Abstract
Background: Pulmonary Arterial Hypertension (PAH) is a deadly and disabling disease for which there is no marketed drug that addresses the underlying disease mechanism and targets to cure patients. The lack of understanding of the disease mechanism represents the main challenges in developing curative therapies. We here report, for the first time, that mice lacking natriuretic peptides clearance receptor develop PAH. Methods and Results: Initial studies assessed cardiac structure and function in NPR-C+/+ (wild type) and age matched, littermate NPR-C-/- mice by echocardiography. Mice lacking NPR-C had right atrial dilation, tricuspid regurgitation as well as echocardiographic signs of right ventricular pressure overload, including flattening and paradoxical bulging of the septum into the left ventricle during systole, and hypertrophy of the right ventricular free wall. Among the 10 NPR-C-/- mice aged between 12 and 20 weeks studied, 8 showed the above typical echocardiographic features of PAH [80%, 95% CI: (0.4439-0.9748)], and only one had pericardial effusion [10%, 95% CI: (0.0025-0.4450)], finding that has a prognostic significance in subjects affected by this clinical entity. To confirm the presence of increased right ventricular systolic pressure (RVSP) among NPR-C-/- mice, right heart catheterization was performed. Strikingly, RVSP was significantly elevated in NPR-C-/- mice compared to their age matched, littermate NPR-C+/+ mice, at baseline (21.95±0.56 mmHg vs. 5.3±0.6 mmHg, respectively (P<0.001)). Conclusion: The above results suggest that NPR-C-mediated signalling pathways play a critical role in the development of PAH, indicating that NPR-C is an important protective receptor in the heart rather than just being a clearance receptor.
Keywords: PAH, pulmonary arterial hypertension, natriuretic peptide clearance receptor
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease, which if not interrupted, leads to progressive right heart failure and death within 2 to 3 years after diagnosis [1]. Despite significant advances in treatment, PAH remains a disease with poor survival and no curative therapy [1]. The failure of current treatment regime indicates that important pathogenic cellular and molecular signalling mechanisms have neither been identified nor therapeutically targeted. Several clinical studies have focused on targeting the nitric oxide (NO) pathway in patients with PAH; however they all fall short as to re-establishment of structural and functional lung vascular integrity, as a basis for handicap-free long-term survival [1,2]. This was an expected outcome as patients with PAH are known to have endothelial dysfunction and thus reduced or loss of NO pathway [1]. Interestingly, evidence also suggests that in the presence of a reduced or loss of NO pathway, there is an enhanced natriuretic peptide (NPs) clearance receptor (NPR-C)-mediated vasorelaxant effect [1,3]. They may therefore be synergistic and complementary cardioprotective roles for NPR-C signalling and NO-mediated signalling in the vasculature [1,3]. The inhibition of one pathway may thus be compensated for by the upregulation of the other [1,3]. These observations raise the question of whether NPR-C signalling pathway may play a critical role in PAH pathobiology. In order to study the roles of NPR-C in PAH, we used experimental animal models lacking NPR-C (NPR-C knockout mice, NPR-C-/-).
Methods
Animals
This study utilized male littermate wild-type (NPR-C+/+) and NPR-C-/- mice between the ages of 12 and 20 weeks. NPR-C-/- mice were obtained from the Jackson Laboratory and subsequently backcrossed into the C57Bl/6 line. These mice contain a 36 base pair deletion that results in a truncated, non-functional NPR-C protein [4]. All the experimental procedures were approved by the local Committee for Laboratory Animals and conformed to the guidelines of the National Council on Animal Care.
Echocardiography
Two-dimensional, Doppler echocardiography measurements and quantification were performed according to recommendations of the European Society of Echocardiography. Wild type (n=10) and NPRC-/- (n=10) mice were scanned at 12-20 weeks of age. Mice were placed in an induction chamber with constant inflow of 5% isoflurane mixed with 100% oxygen. Once the mouse was asleep, it was removed from the induction chamber, weighed and placed on a heating platform with electrocardiogram contact pads and the nose was placed into a nose cone with 1-2% isoflurane in 100% oxygen. Excess gases were evacuated passively using an activated charcoal absorption filter. The eyes were covered with a petroleum based ophthalmic ointment. Electrode gel was placed on the paws and the paws were taped over the electrocardiogram contact pads on the heating platform. A rectal probe was lubricated with gel, placed in the rectum and taped to the platform. The temperature was maintained at 36.5 to 37.5°C. Depilatory cream was applied to the chest of the mouse and removed after two minutes. Ultrasound gel was placed on the chest of the anesthetized mouse. The ultrasound probe was placed in contact with the ultrasound gel and scanning was performed over 20 minutes. B-mode, M-mode and spectral Doppler images were obtained. The temperature and heart rate (HR) were constantly monitored during the scanning. Once completed, all probes and monitors were removed from the mouse. The mouse was cleaned with water and allowed to recover on the heated platform. Once awake, the mouse was returned to its cage. Doppler echocardiography was used to measure tricuspid regurgitation velocity. The right ventricular pressure gradient was measured using the maximum velocity of the tricuspid regurgitation jet. The maximum velocity of the tricuspid regurgitation was measured from the apical 4 chamber view. Other calculations were performed using echocardiographic derived values.
Right heart catheterization
Mice were placed in an induction chamber with constant inflow of 5% isoflurane mixed with 100% oxygen. Once the mouse was asleep, it was removed from the induction chamber, weighed and placed on a heated surgical table and secured with surgical tape. The nose was placed into a nose cone with 2.53% isoflurane in 100% oxygen. Mice were placed in a supine position and T he trachea was exposed via thoracotomy and the animals were intubated (100 cycles/min and 0.4cc volume) using an animal volume-controlled ventilator (Harvard Apparatus Small Animal Ventilator Model 687, Holliston, Massachusetts, USA). A stable body temperature of 37°C was maintained via a rectal temperature sensor connected to a heat-lamp. The animals were then were then shaved to expose the surgical area. An incision of ∼1 in. in length was made extending from the animal’s chin down to the right armpit. The thyroid gland was then blunt-dissected upward to expose the underlying tissue and the right jugular vein. The jugular vein was then separated from surrounding tissue using dissecting forceps until the body of the vessel is completely free from adherent tissues. The cranial end of the jugular was tied off completely, and a loose tie was then made at the caudal end of the exposed jugular using 4-0 braided silk suture. Four-inch microdissecting scissors were then used to make a small incision in the medial aspect of the right jugular vein. A Millar 1.4 French pressure-volume microtip catheter transducer connected to a PowerLab8/30/8s (AD Instruments) was then inserted through the incision and gently threaded down into the right ventricle. Proper placement within the ventricle was determined through observation of the pressure-volume loop obtained from the catheter. The loose caudal suture was then tightened to secure the catheter in place. After a period of stabilization after once the catheter is was properly placed, and the data was acquired when the ventilator was stopped data including HR, and the right ventricular systolic pressure (RVSP) were recorded and analyzed using a data acquisition system (LabChart Pro 7 Analysis software, AD Instruments).
Statistical analysis
All data are presented as means ± SEM. Data were analyzed using Student’s t-test. P<0.05 was considered significant. We also use the Clopper-Pearson Method to calculate the 95% confidence intervals for the prevalence of typical echocardiographic and right heart catheterization’s features of PAH among our NPRC-/- mice [5].
Results and discussion
Initial studies assessed cardiac structure and function in NPR-C+/+ (wild type) and age matched, littermate NPR-C-/- mice by echocardiography. Mice lacking NPR-C had right atrial and ventricular dilation, more severe tricuspid regurgitation (Figure 1) as well as echocardiographic signs of right ventricular pressure overload, including flattening and paradoxical bulging of the septum into the left ventricle during systole (Figure 2), and hypertrophy of the right ventricular free wall and trabeculae. The ejection fraction (EF%)-69±2.4 in NPR-C+/+ vs. 74±2 in NPR-C(-/-) (n=10, p=0.11), and the fractional shortening (FS%)-34±4.3 in NPR-C+/+ vs. 38.3±3.8 (n=10, P=0.13), tended to be greater in NPR-C-/- mice although these did not reach statistical significance as it would be expected in patient with PAH. Among the 10 NPR-C-/- mice aged between 12 and 20 weeks studied, 8 showed the above typical echocardiographic features of PAH [80%, 95% CI: (0.4439-0.9748)], and only one had pericardial effusion [10%, 95% CI: (0.0025-0.4450)], finding that has a prognostic significance in subjects affected by this clinical entity.
Figure 1.
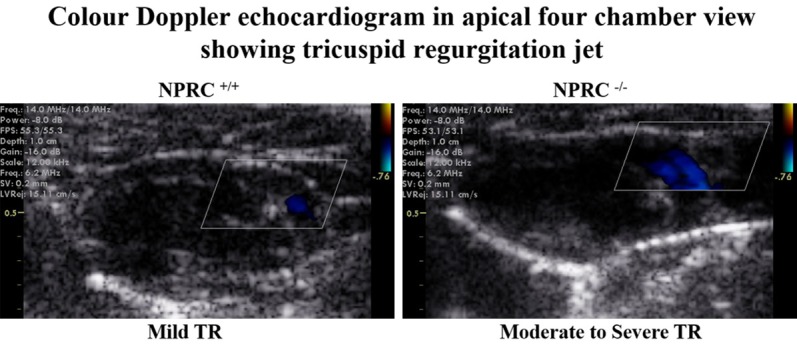
Apical four-chamber echocardiography in NPRC+/+ and NPRC-/- mice illustrating dilation of the right atrium and right ventricle in mice lacking NPRC. While there was trace to mild TR in wild type mice, TR was graded as moderate to severe in mice lacking NPRC. Among the 10 NPR-C-/- mice aged between 12 and 20 weeks studied, 8 showed the above typical echocardiographic features of PAH [80%, 95% CI: (0.4439-0.9748)]. TR denotes tricuspid regurgitation, NPRC denotes natriuretic peptide receptor type C.
Figure 2.
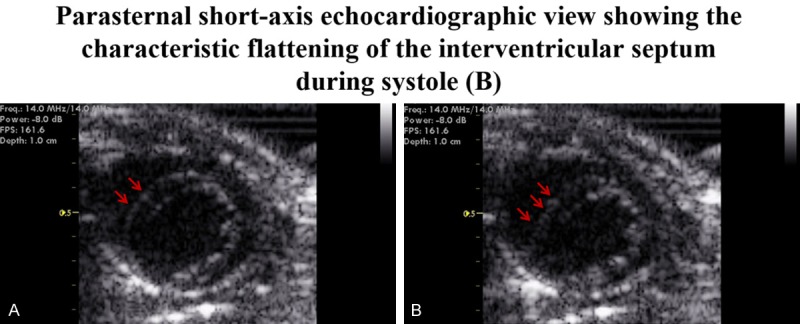
Parasternal short-axis view during diastole (A). Echocardiography data illustrating flattening of the septum into the left ventricle during systole in mice lacking NPRC (B). Among the 10 NPR-C-/- mice aged between 12 and 20 weeks studied, 8 showed the above typical echocardiographic features of PAH [80%, 95% CI: (0.4439-0.9748)]. NPRC denotes natriuretic peptide receptor type C.
Estimation of the right ventricular systolic pressure (RVSP) by Doppler Echocardiography assessment of the tricuspid valve regurgitation (TR) jet peak velocity accurately predicts the pulmonary artery systolic pressure (PASP) observed by invasive measurement [6]. TR was graded as none, trace, mild, moderate, or severe by assessment of the colour-flow jet in relation to the right atrium (RA) area in apical 4 chamber view. Moderate to severe TR was detected in all NPR-C(-/-) mice while most NPR-C(+/+) mice had trace or mild TR. Consistently, Doppler Echocardiography assessment revealed a significantly higher RVSP compared with wild-type littermates (Figure 3). As the ultrasound beam was not parallel to the high velocity jet of the tricuspid regurgitation, estimation of the RVSP (and thus of the PASP) is likely to be an underestimate of the true values (Figure 3). To confirm the presence of increased pulmonary artery pressure among NPR-C-/- mice, right heart catheterization was performed. Consistently, RVSP was significantly elevated in NPR-C-/- mice compared to their age matched, littermate NPR-C+/+ mice, at baseline (21.95±0.56 mmHg vs. 5.3±0.6 mmHg, respectively (n=7, P<0.001)) (Figure 4). Among the 7 NPR-C-/- mice aged between 12 and 20 weeks who underwent right heat catheterization, all of them showed a significantly elevated RVSP [100%, 95% CI: (0.5904-1.0000)]. Yu and colleagues investigated the role of hypoxia-inducible factor 1 in physiological responses to chronic hypoxia [7]. In line with our results, the mean RVSP pressures in wildtype mice were 7.33±0.49 mmHg under normoxic conditions while the mean RVSP pressures in the hypoxia-induced pulmonary hypertension mice model were 18.36±1.88 [7].
Figure 3.
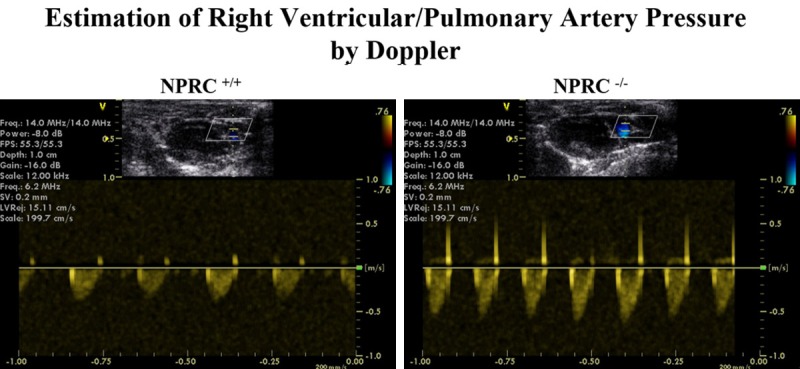
Estimation of Right Ventricular Systolic Pressure (RVSP) by Echocardiography. Estimation of RVSP by using the maximum velocity of the tricuspid regurgitation jet. These are representative examples of right ventricular systolic pressure tracings in the NPRC+/+ and NPRC-/- mice, showing a higher RVSP in the NPRC-/- mice as compared to their littermates’ wildtype. Among the 10 NPR-C-/- mice aged between 12 and 20 weeks studied, 8 showed the above typical echocardiographic features of PAH [80%, 95% CI: (0.4439-0.9748)].
Figure 4.
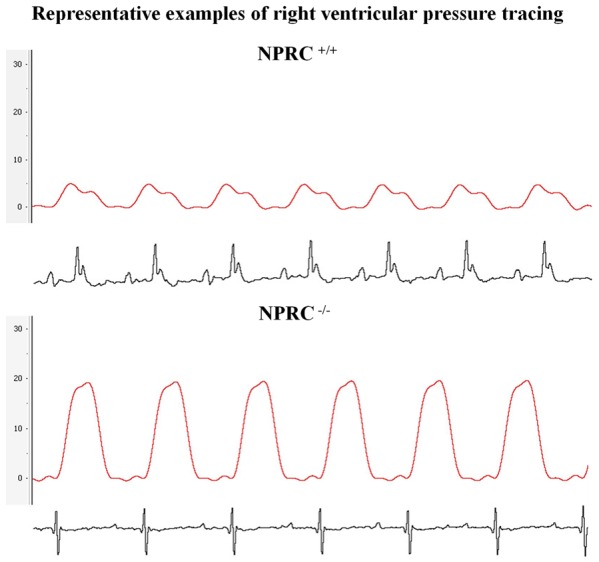
Estimation of Right Ventricular Systolic Pressure (RVSP). Representative examples of right ventricular systolic pressure tracings in the NPRC+/+ and NPRC-/- mice, demonstrating the presence of an increased right ventricular systolic pressure (RVSP) in the NPRC-/- mice (21.95±0.56 mmHg vs. 5.3±0.6 mmHg, respectively (n=7, P<0.001)). Among the 7 NPR-C-/- mice aged between 12 and 20 weeks who underwent right heat catheterization, all of them showed a significantly elevated RVSP [100%, 95% CI: (0.5904-1.0000)].
The above results suggest that the NPs clearance receptor (NPR-C)-mediated signalling pathways play a critical role in the development of PAH, indicating that NPR-C is an important protective receptor in the heart rather than just being a clearance receptor. In fact, NPR-C, which binds all NPs with similar affinity [8,9], does not contain a guanylyl cyclase (GC) domain and was originally classified as a ‘clearance receptor’ with no signalling function [10-12]. Although still commonly called a clearance receptor (and thus largely ignored) [10], evidence suggests that NPR-C is coupled to a pertussis toxin sensitive [13] inhibitory G protein (Gi) and mediates a reduction in adenylyl cyclase activity and intracellular cyclic adenosine monophosphate levels [14-19].
The precise role of NPR-C signalling pathway in pulmonary vasculopathy is not yet fully elucidated. Several basic and clinical research projects have been carried out to understand the roles of NPs in regulating pulmonary vascular tone and remodeling, as well as their roles in the pathogenesis of hypoxia or monocrotaline-induced PH. All the antimitogenic, antifibrotic, and antihypertrophic effects of NPs on pulmonary vascular remodeling and maladaptive hypertrophic responses in the right ventricle were reported to be linked to the GC-linked NPs’ receptors [20-22]. Even the often observed down regulation of NPR-C in hypoxia-associated PH was repeatedly reported to be part of a compensatory mechanism of the lungs aimed at reducing NPs clearance from the circulation, thus enhancing the biological effects of NPs and mitigating the severity of hypoxia-induced PH [20-22]. Interestingly, Sun and colleagues demonstrated that chronic hypoxia causes a significant down regulation of NPR-C expression in several tissues, including pulmonary vascular smooth muscle, independently of changes in NPs levels and expression of other NPs’ receptors [22]. As activation of NPR-C signalling pathway may have anti-proliferative actions [23], hypoxia-induced down regulation of NPR-C expression and associated impaired NPR-C pathways may lead to failure of the NPRC-related antiproliferative effects in the pulmonary vasculature, which may then ultimately lead to PAH. In summary, an impaired NPR-C pathway may be a common underlying cause of all hypoxia-associated vasculopathy, including, but not limited to, pulmonary vasculopathy. This observation suggests that NPR-C pathway may represent, therefore, a therapeutic target to inhibit pulmonary vascular remodeling and maladaptive increases in pulmonary arterial pressures which may complicate various clinical entities.
In conclusion, our data demonstrate, for the first time, that mice lacking NPR-C develop full-blown PAH. As these observations are so dramatic and important, they should stimulate immediate investigations of the roles of agents capable of stimulating the intrinsic NRP-C signalling pathways to treat this devastating human disease. Successful use of such drugs could potentially arrest and reverse the disease process, and this would be an exciting and highly desirable advance in PAH therapy.
Acknowledgements
This study was sponsored by ECTRS Lt and Bonsai Horizons Lt (Dublin, Ireland) (EEE), Grant-in-aid from the Heart and Stroke Foundation of Canada (KP); NSHRF studentship (TF) and Heart and Stroke Foundation of Canada Postdoctoral Fellowship.
Disclosure of conflict of interest
None.
References
- 1.Egom E, Feridooni T, Pharithi R, Maher V, El Hiani Y, Pasumarthi K, Ribama H. New insights and new Hope for pulmonary arterial hypertension: natriuretic peptides clearance receptor as a novel therapeutic target for a complex disease. Journal of the American College of Cardiology. 2017;11:1902. [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954. doi: 10.1093/eurheartj/ehv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–1235. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- 4.Jaubert J, Jaubert F, Martin N, Washburn LL, Lee BK, Eicher EM, Guenet JL. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3) Proc Natl Acad Sci U S A. 1999;96:10278–10283. doi: 10.1073/pnas.96.18.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Statistical science. 2001:101–117. [Google Scholar]
- 6.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 7.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egom EE. BNP and heart failure: preclinical and clinical trial data. J Cardiovasc Transl Res. 2015;8:149–157. doi: 10.1007/s12265-015-9619-3. [DOI] [PubMed] [Google Scholar]
- 9.Johns DG, Ao Z, Heidrich BJ, Hunsberger GE, Graham T, Payne L, Elshourbagy N, Lu Q, Aiyar N, Douglas SA. Dendroaspis natriuretic peptide binds to the natriuretic peptide clearance receptor. Biochem Biophys Res Commun. 2007;358:145–149. doi: 10.1016/j.bbrc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 10.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 11.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 12.Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- 13.Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by isletactivating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anand-Srivastava MB, Cantin M. Atrial natriuretic factor receptors are negatively coupled to adenylate cyclase in cultured atrial and ventricular cardiocytes. Biochem Biophys Res Commun. 1986;138:427–436. doi: 10.1016/0006-291x(86)90299-8. [DOI] [PubMed] [Google Scholar]
- 15.Anand-Srivastava MB, Srivastava AK, Cantin M. Pertussis toxin attenuates atrial natriuretic factor-mediated inhibition of adenylate cyclase. Involvement of inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1987;262:4931–4934. [PubMed] [Google Scholar]
- 16.Anand-Srivastava MB, Sairam MR, Cantin M. Ring-deleted analogs of atrial natriuretic factor inhibit adenylate cyclase/cAMP system. Possible coupling of clearance atrial natriuretic factor receptors to adenylate cyclase/cAMP signal transduction system. J Biol Chem. 1990;265:8566–8572. [PubMed] [Google Scholar]
- 17.Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271:19324–19329. doi: 10.1074/jbc.271.32.19324. [DOI] [PubMed] [Google Scholar]
- 18.Pagano M, Anand-Srivastava MB. Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J Biol Chem. 2001;276:22064–22070. doi: 10.1074/jbc.M101587200. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H, Murthy KS. Identification of the G protein-activating sequence of the singletransmembrane natriuretic peptide receptor C (NPR-C) Am J Physiol Cell Physiol. 2003;284:C1255–1261. doi: 10.1152/ajpcell.00520.2002. [DOI] [PubMed] [Google Scholar]
- 20.Casserly B, Klinger JR. Brain natriuretic peptide in pulmonary arterial hypertension: biomarker and potential therapeutic agent. Drug Des Devel Ther. 2009;3:269–287. doi: 10.2147/dddt.s4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh T, Nagaya N, Murakami S, Fujii T, Iwase T, Ishibashi-Ueda H, Yutani C, Yamagishi M, Kimura H, Kangawa K. C-type natriuretic peptide ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;170:1204–1211. doi: 10.1164/rccm.200404-455OC. [DOI] [PubMed] [Google Scholar]
- 22.Sun JZ, Chen SJ, Li G, Chen YF. Hypoxia reduces atrial natriuretic peptide clearance receptor gene expression in ANP knockout mice. Am J Physiol Lung Cell Mol Physiol. 2000;279:L511–519. doi: 10.1152/ajplung.2000.279.3.L511. [DOI] [PubMed] [Google Scholar]
- 23.Gower WR Jr, Carter GM, McAfee Q, Solivan SM. Identification, regulation and anti-proliferative role of the NPR-C receptor in gastric epithelial cells. Mol Cell Biochem. 2006;293:103–118. doi: 10.1007/s11010-006-9234-3. [DOI] [PubMed] [Google Scholar]


