Abstract
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTCL) is a rare type of lymphoma that accounts for only 5%–18% of all cases of non-Hodgkin lymphoma (NHL). In published series, 60%–90% of NK/T-cell lymphomas are localized to the nasal and upper airway. We describe a 55-year man who presented with cough, sputum, dyspnea on exertion, and a chest computed tomography scan shows diffuse ground glass opacities (GGOs), suggestive of an interstitial lung disease. He was treated with a corticosteroid and his symptoms improved. However, when the corticosteroid was tapered, his symptoms recurred. The patient underwent a surgical lung biopsy and ENKTCL was diagnosed. We present this case because ENKTCL involving only the lung is very rare but very informative. To our knowledge, our patient is the first case that primary pulmonary ENKTCL is presented with GGOs.
Keywords: Lymphoma, Extranodal NK-T-Cell; Lung Involvement; Lung Diseases, Interstitial; Ground Glass Opacities
Graphical Abstract
INTRODUCTION
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTCL) is a rare entity of non-Hodgkin lymphoma (NHL) that typically involves the nasal cavity and upper aerodigestive tract. However, it can also occur at extranasal sites (the skin, liver, spleen, gastrointestinal tract, bone marrow, lung, and so on). A primary presentation of ENKTCL in the lung and ENKTCL involving only the lung is rare (1,2). Furthermore, there is no report which primary pulmonary ENKTCL is presented with ground glass opacities (GGOs). Here, we describe a case of primary pulmonary ENKTCL presenting with pulmonary symptoms and GGOs.
CASE DESCRIPTION
A 55-year-old man without any past medical history presented with cough, sputum, and dyspnea on exertion for three months in March, 2015. He had a smoking history of 30 pack-years, and had quit smoking since his dyspnea developed. On physical examination, he appeared acutely ill, and lung sounds were decreased in bilateral lung fields. He had no peripheral lymphadenopathy, organomegaly, or skin lesion. Laboratory findings were within normal ranges, except for mild elevations of aspartate transaminase (AST) and alanine transaminase (ALT) (AST, 50 IU/L; ALT, 54 IU/L). An arterial blood gas analysis showed mild hypoxia (partial pressure of arterial oxygen, 60.3 mmHg; partial pressure of arterial carbon dioxide, 31.0 mmHg; and pH, 7.455). The result of a pulmonary function test shows decreased diffusing capacity of the lungs for carbon monoxide (DLCO; 5.02 mmol/min/kPa [58.66% of the predicted value]), forced vital capacity (FVC; 3.15 L [85.74% of the predicted value]), forced expiratory volume in 1 second (FEV1; 2.31 L [77.71% of the predicted value]), and a ratio of FEV1/FVC (73.15).
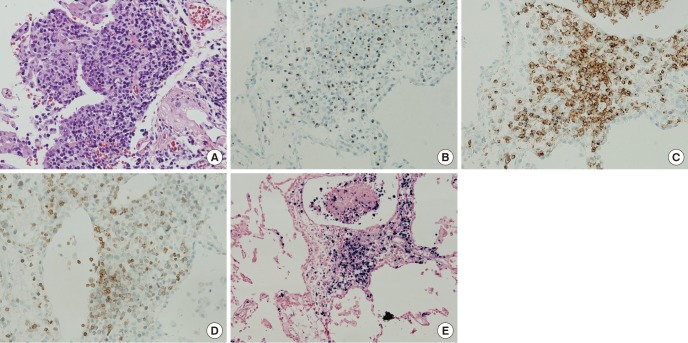
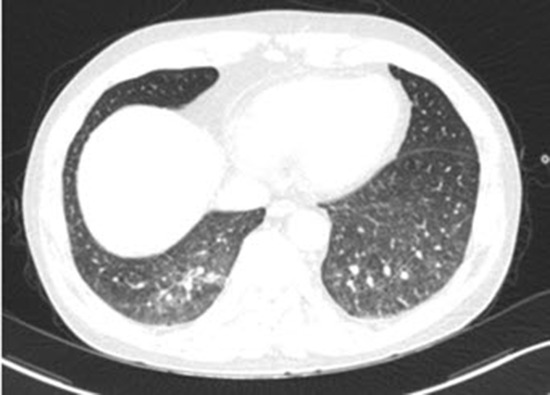
His initial computed tomography (CT) scan shows diffuse GGOs in both lungs with underlying emphysema (Fig. 1). Bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB) via a flexible fiberoptic bronchoscope were performed. The BAL fluid contained 55% lymphocytes with a CD8/CD4 ratio of 1:1, 7% eosinophils, and pathology obtained from the TBLB showed multifocal interstitial and perivascular inflammatory infiltrates, which were predominantly lymphocytes. There was no evidence of infection in the results of TBLB and BAL. Although the pathology results were not diagnostic, and definite exposure to a known offending antigen did not exist, interstitial lung disease (ILD) such as hypersensitivity pneumonitis (HP) or desquamative interstitial pneumonia (DIP) was highly suggested based on the results of the CT scan. With the impression of ILD, he was treated with a corticosteroid (prednisolone 60-mg daily; Cima Labs Inc., Eden Prairie, MN, USA). His symptoms and imaging were improved at the beginning of corticosteroid treatment, but when the corticosteroid was tapered, his symptoms recurred and his imaging worsened. After three months of corticosteroid treatment, he took a follow-up CT scan and it shows diffuse GGOs aggravated compared to initial CT scan (Fig. 1). Therefore, the patient underwent wedge resections of the lung under video-assisted thoracoscopic surgery (VATS) for a definite diagnosis. Wedge resections were done at lateral segment of right middle lobe and posterior basal segment of right lower lobe. The specimen revealed diffuse lymphomatous infiltration, which was composed of medium-sized cells. Immunohistochemically, these cells showed positivity for CD56, granzyme B, CD2, and loss of surface CD3 expression. In situ hybridization for Epstein-Barr virus (EBV) encoded small nuclear RNAs (EBERs) was positive (Fig. 2). The pathology result was consistent with ENKTCL. Since most ENKTCL originates from the nose and paranasal area, including the upper aerodigestive tract, the patient underwent a further imaging work-up (paranasal sinus CT, neck CT, and positron emission tomography-CT [PET-CT]) after pathologic confirmation, even though he did not have any symptom or sign of nose or paranasal area involvement (3,4). PET-CT scan showed no significant fluorodeoxyglucose (FDG) uptake to suggest malignancy (Fig. 3). The only organ that ENKTCL involved was the lung. The patient was treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) chemotherapy.
Fig. 1.

Follow-up CT scan shows diffuse GGOs at both lungs with underlying emphysema. Arrows indicate sites of wedge resections. Posterior basal segment of right lower lobe (A) and lateral segment of right middle lobe (B). (A, B) Axial view. (C) Coronal view.
CT = computed tomography, GGOs = ground glass opacities.
Fig. 2.
Histopathology of the lung biopsy. (A) Angiocentric infiltration of the lung parenchyme (magnification × 200). (B) Neoplastic cells stained for granzyme B (magnification × 200). (C) Neoplastic cells stained for CD2 (magnification × 200). (D) Neoplastic cells with loss of expression for surface CD3 (magnification × 200). (E) EBERs by in situ hybridization (magnification × 100).
EBERs = Epstein-Barr virus encoded small nuclear RNAs.
Fig. 3.

PET-CT scan shows no significant FDG uptake to suggest malignancy. The lung where ENKTCL is confirmed by biopsy also shows no FDG uptake.
PET-CT = positron emission tomography-computed tomography, FDG = fluorodeoxyglucose, ENKTCL = extranodal natural killer/T-cell lymphoma, nasal type.
DISCUSSION
This report concerns a patient with ENKTCL without other involvement, except for the lung, who was suspected as having ILD at the initial visit. ENKTCL commonly presents with midline facial destructive disease and shows a strong association with EBV (5). It occurs typically within the nasal cavity and upper aerodigestive tract (3,4). ENKTCL arises in all geographic regions of the world, although it is much more common in Asian and Hispanic populations and rare in the USA and Europe (6).
Most cases are derived from the malignant transformation of NK cells that express CD56+ and a lack of surface CD3 expression and T-cell receptor (TCR) gene rearrangements. More than 80% of ENKTCL affects the nose and paranasal area, so the initial complaints of ENKTCL include local symptoms such as unilateral nasal cavity obstruction with purulent discharge and bleeding. Moreover, these symptoms can lead to early disease recognition (7).
However, ENKTCL can also occur predominantly at extranasal sites, which makes it hard to diagnose. The sites of predilection of non-upper aerodigestive tract ENKTCL were the skin (37%), liver or spleen (31%), gastrointestinal tract (24%), bone marrow (22%), lung (14%), extremities (8%), and testis (5%) (3,4,8).
When ENKTCL is suspected, a histopathological examination is necessary. The histopathology of ENKTCL comprises a mixed pattern ranging from small-to-medium sized atypical cells to large transformed cells. The tumor cells typically have the immunophenotype of CD56+, CD2+, cytoplasmic CD3+, and are negative to other T-cell antigens such as surface CD3, CD4, CD5, CD57, CD16, and B-cell antigens such as CD20. In addition, cytotoxic molecules such as granzyme B and T-cell intracellular antigen-1 (TIA-1) (76.2% of all ENKTCL), perforin, and the nm23-HI gene (42% of all ENKTCL) can be expressed in this type of lymphoma. ENKTCL seems to be caused by EBV, and EBER in situ hybridization is the most reliable way to demonstrate the presence of EBV, which can be achieved from paraffin-embedded tissues (4,7,8,9).
ENKTCL is rare, and has been recognized as an independent disease relatively recently; thus, the optimal therapeutic approach and prognosis have not been fully defined yet. However, this entity is generally known to have an aggressive clinical course, and standard NHL treatment consisting of anthracycline-based chemotherapy is unsatisfactory (10).
The chest CT patterns of ENKTCL with lung involvement seems to be variable. In the previous reviews of ENKCL patients with lung involvement, patchy consolidations, nodules, and masses are the most frequent chest CT findings. GGOs are relatively rare and may represent an early stage of disease that develops into consolidations at the end stage (1,11,12,13).
We present this case because primary pulmonary ENKTCL is rare and chest CT finding of GGOs is especially rare. To our knowledge, our case is the first case that primary pulmonary ENKTCL is presented with GGOs. Clinicians should know that ENKTCL can involve only the lung and can present as GGOs in CT scan. Surgical lung biopsy should be considered when the clinical course is not according to initial impression.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Investigation: Song M, Kim JY, Choi JS, Yoon B, Kim M, Kim SJ. Visualization: Song M. Writing - original draft: Song M. Writing - review & editing: Kim SY.
References
- 1.Laohaburanakit P, Hardin KA. NK/T cell lymphoma of the lung: a case report and review of literature. Thorax. 2006;61:267–270. doi: 10.1136/thx.2004.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morovic A, Aurer I, Dotlic S, Weisenburger DD, Nola M. NK cell lymphoma, nasal type, with massive lung involvement: a case report. J Hematop. 2010;3:19–22. doi: 10.1007/s12308-009-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hakeem DA, Fedele S, Carlos R, Porter S. Extranodal NK/T-cell lymphoma, nasal type. Oral Oncol. 2007;43:4–14. doi: 10.1016/j.oraloncology.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Tse E, Liang RH. Natural killer cell neoplasms. Clin Lymphoma. 2004;5:197–201. doi: 10.3816/clm.2004.n.027. [DOI] [PubMed] [Google Scholar]
- 5.Kanavaros P, Lescs MC, Brière J, Divine M, Galateau F, Joab I, Bosq J, Farcet JP, Reyes F, Gaulard P. Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood. 1993;81:2688–2695. [PubMed] [Google Scholar]
- 6.Chim CS, Ma SY, Au WY, Choy C, Lie AK, Liang R, Yau CC, Kwong YL. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004;103:216–221. doi: 10.1182/blood-2003-05-1401. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R, Takeuchi K, Ohshima K, Nakamura S. Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hematol Oncol. 2008;26:66–72. doi: 10.1002/hon.847. [DOI] [PubMed] [Google Scholar]
- 8.Aozasa K, Takakuwa T, Hongyo T, Yang WI. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008;87:110–117. doi: 10.1007/s12185-008-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung MM, Chan JK, Wong KF. Natural killer cell neoplasms: a distinctive group of highly aggressive lymphomas/leukemias. Semin Hematol. 2003;40:221–232. doi: 10.1016/s0037-1963(03)00136-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Kim WS, Park YH, Park SH, Park KW, Kang JH, Lee SS, Lee SI, Lee SH, Kim K, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226–1230. doi: 10.1038/sj.bjc.6602502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BH, Kim SY, Kim MY, Hwang YJ, Han YH, Seo JW, Kim YH, Cha SJ, Hur G. CT of nasal-type T/NK cell lymphoma in the lung. J Thorac Imaging. 2006;21:37–39. doi: 10.1097/01.rti.0000179472.46877.28. [DOI] [PubMed] [Google Scholar]
- 12.Oshima K, Tanino Y, Sato S, Inokoshi Y, Saito J, Ishida T, Fukuda T, Watanabe K, Munakata M. Primary pulmonary extranodal natural killer/T-cell lymphoma: nasal type with multiple nodules. Eur Respir J. 2012;40:795–798. doi: 10.1183/09031936.00123911. [DOI] [PubMed] [Google Scholar]
- 13.Fei W, Xiaohong W, Hong Z, Bei H. Pulmonary extranodal natural killer/T-cell lymphoma (nasal type): a case report and radiological image review. Medicine (Baltimore) 2015;94:e1527. doi: 10.1097/MD.0000000000001527. [DOI] [PMC free article] [PubMed] [Google Scholar]