Abstract
Mycoplasma pneumoniae is the major pathogen of community-acquired pneumonia in children. The prevalence of macrolide-resistant M. pneumoniae (MRMP) is important owing to the limited alternative therapies for children. We analyzed 111 M. pneumoniae obtained from 107 children admitted for lower respiratory tract infection at Jeju National University Hospital between 2010 and 2015. Macrolide resistance of M. pneumoniae was searched for using polymerase chain reaction (PCR) and sequencing. Of 107 clinical M. pneumoniae, 11 (10.3%) carried macrolide resistance mutations in the 23S rRNA gene. All macrolide resistance mutations were A2063G transitions. We found an acquired A2063G mutation of M. pneumoniae from a patient during macrolide treatment. Patients' characteristics and clinical severity did not differ between those with MRMP and macrolide-sensitive M. pneumoniae, with the exception of frequent pleural effusion in the MRMP group. The prevalence of MRMP (10.3%) in Jeju Island was relatively lower than those of surrounding countries in East Asia. Previous antimicrobial usage and timing of diagnostic test should be considered when determining of macrolide resistance of M. pneumoniae.
Keywords: Mycoplasma pneumoniae, Macrolide, 23S rRNA
Graphical Abstract
INTRODUCTION
Mycoplasma. pneumoniae is one of the most common causal organisms of community-acquired pneumonia in children and adolescents. Although M. pneumoniae infections are usually mild and self-limited, serious infections requiring hospitalization sometimes occur, especially in children (1). Globally, M. pneumoniae infections occur every 3–7 years (2,3,4), and epidemics in Korea are observed every 3–4 years (5). The first treatment of choice for M. pneumoniae infections in children is macrolides; however, macrolide-resistant M. pneumoniae (MRMP) have increased in Japan, China, Korea, and the USA (6,7,8,9). Quinolones and tetracycline are alternative treatment options for MRMP, but these drugs are not approved for children.
Jeju Island is geographically located between the mainland of Korea and Japan. A high prevalence of MRMP in East Asia has increased interest in M. pneumoniae in Jeju Island. In this study, the prevalence of macrolide resistance among M. pneumoniae isolates from children with lower respiratory tract infections (LRTIs) in Jeju Island was determined. Furthermore, clinical characteristics of MRMP and macrolide-sensitive M. pneumoniae (MSMP) were compared.
MATERIALS AND METHODS
Study design and sample processing
Nasopharyngeal aspirates were obtained from children under the age of 15 years at Jeju National University Hospital (JNUH) on Jeju Island, Korea between 2010 and 2015. Nasopharyngeal aspirates were collected when children were admitted for LRTI before treatment. LRTI was defined as abnormal chest radiography findings or respiratory illness with abnormal lung sound. Demographic information for the patients, such as underlying disease, clinical manifestations, radiological features, and outcomes, were collected retrospectively from medical records.
We used the −80°C frozen DNA after having used it for diagnosis on admission. In JNUH, M. pneumoniae infections were confirmed by polymerase chain reaction (PCR) as follows. DNA extraction from the nasopharyngeal aspirates was performed using the Chemagen® DNA Extraction Kit (Perkin Elmer, Inc., Baesweiler, Germany) according to the manufacturer's instructions. Extracted DNA was amplified with the Veriti® 96-Well Thermocycler (Applied Biosystems, Waltham, MA, USA) using the PneumoBacter ACE Detection Kit (Seegene Inc., Seoul, Korea) which can detect 6 bacterial pathogens including M. pneumoniae. We selected the samples which only M. pneumoniae was detected, and confirmed P1 adhesin amplification by PCR.
Amplification of macrolide resistance genes
The frozen M. pneumoniae DNA was used to detect macrolide resistance genes. Domain V of the 23S rRNA gene was amplified by PCR using primers (forward, 5′-CGCAAGCGAAGCTTTTAACT; reverse, 5′-ATTCCACCTTTCGCATCAAC) (10). PCR was performed in a volume of 20 µL containing 0.2 µL of genomic DNA and 2 µL of 10× buffer in a PCR PreMix Tube (AccuPower; Bioneer Inc., Alameda, CA, USA). The reaction mixture was subjected to denaturation, annealing, and elongation for 5 minutes at 90°C, 30 seconds at 90°C, 40 seconds at 50°C, followed by an extension step of 5 minutes at 90°C for 35 PCR cycles. When the PCR product was uncertain, nested PCR was performed using second primers (forward, 5′-TAACTATAACGGTCCTAAGG; reverse, 5′-ATTCCACCTTTCGCATCAAC). The amplified products were analyzed by electrophoresis on a 1.5% agarose gel. The sizes of PCR products were 306-bp and 263-bp including positions 2063 and 2064.
Statistical analysis
Various characteristics were compared between MRMP and MSMP patient groups. Categorical variables were compared using the chi-square test or Fisher's exact test, and continuous variables were analyzed using the Mann-Whitney U test. A two-tailed P value of less than 0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of the Jeju National University Hospital with an exemption of acquiring informed consent (No. 2016-06-003). There are no potential conflicts of interest relevant to this article.
RESULTS
Patient characteristics
A total of 111 nasopharyngeal aspirates obtained from 107 children were included in the study. Mycoplasma infection was tested twice by PCR in four children to verify M. pneumoniae. The mean age of children was 5.86 years (range, 0.8–16.9 years), and 44 (41.1%) were male. All patients were previously healthy, except 3 children, which had bronchopulmonary dysplasia, dilated cardiomyopathy, and hereditary spherocytosis.
Macrolide-resistant M. pneumoniae
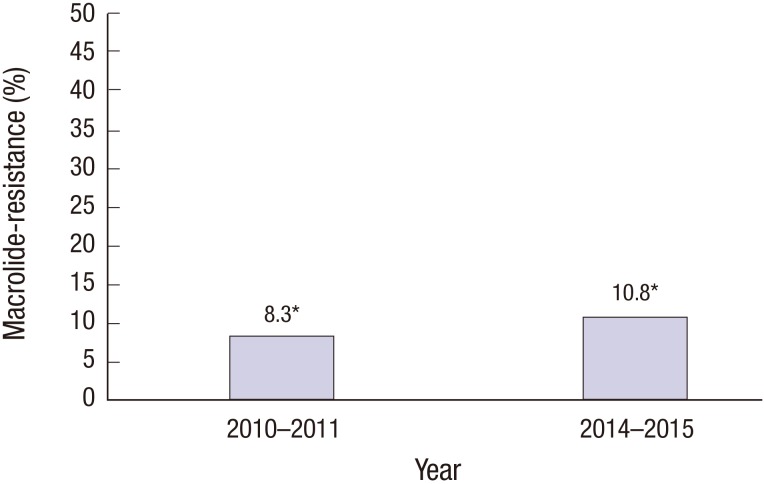
Two nasopharyngeal aspirates from one patient were considered a single clinical mycoplasma infection. Therefore, 107 M. pneumoniae strains were analyzed in this study. Of the 107 samples positive for M. pneumoniae, 12 (10.9%) showed macrolide resistance. Of 8 M. pneumoniae strains in 4 children who were tested twice, 1 strain was initially macrolide-sensitive, but its status changed to macrolide-resistant in the second test. This strain was considered MSMP. All MRMP had the A2063G point mutation in domain V of the 23S rRNA gene. Other mutations in the 23S rRNA were not detected. Epidemics of M. pneumoniae in Jeju Island were observed in 2010–2011 and 2014–2015. Macrolide resistance rates of each epidemics were 8.3% (1/12) and 10.8% (10/93) (Fig. 1)
Fig. 1.
Macrolide resistance rates of M. pneumoniae in 2010–2011 and 2014–2015 epidemics.
*Each number above the bar was represented as a percent (8.3% [1/12] and 10.8% [10/93], respectively).
Comparison of clinical characteristics for MRMP and MSMP
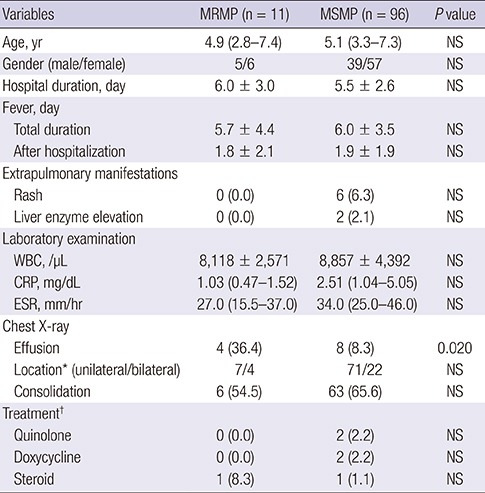
The demographic data, clinical course, and laboratory findings for children with MRMP and MSMP pneumonia are summarized in Table 1. Demographic properties were not significantly different between the two groups. The duration of hospital stay, fever, and time to defeverance were not different between two groups. Inflammatory markers such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and white blood cell (WBC) counts, were not related to macrolide resistance. Most radiological findings of M. pneumoniae pneumonia patients were unilateral lobar pneumonia (72.9%, 78/107) in both groups. Compared with the MSMP group, the MRMP group had more plural effusion on the chest X-ray (33.3% vs. 8.4%, P = 0.028).
Table 1. Comparison of clinical characteristics between MRMP and MSMP patient groups.
| Variables | MRMP (n = 11) | MSMP (n = 96) | P value |
|---|---|---|---|
| Age, yr | 4.9 (2.8–7.4) | 5.1 (3.3–7.3) | NS |
| Gender (male/female) | 5/6 | 39/57 | NS |
| Hospital duration, day | 6.0 ± 3.0 | 5.5 ± 2.6 | NS |
| Fever, day | |||
| Total duration | 5.7 ± 4.4 | 6.0 ± 3.5 | NS |
| After hospitalization | 1.8 ± 2.1 | 1.9 ± 1.9 | NS |
| Extrapulmonary manifestations | |||
| Rash | 0 (0.0) | 6 (6.3) | NS |
| Liver enzyme elevation | 0 (0.0) | 2 (2.1) | NS |
| Laboratory examination | |||
| WBC, /µL | 8,118 ± 2,571 | 8,857 ± 4,392 | NS |
| CRP, mg/dL | 1.03 (0.47–1.52) | 2.51 (1.04–5.05) | NS |
| ESR, mm/hr | 27.0 (15.5–37.0) | 34.0 (25.0–46.0) | NS |
| Chest X-ray | |||
| Effusion | 4 (36.4) | 8 (8.3) | 0.020 |
| Location* (unilateral/bilateral) | 7/4 | 71/22 | NS |
| Consolidation | 6 (54.5) | 63 (65.6) | NS |
| Treatment† | |||
| Quinolone | 0 (0.0) | 2 (2.2) | NS |
| Doxycycline | 0 (0.0) | 2 (2.2) | NS |
| Steroid | 1 (8.3) | 1 (1.1) | NS |
Values are presented as median (IQR), mean ± SD, or number (%).
MRMP = macrolide-resistant M. pneumoniae, MSMP = macrolide-sensitive M. pneumoniae, WBC = white blood cell, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IQR = interquartile range, NS = not significant (P > 0.050), SD = standard deviation.
*Location of abnormality on chest X-ray. Three children in the MSMP group had no abnormalities on chest X-rays. †Alternative treatment after the failure of macrolide treatment.
Antimicrobial agents, such as quinolones or tetracycline, and immunomodulating agents, like steroids, were administered if clinical features did not improve after macrolide uses. These alternative treatments were not increased in the MRMP group compared with the MSMP group (Table 1).
Change in macrolide resistance
Two consecutive tests at an interval of 7 days were performed for a 13-year-old girl. Initial antimicrobial agents were ampicillin/sulbactam and clarithromycin. Despite treatment, the fever was sustained and consolidation on the chest X-ray did not improve. After the administration of minocycline on hospital day 5, the fever disappeared and respiratory symptoms improved. No mutation in domain V of 23S rRNA was found in the first nasal aspirate. However, in a second sample obtained after 7 days of hospitalization, an A2063G mutation was found.
DISCUSSION
M. pneumoniae is the most common bacteria in community-acquired pneumonia requiring hospitalization in children (11). LRTIs caused by M. pneumoniae are initially treated with macrolides because M. pneumoniae lacks a cell wall, the target of β-lactam antibiotics. However, the emergence of MRMP has been reported in many countries. Non-macrolide antibiotics, such as fluoroquinolones and tetracyclines, are candidates for alternative MRMP treatment. Owing to the risk associated with fluoroquinolone and tetracyclines administration in children, it is important to determine MRMP rates to inform the selection of appropriate antibiotics for M. pneumoniae treatment.
Macrolide resistance in M. pneumoniae is caused by point mutations at a few positions in domain V of 23S rRNA, the location of macrolide binding to the 50S ribosome subunit (12). The first nucleotide mutations associated with resistance to macrolides in M. pneumoniae were identified in 2001 (13). Since then, the development of molecular techniques has improved our knowledge of MRMP and its epidemiology (6,8). Known point mutations conferring macrolide resistance include A2063G or T, A2064G, A2067G, and C2617A or G (14,15). The A2063G mutation is the most common mutation associated with macrolide-resistance, and was the only mutation found in our study.
The frequency of macrolide-resistant isolates in M. pneumoniae infections has increased worldwide, especially in Asia. After the first isolation of MRMP, the macrolide resistance rate has increased to 87% among children with mycoplasma in Japan (6,16). In China, the prevalence of MRMP isolates is more than 90% and has increased over time (7,14). The mainland of Korea recently identified a 62.9%–87.2% prevalence of MRMP isolates (8,17). In addition to this high prevalence of MRMP in East Asia, the prevalence of MRMP in the United States and Europe is 13%–30% (9,18,19). This variation indicates regional variation in M. pneumoniae. In our study, a prevalence of 10.9% MRMP was detected in Jeju Island. This prevalence is relatively low when compared with those of geographically surrounding countries, showing a high MRMP prevalence. Although it is difficult to interpret these findings owing to the unique characteristics of the isolated island, they are supported the relatively low MRMP prevalence (23.0%) in Taiwan which has similar geographic conditions to those of Jeju Island (20).
Clinical presentations, such as respiratory symptoms, pneumonia severity, inflammatory markers, and radiographic findings, do not depend on M. pneumoniae resistance to macrolides (21). In our study, no differences in clinical presentation were observed, except for pleural effusion on chest X-rays (Table 1). However, the amount of pleural effusion was minor, and no aspirations were performed in either group. We also found that the incidence of extrapulmonary findings was not different between the two groups.
Macrolide resistance was a subject of controversy as a determinant on clinical course of M. pneumoniae pneumonia. Many studies have found that the therapeutic efficacy of macrolide administration differs between MRMP and MSMP groups. For example, in the MRMP group, the duration of fever, cough, hospitalization was longer and the rate of change to alternative antibiotics was higher than the MSMP group (20,21). However, a recent study reported that macrolide resistance alone has no effect on clinical course of M. pneumoniae pneumonia (22). In our study, the contribution of macrolide resistance on clinical course was not found like the latter report.
We detected many differences in clinical characteristics and the response to macrolides from those observed in previous MRMP studies. We focused on one case in which macrolide resistance changed after the treatment with macrolides for 7 days. The prevalence of MRMP was confirmed not only by the original characteristics of the bacteria, but also by the exposure to antimicrobial agents when considering the observed mutation. Furthermore, the timing of diagnostic tests after disease onset can be an important factor with respect to confirming macrolide-resistance. Most tests for M. pneumoniae macrolide resistance were performed in a tertiary hospital because this test is not a routine laboratory test. Although JNUH is the only university hospital in Jeju Island, it has good accessibility and children with relatively low severity can be admitted. These characteristics of JNUH can explain the low MRMP prevalence and clinical similarities between MRMP and MSMP. When confirming MRMP, previous antimicrobial usage and timing after the disease onset should be considered.
This study had several limitations. First, clinical data were retrospectively collected based on medical charts only. Second, a culture study for M. pneumoniae was not performed because we used previously extracted DNA from nasopharyngeal aspirates. Lastly, the MRMP sample size was not sufficiently large to obtain significance in some statistical analyses.
We found the relatively lower prevalence of MRMP in Jeju Island compared with surrounding countries including the mainland of Korea. Although a recent increase in macrolide resistance was found in East Asia, we believe that total aspects such as previous antimicrobial usage and diagnostic timing should be considered. Also, no significant differences of clinical characteristics between MSMP and MRMP in our study show the importance of other pathogenesis of M. pneumoniae.
Footnotes
Funding: This work was supported by a research grant (No. 201500780001) from Jeju National University Hospital in 2015.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Shin KS, Choi JH. Formal analysis: Kim YJ, Choi JH. Investigation: Lee KH, Kim YR. Writing - original draft: Kim YJ, Choi JH.
References
- 1.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foy HM, Kenny GE, Cooney MK, Allan ID. Long-term epidemiology of infections with Mycoplasma pneumoniae . J Infect Dis. 1979;139:681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- 3.Hauksdottir GS, Love A, Sigurdardottir V, Jonsson T. Outbreaks of Mycoplasma pneumoniae infections in Iceland 1987 to 1997 - a ten and a half years review. Eur J Epidemiol. 1999;15:95–96. doi: 10.1023/a:1007579017695. [DOI] [PubMed] [Google Scholar]
- 4.Ito I, Ishida T, Osawa M, Arita M, Hashimoto T, Hongo T, Mishima M. Culturally verified Mycoplasma pneumoniae pneumonia in Japan: a long-term observation from 1979–99. Epidemiol Infect. 2001;127:365–367. doi: 10.1017/s0950268801005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008;56:326–331. doi: 10.1016/j.jinf.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, Nakayama E, Sunakawa K, Ubukata K, Acute Respiratory Diseases Study Group Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2008;52:348–350. doi: 10.1128/AAC.00779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D, Wang M. Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob Agents Chemother. 2009;53:2160–2162. doi: 10.1128/AAC.01684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, Kang HM, Lee J, Ahn YM, Kang YH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000–2011. Emerg Infect Dis. 2013;19:1281–1284. doi: 10.3201/eid1908.121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng X, Lee S, Selvarangan R, Qin X, Tang YW, Stiles J, Hong T, Todd K, Ratliff AE, Crabb DM, et al. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg Infect Dis. 2015;21:1470–1472. doi: 10.3201/eid2108.150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh CE, Choi EH, Lee HJ. Detection of genetic mutations associated with macrolide resistance of Mycoplasma pneumoniae . Korean J Pediatr. 2010;53:178–183. [Google Scholar]
- 11.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morozumi M, Takahashi T, Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J Infect Chemother. 2010;16:78–86. doi: 10.1007/s10156-009-0021-4. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki N, Narita M, Yamada S, Izumikawa K, Umetsu M, Kenri T, Sasaki Y, Arakawa Y, Sasaki T. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol. 2001;45:617–620. doi: 10.1111/j.1348-0421.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F, Liu G, Wu J, Cao B, Tao X, He L, Meng F, Zhu L, Lv M, Yin Y, et al. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2013;57:1521–1523. doi: 10.1128/AAC.02060-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshaghi A, Memari N, Tang P, Olsha R, Farrell DJ, Low DE, Gubbay JB, Patel SN. Macrolide-resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010–2011. Emerg Infect Dis. 2013;19:1525–1527. doi: 10.3201/eid1909.121466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–1649. doi: 10.1093/cid/cis784. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Cho HJ, Hong SJ, Lee J, Sung H, Yu J. Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr. 2017;60:151–157. doi: 10.3345/kjp.2017.60.5.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chironna M, Sallustio A, Esposito S, Perulli M, Chinellato I, Di Bari C, Quarto M, Cardinale F. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J Antimicrob Chemother. 2011;66:734–737. doi: 10.1093/jac/dkr003. [DOI] [PubMed] [Google Scholar]
- 19.Averbuch D, Hidalgo-Grass C, Moses AE, Engelhard D, Nir-Paz R. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg Infect Dis. 2011;17:1079–1082. doi: 10.3201/eid1706.101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu PS, Chang LY, Lin HC, Chi H, Hsieh YC, Huang YC, Liu CC, Huang YC, Huang LM. Epidemiology and clinical manifestations of children with macrolide-resistant Mycoplasma pneumoniae pneumonia in Taiwan. Pediatr Pulmonol. 2013;48:904–911. doi: 10.1002/ppul.22706. [DOI] [PubMed] [Google Scholar]
- 21.Cardinale F, Chironna M, Chinellato I, Principi N, Esposito S. Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J Clin Microbiol. 2013;51:723–724. doi: 10.1128/JCM.02840-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon IA, Hong KB, Lee HJ, Yun KW, Park JY, Choi YH, Kim WS, Lee H, Eun BW, Ahn YM, et al. Radiologic findings as a determinant and no effect of macrolide resistance on clinical course of Mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2017;17:402. doi: 10.1186/s12879-017-2500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]