, Lysova AA, Codd SL, Seymour JD, Busse SC, Stewart PS, Brown JR. Biopolymer and water dynamics in microbial biofilm extracellular polymeric substance. Biomacromolecules. 2008;9:2322–2328. doi: 10.1021/bm800269h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolker-Nielsen T, Molin S. Spatial organization of microbial biofilm communities. Microb Ecol. 2000;40:75–84. doi: 10.1007/s002480000057. [DOI] [PubMed] [Google Scholar]
- 15.Denotti G, Piga R, Montaldo C, Erriu M, Pilia F, Piras A, Luca MD, Orru G. In vitro evaluation of Enterococcus faecalis adhesion on various endodontic medicaments. Open Dent J. 2009;3:120–124. doi: 10.2174/1874210600903010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monsen T, Lovgren E, Widerstrom M, Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J Clin Microbiol. 2009;47:2496–2501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman HR, McNamara AJ, Nguyen DX, Prausnitz MR, Bioeffects caused by changes in acoustic cavitation bubble density and cell concentration: a unified explanation based on cell-to-bubble ratio and blast radius. Ultrasound Med Biol. 2003;29. [DOI] [PubMed]
- 18.Luca Lombardo, Angela Arreghini, Roberta Maccarrone, Anna Bianchi, Santo Scalia and Giuseppe Siciliani. Optical properties of orthodontic aligners—spectrophotometry analysis of three types before and after aging. Progress in Orthodontics. 2015. DOI10.1186/s40510-015-0111-z. [DOI] [PMC free article] [PubMed]
- 19.Luca Lombardo; Elisa Martines; Valentina Mazzanti; Angela Arreghini; Francesco Mollica; Giuseppe Siciliani. Stress relaxation properties of four orthodontic aligner materials: a 24-hour in vitro study. Angle Orthodontist, DOI:10.2319/113015-813.1. [DOI] [PMC free article] [PubMed]
- 20.Pinheiro, Jose, Douglas Bates, Saikat DebRoy, Deepayan Sarkar, and R Core Team. 2015. nlme: linear and nonlinear mixed effects models. https://cran.r-project.org/web/packages/nlme/index.html.
- 21.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 22.Rediske AM, Roeder BL, Brown MK, Nelson JL, Robison RL, Draper DO, Schaalje GB, Robison RA, Pitt WG. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob Agents Chemother. 1999;43:1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erriu M, Blus C, Szmukler-Moncler S, Buogo S, Levi R, Barbato G, Madonnaripa D, Denotti G, Piras V, Orrù G. Microbial biofilm modulation by ultrasound: current concepts and controversies. Ultrason Sonochem. 2014;21(1):15–22. doi: 10.1016/j.ultsonch.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 24.ter Haar G. Therapeutic ultrasound. Eur J Ultrasound. 1999;9:3–9. doi: 10.1016/S0929-8266(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 25.Robertson VJ, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Phys Ther. 2001;81:1339–1350. [PubMed] [Google Scholar]
- 26.Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 2001;81:1351–1358. [PubMed] [Google Scholar]
- 27.L. Trombelli “Strumentazione parodontale non chirurgica”, 2005, Masson.
- 28.Torlak E, Sert D. Combined effect of benzalkonium chloride and ultrasound against Listeria monocytogenes biofilm on plastic surface. Lett Appl Microbiol. 2013;57(3):220–6. doi: 10.1111/lam.12100. [DOI] [PubMed] [Google Scholar]
- 29.Mermillod-Blondin F, Fauvet G, Chalamet A, Chatelliers MCD. A comparison of two ultrasonic methods for detaching biofilms from natural substrata. Int Rev Hydrobiol. 2001;86:349–360. doi: 10.1002/1522-2632(200106)86:3<349::AID-IROH349>3.0.CO;2-B. [DOI] [Google Scholar]


