Abstract
We investigated the adenoviral etiology and seasonal epidemic trends in intussusception and each adenoviral subgroup. Also we confirmed whether we can use the adenovirus data of Acute Infectious Agents Laboratory Surveillance Report (AIALSR) as an epidemic predictor of intussusception. Patients with intussusception (n = 126), < 5 years old, were enrolled and matched by age and sex with controls suffering acute gastroenteritis without intussusception (n = 106), all recruited at 8 centers. All fecal specimens were assayed for adenovirus, including subgroups A, B, C, E, and F, with reverse transcriptase-polymerase chain reaction (RT-PCR). Adenovirus was detected in 53 cases and 13 controls (P < 0.001). Nonenteric adenoviruses (NEAds) were detected in 51 cases and four controls (P < 0.001). We used Spearman's correlation analysis to analyze the incidence of intussusception and adenoviral epidemic trends, and compared them with fecal and respiratory adenoviral epidemic trends in the AIALSR. The trend of intussusception correlated with total NEAds (r = 0.635; P = 0.011), as did the fecal AIALSR adenovirus trends (r = 0.572; P = 0.026). Among the NEAd subgroups, subgroup C was dominant (P < 0.001), but subgroups B (P = 0.007) and E (P = 0.013) were also significant to intussusception. However, only subgroup C showed a significant epidemic correlation (r = 0.776; P = 0.001) with intussusception. Not respiratory but fecal AIALSR adenovirus trends correlated with the incidence of NEAds and intussusception. We suggest the possibility of using fecal AIALSR adenovirus data as an approximate epidemic predictor of intussusception.
Keywords: Intussusception, Adenoviridae, Epidemics, Reverse Transcriptase-Polymerase Chain Reaction, Child
Graphical Abstract
INTRODUCTION
Intussusception is the invagination of a segment of bowel into the adjacent distal bowel, and it is the most common cause of intestinal obstruction in infancy and early childhood. It constitutes a medical emergency because it impairs the blood flow and induces necrosis of the bowel segment (1). Although the etiology of idiopathic intussusception without a leading point has not yet been clarified, idiopathic intussusception in young children often occurs after respiratory or gastrointestinal infection (2,3). This suggests that the presence of certain viral infections involves the hypertrophy of lymphatic tissue, which can act as the leading point (2,4,5,6,7). However, there is little evidence of a role for viral infection in the development of intussusception. Although the first licensed rotavirus vaccine (RotaShield®, a rhesus-human reassortant rotavirus vaccine; Wyeth Laboratories Inc., Marietta, PA, USA) had been withdrawn, it was recognized as a risk factor for intussusception. However, natural human rotavirus is infrequently detected in cases of intussusception and there is no association between the seasonality and severity of rotaviral enteritis and intussusception (7,8,9). Various infectious pathogens have been investigated in many studies, and several viral agents, including adenovirus, rotavirus, enterovirus, astrovirus, and human herpesviruses 6 and 7, have been associated with intussusception (10,11,12,13,14,15,16,17). The family Adenoviridae has been considered the predominant viral pathogens involved in the disease in recent decades. Among the Adenoviridae, nonenteric adenoviruses (NEAds) have been demonstrated in 20%–70% of cases of idiopathic intussusception in previous studies (3,10,12,18,19).
Most previous studies of intussusception in terms the NEAd subgroups have been comparative studies, with few epidemiological studies. To define an epidemic pathogen, an epidemic correlation analysis between the pathogen and the disease is required. Here, we analyzed the adenovirus subgroups involved in intussusception to establish a correlation between the epidemic seasonal trends in the prevalence of the NEAd subgroups and intussusception.
MATERIALS AND METHODS
This study was based on 2 methodologies. We first used real-time reverse transcriptase-polymerase chain reaction (RT-PCR) to analyze the viruses in fecal samples from patients with intussusception (cases) or with acute gastroenteritis without intussusception (controls). The study was designed as multicenter consisting of 8 centers, annual, prospective, controlled study. We then undertook an epidemiological correlation study based on the ‘Acute Infectious Agents Laboratory Surveillance Report (AIALSR),’ a nationwide report of epidemic respiratory and diarrheal pathogens. AIALSR is issued weekly by the Korea Centers for Disease Control and Prevention (KCDC). We used the fecal adenoviral epidemic data from the ‘weekly viral epidemic reports of acute diarrheal disease (WRAD)’ (20) and respiratory adenoviral epidemic data from ‘weekly viral epidemic report of influenza and respiratory virus (WRIR)’ in the AIALSR (21). For their viral analyses, the KCDC uses enzyme-linked immunosorbent assays (ELISAs) for Adenoviridae and rotavirus, and PCR for norovirus and astrovirus. For the analysis of respiratory and fecal adenoviruses, the KCDC used the Bio Tracer Adenovirus ELISA Kit® (Nano EnTek Inc., Seoul, Korea), which detects the general human Adenoviridae from April 2008 to the present. Previous studies have reported that a large proportion (30%–60%) of the adenoviruses detected from diarrhea in acute gastroenteritis are NEAds (22,23).
Patients and controls
From eight hospitals, we enrolled 126 patients with intussusception as the cases and 106 patients with acute gastroenteritis but without intussusception as the controls; all were < 5 years old between June 2012 and July 2013. To match the cases, the controls were enrolled matching with age and sex in same centers where intussusception patients were enrolled at the same admission period. Patients with intussusception (n = 126), < 5 years old, were enrolled and matched with controls suffering acute gastroenteritis without intussusception (n = 106). Eight hospitals were selected considering the Korea regional distribution: Inje University Sanggye Paik Hospital, Ajou University Hospital, Bundang Jesaeng General Hospital, Gangneung Asan Hospital, Dankook University Hospital, Pusan National University Yangsan Hospital, Ulsan University Hospital, and Kyungpook National University Hospital. We diagnosed intussusception with a diagnostic (or therapeutic) enema or abdominal ultrasonography. We excluded any participant with a history of abdominal surgical or an anatomic anomaly associated with intussusception. We collected demographic data and information on the clinical manifestations of all the participants. The clinical characteristics included a history of rotavirus vaccination, past history of intussusception, preceding symptoms (fever, symptoms of upper respiratory infection, or gastroenteritis), intussusception type, and the therapeutic reduction method used. We compared the data of the case and control groups.
All fecal specimens were assayed with real-time RT-PCR to detect adenovirus, rotavirus, norovirus, astrovirus, and sapovirus. The adenoviral subgroups (A, B, C, E, and F) were then analyzed in the adenovirus-positive cases. We also compared the demographic data and clinical manifestations of the patients in whom no virus was detected, the patients in whom NEAds were detected, and the patients in whom other viruses were detected.
Fecal specimen collection and delivery
The fecal specimens collected from all the subjects were immediately placed in an aseptic container and stored in a laboratory freezer below −20°C at each center. The specimen containers were placed directly into a delivery container containing packed dry ice and transported from each center to the laboratory (Seongnam, Korea) of Bioneer Co. (Daejeon, Korea) within 3–4 days of sampling, by the specialized medical sample delivery system of Bioneer Co.
Assessment and detection of viral pathogens
Extraction of viral nucleic acids
Viral nucleic acids were extracted from the fecal samples. The fecal samples were suspended in phosphate-buffered saline equivalent to 10 times their weight and centrifuged at 12,100 × g for 20 minutes. The supernatant was used for subsequent experiments. The viral nucleic acids in 400 μL of supernatant were extracted with the ExiPrep™ Dx Viral DNA/RNA Kit and the ExiPrep™ 16 Dx Automated Nucleic Acid Extraction System (Bioneer Co.).
Detection and serotyping of adenoviruses
RT-PCR was used to detect adenoviral subgroups A31, F40, and F41 using the Accupower Adenovirus Real-Time RT-PCR Kit® (Bioneer Co.). To detect other adenoviral subgroups, the partial hexon gene was amplified from the extracted DNA with the Accupower ProFi Taq PCR Premix Kit® (Bioneer Co.) and primers Ad1: 5′-TTCCCCATGGCICAYAACAC-3′ and Ad2: 5′-CCCTGGTAKCCRATRTTGTA-3′. The amplified DNA products were analyzed with gel electrophoresis on 1.5% agarose gels. The PCR cycling conditions were: one cycle of 95°C for 5 minutes, 30 cycles of 94°C for 1 minute, 54°C for 45 seconds, and 72°C for 2 minutes, followed by 72°C for 5 minutes. The PCR products were purified using the AccuPrep Gel Purification Kit® (Bioneer Co.). The adenovirus serotypes in the fecal samples from the adenovirus-positive children were determined with the direct DNA sequencing of the polymerase gene, the Basic Local Alignment Search Tool (BLAST) analysis, and the alignment with sequences from GenBank.
Detection of rotavirus, astrovirus, sapovirus, and norovirus
The extracted viral nucleic acids of astrovirus, sapovirus, norovirus, and rotavirus were detected with the Accupower Astro-Sapo Real-Time RT-PCR Kit®, the Accupower Norovirus Real-Time RT-PCR Kit®, and the Accupower Rotavirus RT-PCR Kit®. RT-PCR was performed on a PCR cycler (Exicycler™ 96 Real-Time Quantitative Thermal Block; Bioneer Co.) with the following cycling parameters: reverse transcriptase reaction at 45°C for 15 minutes, predenaturation at 95°C for 10 minutes, 45 cycles of denaturation at 95°C for 30 seconds and annealing/extension at 55°C for 30 seconds.
Analysis of seasonal epidemic trends in the fecal and respiratory AIALSR
The correlation between the seasonal epidemic trends in intussusception and each NEAd subgroup was analyzed with Spearman's correlation analysis.
We compared the seasonal trends in the incidence of intussusception with our NEAd results obtained with RT-PCR and with the WRAD (fecal) and WRIR (respiratory) adenovirus data. We divided the study period into 15 subperiods by 4-week, extending from week 23 in 2012 to week 30 in 2013. We analyzed the seasonal epidemic trends for the adenoviral subgroups in our data and the incidence of intussusception in these 15 subperiods. The adenoviral epidemic incidence data from WRAD and WRIR were also analyzed in these 15 subperiods.
The trends of the intussusception incidence, NEAd of case, AIALSR (WRAD and WRIR) adenovirus data and each NEAd subgroup (A, B, C, and E) were analyzed to investigate the correlation between each data and the incidence of intussusception, using Spearman's correlation analysis.
Statistical analysis
IBM SPSS ver. 23 (IBM Co., Armonk, NY, USA) was used for all statistical analyses. An independent two-sample t-test was used to analyze parametric continuous variables, and a cross-tabulation analysis with the χ2 test was used to analyze nominal variables. Because of our relatively small sample sizes, we used a nonparametric Spearman's correlation analysis to determine the correlation between the seasonal epidemic variations in the incidence of intussusception, the viruses detected in the case samples, and the AIALSR (WRAD and WRIR) virus data. For all analyses, a two-tailed P value of < 0.05 was considered significant.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board (IRB) of Gangneung Asan Hospital, Ulsan University College of Medicine (2012-020). And this study protocol was approved by each center's IRB, too. They were followings as Inje University Sanggye Paik Hospital (12-041), Ajou University Hospital (MED-SMP-12-167), Bundang Jesaeng General Hospital (PD 12-01), Dankook University Hospital (2012-05-007), Pusan National University Yangsan Hospital (05-2012-043), Ulsan University Hospital (UUH-IRB-13-050), and Kyungpook National University Hospital (KNUH 2012-06-004). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
Demographic data and clinical characteristics of cases and controls
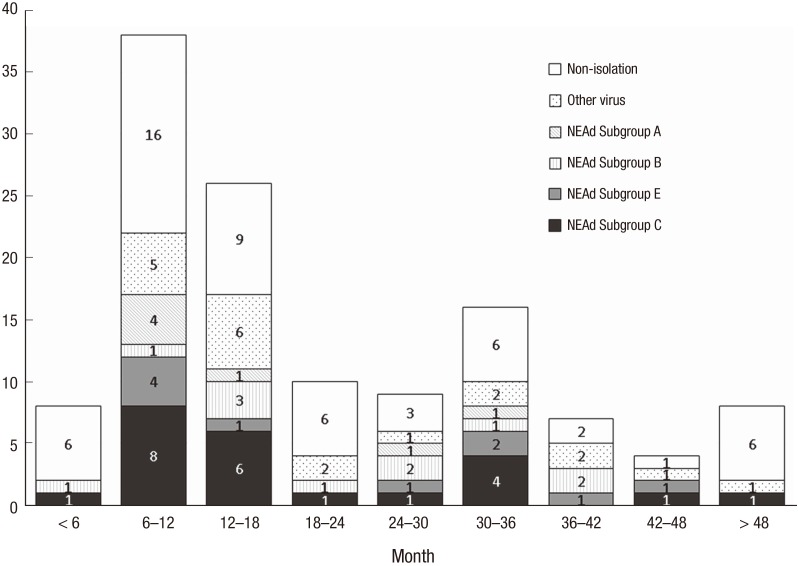
The mean ages were 1.74 ± 1.20 years in the cases and 1.66 ± 1.35 years in the controls, which were not significantly different (P = 0.622) (Table 1). There were two age peaks (6–18 months and 30–36 months) among the cases. NEAd subgroup C was the predominant pathogen in most age groups. The incidence of NEAd subgroup C did not differ significantly between the < 2-year-old group and the > 2-year-old group (P = 0.197; Fig. 1).
Table 1. Comparison of the demographic data and clinical manifestations of intussusception patients and controls.
| Characteristics | Intussusception (n = 126) | Control (n = 106) | P value |
|---|---|---|---|
| Age, yr | 1.74 ± 1.20 | 1.66 ± 1.35 | 0.622* |
| Male:female | 82:44 | 65:41 | 0.499† |
| Rotavirus vaccination | 81 (n = 119) | 64 (n = 96) | 0.884† |
| Previous history of intussusception | 13 (n = 125) | 0 (n = 106) | < 0.001† |
| Preceding symptoms | |||
| Fever | 16 (n = 117) | 34 (n = 104) | 0.001† |
| Upper respiratory infection‡ | 28 (n = 117) | 22 (n = 104) | 0.750† |
| Acute gastroenteritis§ | 37 (n = 117) | 86 (n = 104) | < 0.001† |
*Using the t-test; †Using the χ2 test; ‡Cough, sputum, rhinorrhea; §Vomiting, not bloody diarrhea, not colicky abdominal pain.
Fig. 1.
Cases of intussusceptions and the virus detected, according to age.
NEAd = nonenteric adenovirus.
The proportion of males did not differ significantly (P = 0.499) between the cases (65.1%) and the controls (61.3%) (Table 1). Nor did the rotavirus vaccination histories of the two groups differ significantly. A previous history of intussusception was significantly more frequent in the case group than in the control group (10.4% vs. 0%, P < 0.001). Fever (P = 0.001) and preceding gastroenteritis symptoms (P < 0.001) were more frequent among the controls than among the cases, but preceding upper respiratory infection symptoms did not differ significantly between the two groups (23.9% vs. 21.1%, P = 0.750) (Table 1).
Fecal virus detection with real-time PCR
Viruses were detected in 71 (56.3%) of the 126 intussusception-associated fecal samples. Among the 71 virus-positive samples, two species of viruses were detected in 14 samples and three species were detected in two samples, and thus 89 viruses were detected in the 71 samples. Viruses were detected in 65 (61.3%) of the 106 control fecal samples. Among these 65 virus-positive samples, two species of virus were detected in eight samples, and thus 73 viruses were detected in the 65 samples. The number of negative samples did not differ significantly between the case and control groups (43.7% vs. 38.7%, P = 0.504) (Table 2).
Table 2. Fecal viruses detected with real time RT-PCR in pediatric patients with intussusception.
| Virus information | Intussusception group | Control group | P value* |
|---|---|---|---|
| Virus identification | (n = 126) | (n = 106) | |
| Non-isolation | 55 (43.7 %) | 41 (38.7 %) | 0.504 |
| Adenoviridae | 53 (42.1 %) | 13 (12.3 %) | < 0.001 |
| Non-enteric | 51 (40.5 %) | 4 (3.8 %) | < 0.001 |
| Enteric | 2 (1.6 %) | 9 (8.5 %) | 0.025 |
| Norovirus | 16 (12.7 %) | 25 (23.6 %) | 0.038 |
| Rotavirus | 2 (1.6 %) | 20 (18.7 %) | < 0.001 |
| Astrovirus | 13 (10.3 %) | 7 (6.6 %) | 0.356 |
| Sapovirus | 3 (2.4 %) | 8 (7.5 %) | 0.117 |
| Adenoviridae subgroup | (n = 53) | (n = 13) | |
| Non-enteric adenovirus | |||
| A31 | 7 | 1 | 0.074 |
| B | 11 | 1 | 0.007 |
| B3 | 9 | 1 | 0.023 |
| B35 | 1 | 0 | |
| B55 | 1 | 0 | |
| C | 23 | 1 | < 0.001 |
| C1 | 9 | 1 | 0.023 |
| C2 | 7 | 0 | |
| C5 | 5 | 0 | |
| C6 | 2 | 0 | |
| E4 | 10 | 1 | 0.013 |
| Enteric adenovirus | |||
| F41 | 2 | 9 | 0.025 |
Values are presented as number (%). Number of viruses detected in intussusception patients (89 viruses) and controls (73 viruses).
RT-PCR = reverse transcriptase-polymerase chain reaction.
*Using the χ2 test.
Members of the family Adenoviridae were the predominant pathogens in the children with intussusception, and were detected significantly more often in the cases than in the controls (42.1% vs. 12.3%, P < 0.001) (Table 2). NEAds (subgroups A, B, C, and E) were detected considerably more often in the patients with intussusception than in the controls (40.5% vs. 3.8%, P < 0.001), whereas enteric adenoviruses (subgroup F) were more often detected in the controls than in the cases (8.5% vs. 1.6%, P = 0.025). Among the NEAds, subgroup C was predominant (23/126 vs. 1/106, P < 0.001) and subgroups B (11/126 vs. 1/106, P = 0.007) and E (10/126 vs. 1/106, P = 0.013) were statistically significantly more frequent in the intussusception patients than in the controls. NEAd subgroup A was more often detected in the cases than in the controls, but not significantly so (7/126 vs. 1/106, P = 0.074). Rotavirus (18.7% vs. 1.6%, P < 0.001) and norovirus (23.6% vs. 12.7%, P = 0.038) were detected significantly more often in the controls than in the cases, but the incidences of astrovirus and sapovirus did not differ significantly between the two groups.
Demographic data and clinical characteristics of cases with no virus, NEAds, or another enteritis viruses
The patients with intussusception were divided into three groups: those in whom no virus was detected (group 1, n = 55), those in whom NEAds were detected (group 2, n = 51), and those in whom other enteritis viruses were detected (group 3, n = 20) (Table 3). No demographic or clinical characteristics differed significantly between groups 1 and 2 or between groups 2 and 3.
Table 3. Clinical manifestations of intussusception patients in whom NEAds, other viruses, or no viruses were detected.
| Characteristics (respondent) | Group 1 (n = 55; no viruses) |
Group 2 (n = 51; NEAds) |
Group 3 (n = 20; other viruses*) |
P value | |
|---|---|---|---|---|---|
| Group 1 vs. 2 | Group 2 vs. 3 | ||||
| Age, yr (n = 126) | 1.76 ± 1.40 | 1.68 ± 1.00 | 1.86 ± 1.14 | 0.724† | 0.506† |
| Male (n = 126) | 35 | 33 | 14 | 1.000‡ | 0.784‡ |
| Rotavirus vaccination (n = 119) | 38 (n = 51) | 32 (n = 48) | 11 (n = 20) | 0.508‡ | 0.415‡ |
| Previous history of intussusception | 8 (n = 55) | 3 (n = 50) | 2 (n = 20) | 0.200‡ | 0.619‡ |
| Preceding symptoms (n = 117) | |||||
| Fever | 6 (n = 48) | 8 (n = 49) | 2 (n = 20) | 0.774‡ | 1.000‡ |
| Upper respiratory infection§ | 9 (n = 48) | 15 (n = 49) | 4 (n = 20) | 0.240‡ | 0.122‡ |
| Acute gastroenteritis‖ | 18 (n = 48) | 12 (n = 49) | 7 (n = 20) | 0.192‡ | 0.389‡ |
| Type (n = 126) | |||||
| Ileocolic type | 53 | 48 | 16 | 0.670‡ | 0.092‡ |
| Ileoileal | 1 | 0 | 2 | - | - |
| Colon to colon | 1 | 3 | 2 | - | - |
| Reduction (n = 126) | |||||
| Enema | 52 | 48 | 19 | 1.000‡ | 1.000‡ |
| Surgical | 2 | 2 | 0 | - | - |
| Self-reduction | 1 | 1 | 1 | - | - |
NEAds = nonenteric adenoviruses.
*Other viruses consisted of enteric adenovirus, rotavirus, norovirus, astrovirus, and sapovirus; †Using the t-test; ‡Using the χ2 test; §Cough, sputum, rhinorrhea; ‖Vomiting, not bloody diarrhea, not colicky abdominal pain.
Comparison of our seasonal epidemic trends in adenovirus with AIALSR data
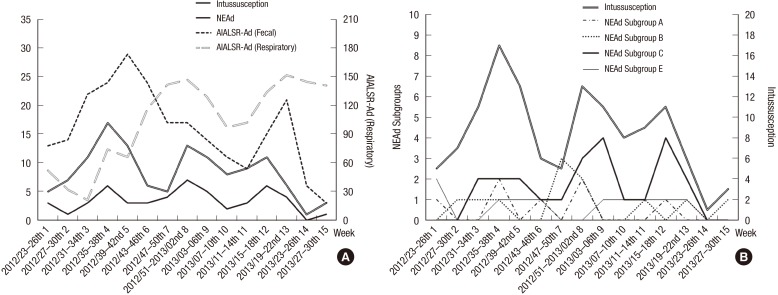
We compared the seasonal epidemic trends in our NEAd data with respiratory and fecal adenoviral data from the WRIR and WRAD in the AIALSR. Not respiratory (WRIR) but fecal (WRAD) adenovirus epidemic data were similar to epidemic trend of NEAd and the incidence of intussusception (Fig. 2A). The seasonal trends in the incidence of intussusception and in the presence of NEAd showed three congruent seasonal peaks (Fig. 2A). All these peaks occurred within two WRAD adenoviral epidemic periods (weeks 31–46 in 2012 and weeks 15–22 in 2013).
Fig. 2.
Comparisons of the seasonal epidemic trends. (A) Adenovirus data from AIALSR, intussusception, and NEAd, and (B) intussusception and NEAd subgroups.
AIALSR = Acute Infectious Agents Laboratory Surveillance Report, NEAd = nonenteric adenovirus, Ad =adenovirus.
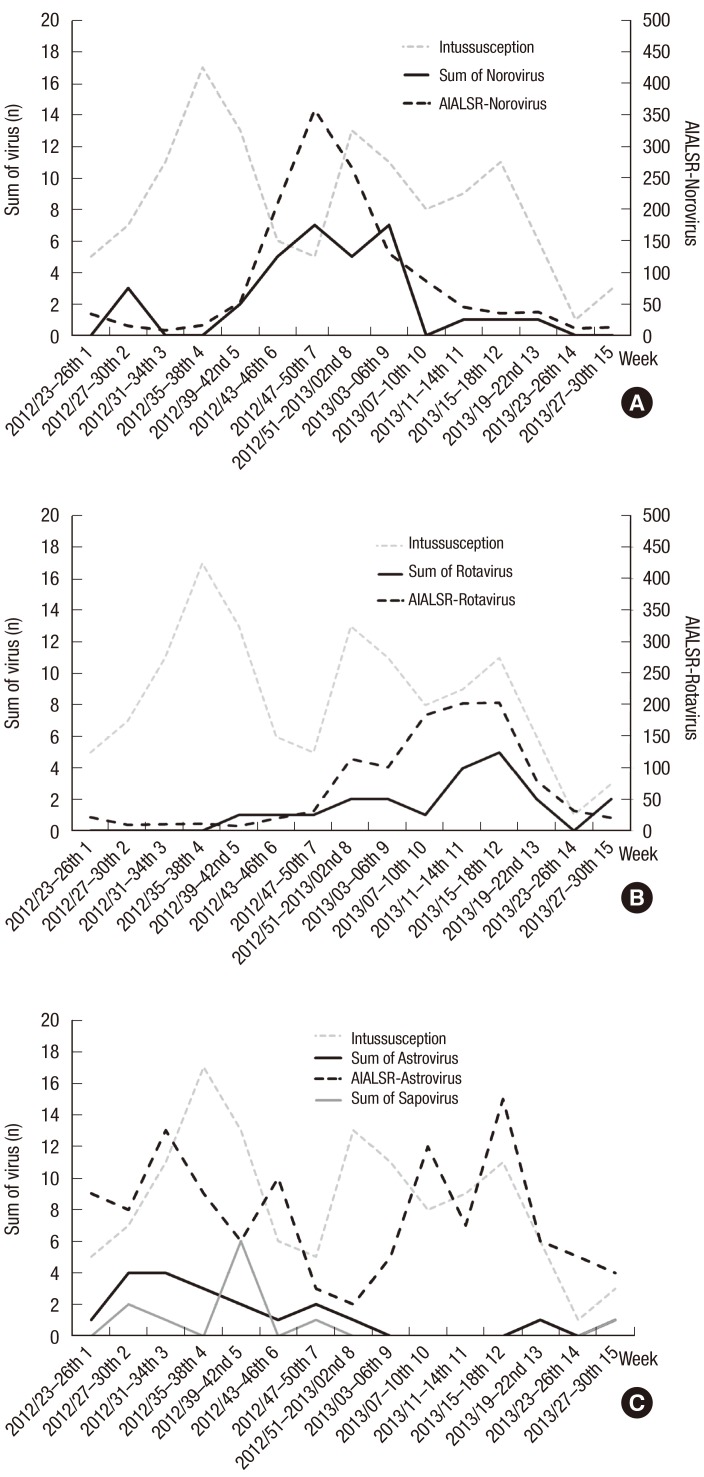
Adenoviral subgroups B and E were detected significantly more often in the case group than in the control group with RT-PCR. However, only subgroup C correlated with intussusception epidemics, in contrast to the relatively perennial trends in subgroups B and E (Fig. 2B). The trends in rotavirus and norovirus in our data were also similar to the corresponding trends reported in the WRAD (Fig. 3). The seasonal trend in rotavirus (Fig. 3A) showed one distinctive prominent epidemic peak in weeks 7–26 in 2013, which was similar to the rotaviral seasonal trend in the WRAD rotavirus data (Fig. 3C). The seasonal trend in norovirus (Fig. 3A) also showed one distinctive prominent epidemic peak from week 43, 2012, to week 6, 2013, which was similar to the noroviral seasonal trend in the WRAD norovirus data (Fig. 3C). However, the trend for astrovirus in our data, which had one epidemic peak, did not correlate with the WRAD astroviral data. Sapovirus was only detected in this study (Fig. 3B), and not in the WRAD.
Fig. 3.
Seasonal epidemic trends in each virus in the fecal AIALSR data and our results (sum of cases and controls). (A) Norovirus, (B) rotavirus, and (C) astrovirus and sapovirus in this study.
AIALSR = Acute Infectious Agents Laboratory Surveillance Report.
Correlations between the incidence of intussusception and adenoviral subgroups
Spearman's analysis was used to correlate the trends in the incidence of intussusception and the seasonal variations in viral epidemics based on the adenoviral data from our RT-PCR results and the AIALSR data, after the study period was divided into 15 subperiods (Table 4). The correlations between the incidence of intussusception and the trends in total NEAds (r = 0.635; P = 0.011), NEAd subgroup C (r = 0.776; P = 0.001), and in the WRAD adenoviruses (r = 0.572; P = 0.026) were all statistically significant. However, the correlation between the incidence of intussusception and epidemic trends in the WRIR adenovirus was not significant (r = −0.298; P = 0.280). Also, the WRIR adenovirus did not show the correlation with any trend of total NEAds (r = 0.272; P = 0.327) or NEAd subgroup C (r = 0.173; P =0.538).
Table 4. Correlation of seasonal epidemic variations in intussusception, NEAd subgroups, and AIALSR adenovirus data (fecal and respiratory), determined with Spearman's correlation analysis.
| Characteristics | Intussusception | Case | AIALSR Ad | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total NEAd | Ad subgroup | Fecal | Resp | |||||||||
| A | B | C | E | EAd (F) | ||||||||
| Intussusception | r | 1 | ||||||||||
| P | - | |||||||||||
| Case | Total NEAd | r | 0.635 | 1 | ||||||||
| P | 0.011 | - | ||||||||||
| Ad subgroup | A | r | 0.373 | 0.591 | 1 | |||||||
| P | 0.171 | 0.020 | - | |||||||||
| B | r | 0.060 | 0.259 | −0.007 | 1 | |||||||
| P | 0.832 | 0.351 | 0.980 | - | ||||||||
| C | r | 0.776 | 0.831 | 0.283 | 0.004 | 1 | ||||||
| P | 0.001 | 0.000 | 0.307 | 0.989 | - | |||||||
| E | r | 0.201 | 0.255 | 0.289 | −0.587 | 0.202 | 1 | |||||
| P | 0.471 | 0.359 | 0.296 | 0.021 | 0.471 | - | ||||||
| EAd (F) | r | −0.156 | −0.063 | 0.296 | −0.270 | −0.095 | 0.174 | 1 | ||||
| P | 0.580 | 0.823 | 0.283 | 0.330 | 0.735 | 0.534 | - | |||||
| AIALSR Ad | Fecal | r | 0.572 | 0.520 | 0.340 | 0.098 | 0.537 | 0.157 | 0.341 | 1 | ||
| P | 0.026 | 0.047 | 0.215 | 0.729 | 0.039 | 0.575 | 0.213 | - | ||||
| Resp | r | −0.298 | 0.272 | 0.032 | 0.228 | 0.173 | −0.231 | 0.000 | −0.186 | 1 | ||
| P | 0.280 | 0.327 | 0.910 | 0.413 | 0.538 | 0.407 | 1.000 | 0.506 | - | |||
Significant findings at P < 0.05 are in bold.
NEAd = nonenteric adenovirus, EAd = enteric adenovirus, Ad = adenovirus, r = correlation coefficient, AIALSR=Acute Infectious Agents Laboratory Surveillance Report, Resp = respiratory.
The epidemic trend in total NEAds correlated significantly with NEAd subgroup C (r = 0.831; P = 0.000) and subgroup A (r = 0.591; P = 0.020). The epidemic trend in NEAd subgroup C also correlated significantly with the trends in the WRAD adenoviruses (r = 0.537; P = 0.039), but not correlated with WRIR adenovirus trend (r = 0.173; P = 0.538). In contrast, the epidemic trends in NEAd subgroups B and E did not correlate significantly with any trends in intussusception, total NEAds, the WRAD or WRIR adenovirus. The seasonal trends in enteric adenovirus (subgroup F) did not correlate with the incidence of intussusception (r = −0.156; P = 0.580), adenovirus data from WRAD (r = 0.341; P = 0.213) or WRIR (r = 0.000; P = 1.000).
DISCUSSION
The aim of this annual multicenter case-control study was a comparative analysis of the etiology and epidemic correlations between subgroups of the family Adenoviridae and pediatric idiopathic intussusception. Importantly, a multicenter study minimizes any regional endemic selection bias in a study of epidemic pathogens. Annual observations also reduce the bias caused by seasonal epidemic variations.
The proportion of males did not differ significantly between the intussusception and control patients (65.1% vs. 61.3%, P = 0.499) (Table 1). Although the proportion of males was reported to be about 70% in a textbook (1), in two Asian studies based on national health data, the proportions of males with childhood intussusception were 62.5% (24) and 66.3% (25), which are more similar to our result. The proportion of males in the control group was 61.3% because the controls were selected to match the sexes of the patients with intussusception. In this study, 10.3% of intussusception patients had a previous history of intussusception, whereas none in the control group had a previous history of intussusception. Intussusception is known to recur at a rate of about 10%–20% (26,27). Because we used patients with acute gastroenteritis as the controls, fever and preceding acute gastroenteritis symptoms were more common in the controls than in the cases. However, preceding symptoms of upper respiratory infection did not differ significantly between the two groups (Table 1).
Several other studies have shown that adenoviruses are the viruses predominantly detected in intussusception (3,10,11,12,17,18,19). In this study, adenoviruses were the pathogens predominantly associated with intussusception, and were detected significantly more often in patients with intussusception than in the controls (42.1% vs. 12.3%, P < 0.001) (Table 2). A 1-year observational study showed a similar result, in that 50% of cases of intussusception were caused by a virus in the intestinal epithelium, and the predominant virus (33%) was a member of the Adenoviridae (28). Another study reported similar results, with 36 (50.7%) of 71 fecal sample from intussusception patients positive for adenovirus (12). In an analysis of fecal adenoviruses in intussusception patients, adenovirus was detected in 34% of patients in Vietnam (odds ratio [OR], 8.2) and in 40% of patients in Australia (OR, 44) (18). In a Taiwanese study, NEAds were detected 44.3% of intussusception patients but in only 3.8% of healthy controls (10). Similarly, NEAds were detected significantly more often in patients with intussusception than in the controls in this study (40.5% [51/126] vs. 3.8% [4/106], P < 0.001). Our results show that 96.3% (51/53) of the adenovirus associated with intussusception were NEAds. Intussusception has been particularly associated with adenoviral types 1, 2, 5, and 6, all belonging to NEAd subgroup C (3,19,29,30). In our adenoviral subgroup analysis, the predominant subgroup was also adenoviral subgroup C, and 45.1% (23/51) of the NEAds detected were subgroup C in the NEAd-positive patients with intussusception.
In contrast, adenovirus was detected in only 12.3% (13/106) of the controls; 30.8% (4/13) of these adenoviruses were NEAds, and only 7.7% (1/13) of the NEAds in the controls were adenoviral subgroup C.
Subgroup C was the NEAd subgroup most frequently detected (P < 0.001) in this study. NEAd subgroups A, B, and E were also detected more often in the cases than the controls, but only subgroups B (20.8%, 11/53) and E (18.9%, 10/53) showed statistical significance in adenovirus-positive intussusception. NEAd subgroup B has been detected in several previous intussusception studies (3,12,19). Okimoto et al. (12) detected NEAd subgroup B in patients with intussusception (30.6%, 11/36), although it is unclear whether it was a perennial or outbreak epidemic pattern in that study. Two Korean studies identified subgroup B in 2.3% (1/44) (19) and 6.3% (2/32) (3) of intussusception patients. It seems that the discrepancies in the incidence of subgroup B reported in previous studies were caused by a viral epidemic event during the study period. Only few studies have examined the association between NEAd subgroup E and intussusception. NEAd subgroup E is associated with respiratory infections and keratoconjunctivitis (31). A lower incidence than other NEAd subgroups is characteristic of subgroup E (22,32), which constitutes about 1%–3% of all adenoviral infections. In the present study, the incidence of NEAd subgroup E was low (0.95%, 1/106) in the control group, as in previous studies, but it was detected significantly frequently in the intussusception patients (7.9%, 10/126). Another characteristic of NEAd subgroup E is that its epidemic period (< 1 month) is shorter than those of the other adenoviral subgroups (32). Its short-term epidemics and low incidence of infection are insufficient to show an epidemic trend, especially if the sample is small, as in this study.
NEAd subgroup C was the predominant pathogen among NEAd subgroups, consistently across most age groups (Fig. 1). A Japanese study reported that NEAd is a significant risk factor for intussusception in children, particularly those aged > 2 years (12). However, in our study, the incidence of NEAd subgroup C did not differ significantly between children < 2 years old and those > 2 years old (P = 0.197) (Fig. 1).
Although a proportion of NEAd-infected intussusception patients had preceding upper respiratory symptoms, the clinical manifestations of the intussusception patients infected with different pathogens did not differ significantly in this study (Table 3). Therefore, clinical respiratory symptoms were insufficient to distinguish the involvement of different viral species in intussusception, although the predominant adenoviruses were NEAds, which also cause respiratory infections.
In this study, the seasonal epidemic trends correlated well with our RT-PCR results and with the WRAD data for all viruses (adenovirus, rotavirus, and norovirus), except astrovirus (Figs. 2 and 3). This confirms that our fecal sampling properly reflected the annual epidemic variations in these viruses. The seasonal epidemic trends in the incidence of intussusception and NEAds displayed congruent seasonal peaks, whereas the peaks of norovirus, rotavirus, and astrovirus were never congruent with the peaks of intussusception (Fig. 3).
In this study, the incidence intussusception also increased during the periods of the WRAD-recorded adenovirus epidemics (Fig. 2A). The epidemic episodes of intussusception and NEAds were observed in September 2012 (subperiod No. 4), mid-December 2012, to mid-January 2013 (subperiod No. 8), and April 2013 (subperiod No. 12). All of these peaks in NEAd corresponded to two adenoviral epidemic peaks recorded in the WRAD. The correlations between the seasonal variations in the incidence of intussusception and each virus were analyzed with Spearman's correlation analysis (Table 4). Although both NEAd subgroup C and subgroups B and E were significantly associated with intussusception in comparative analysis (Table 2), only the epidemic trend in subgroup C correlated with the trend in the incidence of intussusception. The epidemic trends in subgroups B and E did not correspond to outbreaks, but formed relatively perennial patterns (Fig. 2B). We also confirmed these discrepancies with a Spearman's correlation analysis. In the correlation with NEAd, the epidemic trend in NEAd subgroup C and subgroup A correlated with that of NEAd. We guess that epidemic trend of NEAd was influenced by the epidemic episodes of subgroup A and subgroup C (Fig. 2B). And the epidemic trend in NEAd subgroup C correlated significantly with the trend in the WRAD adenoviruses.
In the correlation with the incidence of intussusception, the trends in total NEAds, NEAd subgroup C, and the WRAD adenoviruses correlated statistically significantly (Table 4). However, WRIR adenovirus showed insignificant correlation with total NEAds, NEAd subgroup C and the incidence of intussusception. We guess that the incidence of intussusception is associated with not respiratory but gastrointestinal involved NEAd.
Our study had several limitations. One of the major limitations was that we could not avoid the loss of a large proportion of intussusception patients. Because many intussusception patients were immediately discharged from the emergency room after reduction, we could not obtain samples from the patients or consent from their parents. Second, the WRAD data do not refer specifically to NEAds but data on general adenoviruses. A comparison of our NEAd results with nationwide NEAd data might allow a more accurate epidemic correlation analysis. Unfortunately, we could not obtain nationwide NEAd data. Finally, although both NEAd subgroup C and subgroups B and E were statistically significantly associated with intussusception in comparative analysis (Table 2), only the epidemic trend in NEAd subgroup C correlated significantly with the trend in the incidence of intussusception. The epidemic trends in subgroups B and E did not indicate outbreaks, but relatively perennial patterns. We attribute this to our small sample size or the lack of subgroup B and E epidemic events during our study period. The detection of NEAd subgroup A correlated marginally significantly with the incidence of intussusception. We anticipate that in future large-scale studies, this correlation with subgroup A will be significant. We hope that future studies will be based on larger samples across long periods that include epidemic episodes involving these NIEAd subgroups. The identification of a correlation between NEAd subgroup B or E and the incidence of intussusception will require a larger sample size and longer study period, including viral epidemic episodes, than were analyzed in this study.
In conclusion, we suggest that NEAd is the major risk factor for intussusception, based on both an RT-PCR analysis and annual epidemiological trends. The seasonal trend in the incidence of intussusception correlated significantly with the trends in NEAds and the WRAD adenoviruses. Only the epidemic trend in NEAd subgroup C correlated significantly with the incidence of intussusception in correlation analysis, although subgroups B, C and E correlated statistically significantly with the intussusception in comparative analysis. Therefore, we suggest that the nationwide adenoviral data from the WRAD presented in the AIALSR could be used as an approximate epidemic predictor of intussusception, a critical medical condition in infancy and childhood requiring urgent attention.
ACKNOWLEDGMENT
On behalf of all authors, I thank to all contributors who supported this study in eight centers, Korea Centers for Disease Control and Prevention, and Bioneer Co.
This study was performed in collaboration of the Inje University Sanggye Paik Hospital, Ajou University Hospital, Bundang Jesaeng General Hospital, Gangneung Asan Hospital, Dankook University Hospital, Pusan National University Yangsan Hospital, Ulsan University Hospital, and Kyungpook National University Hospital.
Footnotes
Funding: This work was funded by Gangneung Asan Hospital Biomedical Research Center Promotion Fund (grant number: 2012-003).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Chung JY, Choe BH, Park KY. Data curation: Jang J, Lee YJ, Kim JS, Chung JY, Chang S, Lee K, Choe BH, Hong SJ, Park KY. Formal analysis: Jang J, Song JS, Park KY. Funding acquisition: Park KY. Investigation: Jang J, Song JS, Park KY. Writing - original draft: Jang J, Park KY. Writing - review & editing: Jang J, Lee YJ.
References
- 1.Kennedy M, Liacouras CA. Intussusception. In: Kliegman RM, Stanton BF, St. Geme JW 3rd, Schor NF, editors. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016. pp. 1812–1814. [Google Scholar]
- 2.Bell TM, Steyn JH. Viruses in lymph nodes of children with mesenteric adenitis and intussusception. BMJ. 1962;2:700–702. doi: 10.1136/bmj.2.5306.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JS, Lee SK, Ko DH, Hyun J, Kim HS, Song W, Kim HS. Associations of adenovirus genotypes in Korean acute gastroenteritis patients with respiratory symptoms and intussusception. Biomed Res Int. 2017;2017:1602054. doi: 10.1155/2017/1602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore SW, Kirsten M, Müller EW, Numanoglu A, Chitnis M, Le Grange E, Banieghbal B, Hadley GP. Retrospective surveillance of intussusception in South Africa, 1998–2003. J Infect Dis. 2010;202(Suppl):S156–S161. doi: 10.1086/653563. [DOI] [PubMed] [Google Scholar]
- 5.Bruce J, Huh YS, Cooney DR, Karp MP, Allen JE, Jewett TC., Jr Intussusception: evolution of current management. J Pediatr Gastroenterol Nutr. 1987;6:663–674. [PubMed] [Google Scholar]
- 6.Pang LC. Intussusception revisited: clinicopathologic analysis of 261 cases, with emphasis on pathogenesis. South Med J. 1989;82:215–228. [PubMed] [Google Scholar]
- 7.Arbizu RA, Aljomah G, Kozielski R, Baker SS, Baker RD. Intussusception associated with adenovirus. J Pediatr Gastroenterol Nutr. 2014;59:e41. doi: 10.1097/MPG.0b013e3182868971. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, et al. Rotavirus Intussusception Investigation Team Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 9.El-Hodhod MA, Nassar MF, Ezz El-Arab S, Ahmed EF. Rotavirus fecal antigen retrieval in infantile intussusception. Eur J Clin Microbiol Infect Dis. 2008;27:879–881. doi: 10.1007/s10096-008-0506-6. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HY, Kao CL, Huang LM, Ni YH, Lai HS, Lin FY, Chang MH. Viral etiology of intussusception in Taiwanese childhood. Pediatr Infect Dis J. 1998;17:893–898. doi: 10.1097/00006454-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Aminu M, Ameh EA, Geyer A, Esona MD, Taylor MB, Steele AD. Role of astrovirus in intussusception in Nigerian infants. J Trop Pediatr. 2009;55:192–194. doi: 10.1093/tropej/fmn101. [DOI] [PubMed] [Google Scholar]
- 12.Okimoto S, Hyodo S, Yamamoto M, Nakamura K, Kobayashi M. Association of viral isolates from stool samples with intussusception in children. Int J Infect Dis. 2011;15:e641–e645. doi: 10.1016/j.ijid.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Khalifa AB, Jebali A, Kedher M, Trabelsi A. Infectious etiology of acute idiopathic intussusception in children. Ann Biol Clin (Paris) 2013;71:389–393. doi: 10.1684/abc.2013.0859. [DOI] [PubMed] [Google Scholar]
- 14.Kaemmerer E, Tischendorf JJ, Steinau G, Wagner N, Gassler N. Ileocecal intussusception with histomorphological features of inflammatory neuropathy in adenovirus infection. Gastroenterol Res Pract. 2009;2009:579501. doi: 10.1155/2009/579501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhabra P, Payne DC, Szilagyi PG, Edwards KM, Staat MA, Shirley SH, Wikswo M, Nix WA, Lu X, Parashar UD, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis. 2013;208:790–800. doi: 10.1093/infdis/jit254. [DOI] [PubMed] [Google Scholar]
- 16.Jakab F, Péterfai J, Verebély T, Meleg E, Bányai K, Mitchell DK, Szûcs G. Human astrovirus infection associated with childhood intussusception. Pediatr Int. 2007;49:103–105. doi: 10.1111/j.1442-200X.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- 17.Lappalainen S, Ylitalo S, Arola A, Halkosalo A, Räsänen S, Vesikari T. Simultaneous presence of human herpesvirus 6 and adenovirus infections in intestinal intussusception of young children. Acta Paediatr. 2012;101:663–670. doi: 10.1111/j.1651-2227.2012.02616.x. [DOI] [PubMed] [Google Scholar]
- 18.Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, de Campo M, Barnett P, Bishop RF, Robins-Browne R, Carlin JB, Intussusception Study Group Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr. 2006;149:452–460. doi: 10.1016/j.jpeds.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Lee YW, Yang SI, Kim JM, Kim JY. Clinical features and role of viral isolates from stool samples of intussuception in children. Pediatr Gastroenterol Hepatol Nutr. 2013;16:162–170. doi: 10.5223/pghn.2013.16.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korea Centers for Disease Control & Prevention. Weekly viral epidemic reports of acute diarrheal disease: no. 213–278 [Internet] [accessed on 6 June 2017]. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0503.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0048-MNU0051<fid=478<q_type=<q_value=&pageNum=
- 21.Korea Centers for Disease Control & Prevention. Weekly viral epidemic report of influenza and respiratory virus: no. 209–268 [Internet] [accessed on 6 June 2017]. Available at http://www.cdc.go.kr/CDC/info/CdcKrInfo0502.jsp?menuIds=HOME001-MNU1154-MNU0005-MNU0048-MNU0050&fid=477&q_type=&q_value=&pageNum=26.
- 22.Liu L, Qian Y, Zhang Y, Deng J, Jia L, Dong H. Adenoviruses associated with acute diarrhea in children in Beijing, China. PLoS One. 2014;9:e88791. doi: 10.1371/journal.pone.0088791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JI, Lee GC, Chung JY, Han TH, Lee YK, Kim MS, Lee CH. Detection and molecular characterization of adenoviruses in Korean children hospitalized with acute gastroenteritis. Microbiol Immunol. 2012;56:523–528. doi: 10.1111/j.1348-0421.2012.00469.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen SC, Wang JD, Hsu HY, Leong MM, Tok TS, Chin YY. Epidemiology of childhood intussusception and determinants of recurrence and operation: analysis of national health insurance data between 1998 and 2007 in Taiwan. Pediatr Neonatol. 2010;51:285–291. doi: 10.1016/S1875-9572(10)60055-1. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi M, Osamura T, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S. Intussusception among Japanese children: an epidemiologic study using an administrative database. BMC Pediatr. 2012;12:36. doi: 10.1186/1471-2431-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang CM, Hsu HY, Tsao PN, Chang MH, Lin FY. Recurrence of intussusception in childhood. Acta Paediatr Taiwan. 2001;42:158–161. [PubMed] [Google Scholar]
- 27.Wang GD, Liu SJ. Enema reduction of intussusception by hydrostatic pressure under ultrasound guidance: a report of 377 cases. J Pediatr Surg. 1988;23:814–818. doi: 10.1016/s0022-3468(88)80229-x. [DOI] [PubMed] [Google Scholar]
- 28.Nicolas JC, Ingrand D, Fortier B, Bricout F. A one-year virological survey of acute intussusception in childhood. J Med Virol. 1982;9:267–271. doi: 10.1002/jmv.1890090404. [DOI] [PubMed] [Google Scholar]
- 29.Guarner J, de Leon-Bojorge B, Lopez-Corella E, Ferebee-Harris T, Gooding L, Garnett CT, Shieh WJ, Dawson J, Erdman D, Zaki SR. Intestinal intussusception associated with adenovirus infection in Mexican children. Am J Clin Pathol. 2003;120:845–850. doi: 10.1309/LBRN-GF9M-JW2M-HT97. [DOI] [PubMed] [Google Scholar]
- 30.Selvaraj G, Kirkwood C, Bines J, Buttery J. Molecular epidemiology of adenovirus isolates from patients diagnosed with intussusception in Melbourne, Australia. J Clin Microbiol. 2006;44:3371–3373. doi: 10.1128/JCM.01289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghebremedhin B. Human adenovirus: viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp) 2014;4:26–33. doi: 10.1556/EuJMI.4.2014.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelboim M, Dror P, Azar R, Bromberg M, Mendelson E. Adenovirus infections in hospitalized patients in Israel: epidemiology and molecular characterization. J Clin Microbiol. 2011;49:597–601. doi: 10.1128/JCM.00979-10. [DOI] [PMC free article] [PubMed] [Google Scholar]