Summary
Background
Tobacco smoking and smoke exposure during pregnancy and childhood cause considerable childhood morbidity and mortality. We did a systematic review and meta-analysis to investigate whether implementation of WHO's recommended tobacco control policies (MPOWER) was of benefit to perinatal and child health.
Methods
We searched 19 electronic databases, hand-searched references and citations, and consulted experts to identify studies assessing the association between implementation of MPOWER policies and child health. We did not apply any language restrictions, and searched the full time period available for each database, up to June 22, 2017. Our primary outcomes of interest were perinatal mortality, preterm birth, hospital attendance for asthma exacerbations, and hospital attendance for respiratory tract infections. Where possible and appropriate, we combined data from different studies in random-effects meta-analyses. This study is registered with PROSPERO, number CRD42015023448.
Findings
We identified 41 eligible studies (24 from North America, 16 from Europe, and one from China) that assessed combinations of the following MPOWER policies: smoke-free legislation (n=35), tobacco taxation (n=11), and smoking cessation services (n=3). Risk of bias was low in 23 studies, moderate in 16, and high in two. Implementation of smoke-free legislation was associated with reductions in rates of preterm birth (–3·77% [95% CI −6·37 to −1·16]; ten studies, 27 530 183 individuals), rates of hospital attendance for asthma exacerbations (–9·83% [–16·62 to −3·04]; five studies, 684 826 events), and rates of hospital attendance for all respiratory tract infections (–3·45% [–4·64 to −2·25]; two studies, 1 681 020 events) and for lower respiratory tract infections (–18·48% [–32·79 to −4·17]; three studies, 887 414 events). Associations appeared to be stronger when comprehensive smoke-free laws were implemented than when partial smoke-free laws were implemented. Among two studies assessing the association between smoke-free legislation and perinatal mortality, one showed significant reductions in stillbirth and neonatal mortality but did not report the overall effect on perinatal mortality, while the other showed no change in perinatal mortality. Meta-analysis of studies on other MPOWER policies was not possible; all four studies on increasing tobacco taxation and one of two on offering disadvantaged pregnant women help to quit smoking that reported on our primary outcomes had positive findings. Assessment of publication bias was only possible for studies assessing the association between smoke-free legislation and preterm birth, showing some degree of bias.
Interpretation
Smoke-free legislation is associated with substantial benefits to child health. The majority of studies on other MPOWER policies also indicated a positive effect. These findings provide strong support for implementation of such policies comprehensively across the world.
Funding
Chief Scientist Office Scotland, Farr Institute, Netherlands Lung Foundation, Erasmus MC.
Introduction
Almost half of children worldwide are regularly exposed to second-hand smoke, and 28% of the 600 000 deaths each year related to second-hand smoke occur in children.1, 2 Maternal smoking and second-hand smoke exposure during pregnancy are detrimental to fetal growth and development, leading to adverse birth outcomes such as preterm birth, low birthweight, being small for gestational age, and perinatal and infant mortality.3, 4, 5, 6, 7, 8 Additionally, second-hand smoke exposure presents substantial health risks postnatally by increasing the risk of asthma and respiratory tract infections.1, 9
Protection of children from the adverse health implications of second-hand smoke during important phases of development and the subsequent disease burden carried on into adulthood is crucial. The WHO Framework Convention on Tobacco Control (FCTC) aims to reduce tobacco consumption and second-hand smoke exposure through national tobacco control programmes.2 In 2008, six MPOWER measures were introduced to guide FCTC implementation (panel).2, 10 With tobacco use increasingly becoming a problem of developing countries already experiencing the largest burden of early-life morbidity and mortality, the absence of tobacco regulation is set to be a big driver of between-country inequality in child health outcomes.11 However, evaluations of the effectiveness of tobacco control interventions have generally excluded children, focusing instead on smoking rates and adult health outcomes.12, 13, 14
Panel. MPOWER policies2.
Monitor tobacco use
Eligible policies include those that enforce accurate measurement of the extent of the tobacco epidemic and of the interventions to control it.
Protect people from smoke
Eligible policies include legislation to create smoke-free public environments (both indoors and outdoors).
Offer help to quit tobacco use
Eligible policies include tobacco cessation advice or interventions offered through health-care services, free telephone quit lines, and providing access to free or low-cost cessation medicines.
Warn about the dangers of tobacco
Eligible policies include health warnings on tobacco products, plain packaging of tobacco products, and mass media campaigns to educate the public about the dangers of tobacco.
Enforce bans on tobacco advertising, promotion and sponsorship
See WHO Framework Convention on Tobacco Control (FCTC) guidelines for implementation of Article 13, which provides a non-exhaustive list of advertising, promotion, and sponsorship within the terms of the FCTC.11
Raise taxes on tobacco
Eligible policies include increasing percentage excise tax share in final tobacco.
Research in context.
Evidence before this study
Tobacco smoke exposure is the world's leading cause of preventable morbidity and premature mortality. Children cannot control their tobacco smoke exposure and therefore need protection through tobacco control measures. In a previous systematic review, we investigated the associations between smoke-free legislation and perinatal and child health outcomes. We searched 14 online medical research databases, the WHO International Clinical Trials Registry Platform, hand-searched references and citations, and consulted a panel of experts in the field to identify published and unpublished literature in any language from January, 1975, to May, 2013, on the associations between smoke-free legislation and our outcomes of interest. The primary outcomes were preterm birth, low birthweight, and hospital attendance for asthma. We identified 11 studies showing that smoke-free legislation was associated with significant reductions in preterm birth and severe asthma exacerbations. Studies have since addressed various knowledge gaps identified in our previous review, including assessments of the effect of smoke-free legislation on respiratory tract infections, the most important contributor to the global burden of paediatric morbidity and mortality associated with tobacco smoke exposure. The increased number of studies now available was also anticipated to allow investigation of another knowledge gap: exploration of a potential dose–response association between the comprehensiveness of smoke-free laws and their effect on child health. Furthermore, we sought to substantially broaden the focus of our study by evaluating the early-life health effect of the entire range of WHO-recommended tobacco control policies (ie, MPOWER). Following a prespecified and peer-reviewed protocol, we did a comprehensive literature search for experimental and quasi-experimental studies assessing associations between implementation of MPOWER policies and key perinatal and childhood outcomes associated with tobacco smoke exposure.
Added value of this study
To our knowledge, this is the first systematic review examining the association between the full spectrum of MPOWER policies and perinatal and child health. Our findings add value to the existing evidence base by identifying a link between smoke-free legislation and a substantial reduction in severe paediatric respiratory tract infections, providing consistent evidence that comprehensive smoke-free laws are associated with broad health effects, and collating evidence supporting the potential for other MPOWER measures to benefit child health. We also identified several key knowledge gaps, including a shortage of studies in low-income and middle-income countries, and of studies assessing MPOWER measures other than smoke-free legislation, tobacco tax increases, and smoking cessation services.
Implications of all the available evidence
With most of the world's population currently not covered by comprehensive tobacco control policies, there is great potential for global public health gains by protecting unborn babies and children from tobacco smoke exposure. Future efforts should focus on increasing the uptake of comprehensive MPOWER policies worldwide to protect the health of children, while developing and evaluating new and ongoing tobacco control policy initiatives around the world.
In a previous systematic review,15 we partly addressed this gap in the literature by synthesising available evidence on the effect of smoke-free legislation (ie, “P” in MPOWER, for ”Protect people from tobacco smoke”) on perinatal and child health. By combining data from 11 studies, we found smoke-free legislation to be associated with substantial reductions in preterm birth and hospital admissions for asthma among children. Studies have since addressed various knowledge gaps identified in our review, including assessments of the effect of smoke-free legislation on respiratory tract infections and on general practitioner (GP) consultations.16, 17, 18, 19 The increased number of studies now available was also anticipated to allow investigation of another knowledge gap: exploration of a potential dose–response association between the comprehensiveness of smoke-free laws and their effect on child health. In addition to addressing this association, we sought to substantially broaden the focus of our systematic review by systematically evaluating the early-life health effect of the entire range of MPOWER measures. This analysis has implications for the Sustainable Development Goal 3 (SDG 3) aims to strengthen FCTC implementation and reduce child mortality. As such, findings from this study can guide policy making for prioritisation of the most effective tobacco control policies to protect child health, especially in parts of the world where MPOWER implementation is lagging behind, while identifying the key remaining knowledge gaps that need to be addressed.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was done according to a peer-reviewed protocol that is published20 and registered with PROSPERO (CRD42015023448). We followed the PRISMA checklist when reporting our findings.21 Ethical approval was not required for this study.
Studies were eligible for inclusion if they investigated the association between one or more MPOWER tobacco control policies and health outcomes among fetuses, neonates, or children (ie, the majority of the study population aged <12 years).
We searched for published studies in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), WHO Global Health Library (in addition to MEDLINE, covering African Index Medicus [AIM], LILACS, Index Medicus for the Eastern Mediterranean Region [IMEMR], Index Medicus for South-East Asia Region [IMSEAR], Western Pacific Region Index Medicus [WPRIM], WHO Library Database [WHOLIS], and Scientific Electronic Library Online [SciELO]), IndMED, ISI Web of Science, KoreaMed, EconLit, Paediatric Economic Database Evaluation (PEDE), Google Scholar, and the ProQuest database of PhD dissertations. We searched the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished studies.
The appendix (p 1) contains an overview of the search strategies for each database. We did not apply any language restrictions, and searched the full time period available for each database. Searches were updated on June 22, 2017. To identify any additional relevant studies, we hand-searched reference lists of, and citations to, included studies and relevant review papers, and consulted experts in the field (appendix p 2).
We focused on studies that evaluated governmental public health interventions that could be classified according to the MPOWER acronym (panel), with the exception of “M” since “Monitoring tobacco use and prevention policies” itself was not expected to affect health outcomes. We followed the methodological approach recommended by the Cochrane Effective Practice and Organization of Care (EPOC) group to select studies with the most robust designs for our primary analyses: randomised controlled trials (including cluster randomised controlled trials), controlled clinical trials (including cluster controlled clinical trials), interrupted time series studies (including difference-in-difference designs, which were categorised as controlled interrupted time series studies),22 and controlled before-and-after studies. To assess the robustness of our findings, we also included non-EPOC study designs in sensitivity analyses: uncontrolled before-and-after studies, prospective or retrospective cohort studies, and case-control and nested case-control studies. Primary and secondary outcomes were selected on the basis of their established associations with maternal smoking during pregnancy and prenatal or childhood second-hand smoke exposure,23, 24 and their relative contributions to the global burden of adverse child health.1, 25 Our primary outcomes of interest were perinatal mortality, preterm birth, asthma exacerbations requiring hospital attendance, and respiratory tract infections requiring hospital attendance. Secondary outcomes of interest were stillbirth, early neonatal mortality, neonatal mortality, late neonatal mortality, post-neonatal mortality, infant mortality, child mortality, extremely low birthweight, very low birthweight, low birthweight, birthweight (continuous scale), very small for gestational age, small for gestational age, extremely preterm birth, very preterm birth, gestational age (continuous scale), congenital anomalies, asthma, wheezing, respiratory tract infections, upper respiratory tract infections, lower respiratory tract infections, otitis media with effusion, and chronic cough. Studies were excluded if they only measured smoking prevalence, smoking behaviour, second-hand smoke exposure, surrogate outcomes, or economic outcomes. Studies that reported outcomes for both adults and children were included if paediatric subgroup data were available.
Data analysis
Two reviewers (TF and AK) independently assessed all search results by title and abstract, and by full text for potential eligible studies identified. Any disagreements were resolved through joint discussion or via an adjudicator (JVB).
Relevant data were extracted with a customised data extraction form (appendix, pp 3–5). Study authors were contacted for clarification where necessary and to obtain relevant data that were missing from the reports.
A risk-of-bias assessment form was created on the basis of EPOC criteria for interrupted time series and controlled before-and-after studies.26 The Effective Public Health Practice Project (EPHPP) tool was adapted to assess the risk of bias of observational studies.27 Two reviewers (TF and AK) independently extracted data and assessed risk of bias, with disagreements resolved through discussion or arbitration (JVB).
Point estimates and corresponding 95% CIs for effect sizes or association measures were extracted. For dichotomous outcomes, risk ratios (RRs) were extracted. Where RRs were not available, we calculated RRs from odds ratios (ORs) using the following formula, where PEER is the patient-expected event rate in the control group:
When PEER was not available in interrupted time series studies we used the overall event rate across the study population as an approximation. For outcomes that could occur more than once (eg, hospital attendances for asthma and respiratory tract infections), we used incidence rate ratios (IRRs).
Aggregated effect estimates were calculated to assess the association between each tobacco control policy and individual health outcomes, where feasible. Relative risk differences were extracted or calculated from absolute risk differences and were pooled in random-effects meta-analyses given anticipated heterogeneity. Step changes (ie, immediate risk changes) following introduction of an intervention were pooled in separate analyses from slope changes (ie, gradual risk changes). Heterogeneity was assessed by the I2 statistic. For the meta-analyses, we selected the effect estimate of the most comprehensive intervention within each MPOWER category from each study. In case of overlapping populations between studies, we selected one study according to the following hierarchy: the lowest risk of bias, the most comprehensive intervention, or the largest study population. We also extracted data on changes in smoking behaviour and second-hand smoke exposure if reported. The comprehensiveness of smoke-free legislation was assessed by counting the number of locations that were made completely smoke-free, out of eight prespecified options as suggested by WHO.2 Policies that were completely smoke-free in all eight locations were considered to be comprehensive.
We did sensitivity analyses to explore the robustness of our findings by reanalysing the data for the primary outcomes with the addition of non-EPOC studies, and by restricting analyses to studies with low risk of bias and moderate risk of bias. Where possible, we did subgroup analyses according to the comprehensiveness of each intervention. Where possible, the effect of each intervention was reported according to socioeconomic status, alongside its overall effect.
We assessed risk of bias across studies using funnel plots when ten or more studies were included in a meta-analysis.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
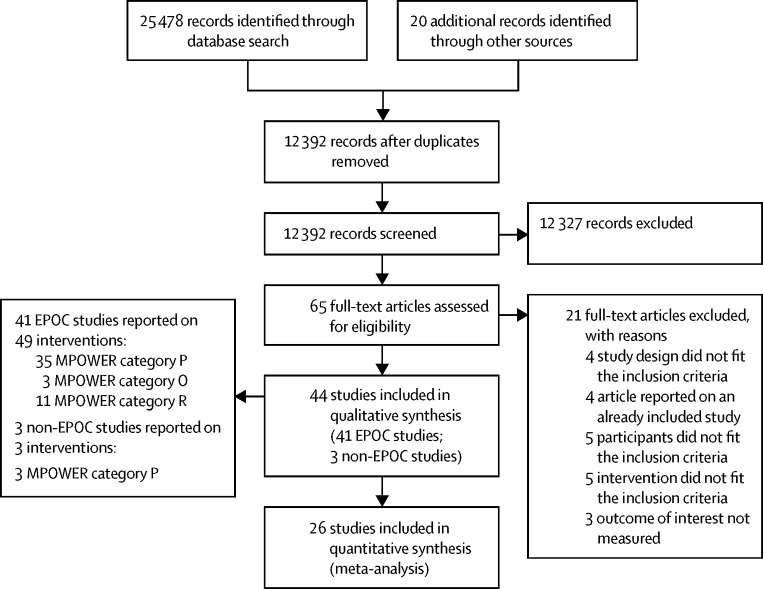
We identified 25 478 citations from bibliographic databases and an additional 20 from other sources. After removal of duplicates, 12 392 unique citations were screened by title and abstract, and 65 full texts were sourced. Of these, 41 EPOC studies16, 17, 18, 19, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 and three non-EPOC studies65, 66, 67 fit the inclusion criteria (figure 1; appendix, pp 6, 7). The EPOC studies included data from more than 57 million births, and from 4·6 million GP diagnoses and 2·7 million hospital admissions for respiratory conditions.
Figure 1.
PRISMA flow diagram
EPOC= Effective Practice and Organization of Care (a Cochrane Review Group). MPOWER=WHO's recommended tobacco control policies (see panel).
The appendix (pp 8–20) details the main characteristics of the EPOC studies. Among these, 26 were interrupted time series studies,16, 17, 18, 19, 31, 32, 37, 38, 40, 42, 43, 45, 46, 48, 50, 51, 52, 54, 55, 57, 58, 59, 61, 62, 63, 64 14 were controlled interrupted time series studies,28, 29, 33, 34, 35, 36, 39, 41, 44, 47, 49, 53, 56, 60 and one had a regression discontinuity design,30 a quasi-experimental design bearing close resemblance to interrupted time series methodology.68 The three non-EPOC studies were uncontrolled before-and-after studies (appendix, p 21).65, 66, 67 Model characteristics of individual studies can be found in the appendix (pp 22–31). The EPOC studies were done in 14 countries across North America (24 studies)18, 28, 29, 31, 33, 35, 36, 38, 39, 41, 42, 43, 45, 46, 47, 49, 50, 53, 54, 56, 57, 59, 60, 61 and Europe (16 studies),16, 17, 30, 32, 34, 37, 40, 44, 48, 51, 52, 55, 58, 62, 63, 64 with one study from Hong Kong, China.19 Several US studies assessed the same outcomes in partially overlapping study populations.18, 29, 31, 36, 38, 43, 45, 46, 49, 53, 56, 59, 61
Risk of bias of individual studies is reported in detail in the appendix (pp 32, 33). For the EPOC studies, risk of bias was low in 23 studies,16, 17, 18, 28, 30, 31, 32, 33, 36, 37, 39, 40, 44, 45, 46, 47, 48, 51, 52, 53, 58, 63, 64 moderate in 16,19, 34, 35, 41, 42, 43, 49, 50, 54, 55, 56, 57, 59, 60, 61, 62 and high in two.29, 38 For the non-EPOC studies, risk of bias was high for two studies66, 67 and unclear for one.65
28 studies assessed the association between smoke-free legislation and one or more primary outcomes (ie, perinatal mortality, preterm birth, asthma exacerbations requiring hospital attendance, and respiratory tract infections requiring hospital attendance), five assessed the association between tobacco taxation and primary outcomes, and two assessed the association between policies providing smoking cessation services and our primary outcomes (Table 1, Table 2, Table 3); four studies assessed a combination of these interventions, and ten studies only assessed secondary outcomes. A meta-analysis was only possible for studies on smoke-free legislation because studies on tax increases and smoking cessation services had variable outcome reporting and overlapping study populations.
Table 1.
Association between implementation of smoke-free legislation and primary outcomes
| Details of intervention | Population at risk (n) | Events (n) | Slope before intervention (% change in events per year) | Direct change in events (step change, %; 95% CI) | Sustained change in events per year (slope change, %; 95% CI) | Summary of findings | |
|---|---|---|---|---|---|---|---|
| Perinatal mortality | |||||||
| Peelen (2016)58* | First smoke-free law: workplaces and public transport except for restaurants and bars† (allowing designated smoking areas) Second smoke-free law: expansion of first smoke-free law to include restaurants and bars‡(allowing designated smoking areas) |
1 980 727 | 13 027 | NA because of non-linear time trend | First smoke-free law: −1·99% (−8·95 to 5·96) Second smoke-free law: −5·96% (−12·93 to 1·99) |
NA | National smoke-free workplaces and public transport, and smoke-free restaurants and bars, were not associated with significant changes in perinatal mortality |
| Preterm birth | |||||||
| Bakolis (2016)30 | Public places and workplaces (including restaurants and bars) | 1 800 906 | 126 527 | NR | Analysis of a 1, 2, 3, or 5 month time window around the intervention cutoff date (July 1, 2007): ±1 month, −4·67% (−16·00 to −0·93); ±2 months, −8·42% (−15·05 to −1·86); ±3 months, −5·60% (−10·31 to −0·93); ±5 months, −3·73% (−7·48 to −0·93) | NA | National comprehensive smoke-free legislation was associated with an immediate 4–9% decrease in preterm births |
| Bartholomew (2016)31 | Comprehensive (workplaces, restaurants, and bars) Restrictive (workplaces and restaurants, no restriction in bars) Moderate (workplaces, partial restriction in restaurants, and no restriction in bars) Limited (partial restriction in workplaces, any restriction in restaurants, and no restriction in bars) |
293 715 | 32 250 | NR | Comprehensive: −0·015%§ (−0·022 to −0·008) Restrictive: 0·003%§ (−0·005 to 0·011) Moderate: 0·004%§ (−0·002 to 0·010) Limited: 0·001%§ (−0·006 to 0·007) |
NA | County-wide, comprehensive smoke-free legislation was associated with a 0·015 percentage point decrease in preterm births, whereas less restrictive laws were not associated with changes in incidence of preterm births |
| Bharadwaj (2014)34 | Restaurants and bars (in addition to existing smoke-free laws in public places and workplaces) | 822 (intervention group), 3185 (control group) | 46 (intervention group), 189 (control group) | NR | −2·55%§ (−5·52 to 0·42) | NA | National smoke-free restaurants and bars were not associated with significant changes in preterm births among women working in restaurants and bars |
| Cox (2013)37 | Public places and workplaces (excluding catering industry); restaurants (in addition to existing smoke-free laws in public places and workplaces); and bars serving food (in addition to existing smoke-free laws in public places and workplaces, including restaurants) | 606 877 | 36 663 | NR | Public places and workplaces: single smoke-free law¶, −0·59% (−2·63 to 1·49); final model‖, no significant changes Restaurants (in addition to public places and workplaces): single smoke-free law¶, −2·28% (−4·73 to −0·15); final model‖, −3·18% (−5·38 to −0·94) Bars serving food (in addition to restaurants and public places and workplaces): single smoke-free law¶, −1·24% (−3·05 to 0·60); final model‖, no significant changes |
Public places and workplaces: single smoke-free law†, −1·95% (−3·50 to −0·37); final model‡, no significant changes Restaurants (in addition to public places and workplaces): single smoke-free law†, −1·42% (−2·87 to 0·05); final model‡, no significant changes Bars serving food (in addition to restaurants and public places and workplaces): single smoke-free law†, −2·10% (−4·82 to 0·69); final model‡, −3·50% (−6·35 to −0·57) |
National smoke-free public places and workplaces were not associated with significant changes in preterm births; expansion of national smoke-free legislation to include restaurants was associated with an immediate 3·2% reduction in preterm births; and expansion of national smoke-free legislation to include bars was associated with a gradual 4% per year decrease in preterm births |
| Hade (2011)43 | Public places and workplaces (including restaurants and bars) | 583 530 | NR | NR | No significant changes** | No significant changes** | State-wide, smoke-free public places and workplaces were not associated with significant changes in preterm birth |
| Hajdu (2017)44 | Public places and workplaces (including restaurants and bars) | 18 755 | NR | NR | –1·9%§ (–4·3 to 0·5) | NA | National smoke-free legislation was not associated with significant changes in preterm birth among female restaurant and bar workers compared with women working in places other than restaurants and bars |
| Hankins (2016)45 | Workplaces, restaurants, and bars | NR | NR | NR | Workplaces: 0·07%§ (−0·11 to 0·25) Restaurants: 0·09%§ (−0·13 to 0·31) Bars: −0·29%§ (−0·49 to −0·09) |
NA | State-wide or county smoke-free workplaces and restaurants were not associated with significant changes in preterm births; state-wide or county smoke-free bars were associated with an immediate 0·3 percentage point decrease in preterm births |
| Hawkins (2014)46 | 100% smoke-free workplaces and restaurants | 16 198 654 | 1 555 071 | NR | 0·72%§ (−0·11 to 1·55) | NA | State-wide smoke-free workplaces and restaurants were not associated with significant changes in preterm births |
| Mackay (2012)52 | Public places and workplaces (including restaurants and bars) | 709 756 | 41 998 | NR | Crude: −11·07% (−15·15 to −6·79) Adjusted: −11·72% (−15·87 to −7·35) |
Crude: 2·28% (−0·03 to 4·66) Adjusted: 3·83% (1·42 to 6·30) |
National smoke-free public places and workplaces were associated with an immediate 12% decrease in preterm births, and a subsequent gradual 4% increase per year |
| Markowitz (2013)53 | Workplaces with complete smoke-free law Workplaces with smoking restrictions (requiring designated smoking areas) Restaurants with complete smoke-free laws Restaurants with smoking restrictions (requiring designated smoking areas) |
Maternal age <20 years: 54 132Maternal age 20–24 years: 101 723Maternal age 25–34 years: 183 763Maternal age >34 years: 53 109 | Maternal age <20 years: 5413Maternal age 20–24 years: 7120Maternal age 25–34 years: 11 026Maternal age >34 years: 3718 | NR | Workplaces with complete smoke-free laws: NRWorkplaces with smoking restrictions: NRRestaurants with complete smoke-free laws: maternal age <20 years, 0·7%§ (−3·5 to 4·9);20–24 years, −0·2%§ (−1·5 to 1·1); 25–34 years, −0·3%§ (−0·8 to 0·2); >34 years, −0·6%§ (−1·9 to 0·7) Restaurants with smoking restrictions: maternal age <20 years, −0·6%§ (−3·8 to 2·6); 20–24 years, −0·1%§ (−1·1 to 0·9); 25–34 years, −0·8%§ (−1·2 to −0·4); >34 years, −0·3%§ (−1·7 to 1·1) |
NA | State-wide complete smoke-free laws were not associated with significant changes in preterm births, but state-wide restaurant smoking restrictions were associated with a 0·8 percentage point decrease in preterm births among women aged 25–34 years |
| McKinnon (2015)54 | Public places and workplaces (including restaurants and bars) | 470 199 | 19 321 | NR | Crude: −6% (−10 to −1) Adjusted: −5% (−10 to 0) |
NA | State-wide smoke-free legislation was associated with a 5% decrease in preterm births 9 months after its implementation |
| Page (2012)56 | Public places and workplaces (including restaurants and bars) | 6717 (intervention group), 32 293 (control group) | 515 (intervention group), 2767 (control group) | NR | Crude: −20·6% (−34·7 to −3·4) Adjusted: −23·1% (−40·1 to −1·3) |
NA | City-wide smoke-free public places and workplaces were associated with a 23% decrease in preterm births |
| Peelen (2016)58* | First smoke-free law†: workplaces and public transport except for restaurants and bars (allowing designated smoking areas) Second smoke-free law‡: expansion of first smoke-free law to include restaurants and bars (allowing designated smoking areas) |
1 972 163 | 116 043 | NA because of non-linear time trend | First smoke-free law: 0·94% (−1·89 to 3·77) Second smoke-free law: −0·94% (−3·78 to 2·83) |
NA | National smoke-free workplaces and public transport, and smoke-free restaurants and bars, were not associated with significant changes in preterm births |
| Simón (2017)62 | First smoke-free law: complete smoke-free workplaces and partial smoke-free restaurants and bars Second smoke-free law: public places and workplaces (including restaurants and bars) |
5 302 374 | 416 595 | NR | First smoke-free law: 4·6% (2·9 to 6·2) Second smoke-free law: −4·5% (–6·1 to −2·9) |
NA | National partial smoke-free legislation was associated with a 5% increase in preterm births; the subsequent national comprehensive smoke-free legislation was associated with a 5% decrease in preterm births |
| Vicedo-Cabrera (2016)63 | Public places and workplaces (including restaurants and bars), with several exceptions in the hospitality sector†† | 446 492 | 24 482 | NR | −3·56% (−9·29 to 2·53) | NA | Federal smoke-free legislation was not associated with a significant change in preterm births |
| Asthma exacerbations requiring hospital attendance | |||||||
| Ciaccio (2016)36 | Public places and workplaces (including restaurants and bars) | 13 246 809 | 335 588 | NR | –17% (–18 to −15) | NA | State or local smoke-free legislation was associated with an immediate 17% decrease in emergency department visits for asthma |
| Croghan (2015)38 | Public places and workplaces (including restaurants and bars) | NR | 1531 | 1·1% (0·2 to 2·0) | −24·9% (−40·5 to −5·3) | −1·5% (−2·9 to −0·1) | National smoke-free legislation was associated with an immediate 25% decrease in emergency department visits for children with asthma, and a subsequent gradual 1·5% decrease per year |
| Galán (2017)40 | First smoke-free law: complete smoke-free workplaces and partial smoke-free restaurants and bars Second smoke-free law: public places and workplaces (including restaurants and bars) |
NR | NR | NR | First smoke-free law: 25·0% (–2·6 to 60·4) Second smoke-free law: −11·0% (–28·6 to 11·1) |
NA | Partial and comprehensive national smoke-free legislation were not associated with significant immediate changes in asthma-related hospital admissions via emergency departments |
| Gaudreau (2013)42 | Public places and workplaces (including restaurants and bars), allowing designated smoking areas | NR | 3050 | NR | 11% (−37 to 95) | 0% (−2 to 2) | Provincial smoke-free public places and workplaces were not associated with significant changes in hospital admissions for paediatric asthma |
| Hawkins (2016)18 | State or local 100% smoke-free workplaces or restaurants, or both | NR | 128 807 | NR | State: −3% (−8 to 2) Local: 2% (−6 to 11) |
NA | State or local smoke-free workplaces or restaurants were not associated with significant changes in emergency department visits for paediatric asthma |
| Landers (2014)49 | 100% smoke-free workplaces, restaurants, and bars‡‡ | NR | NR | Mean rate across all states and years: 9·02 per 10 000 per quarter (SD 9·66; range 0·00–144·47) | Any state law: 0·12%§ (−0·38 to 0·62) Any county law: −1·32%§ (−2·64 to 0·00) Interaction term of state law and county law: 0·51%§ (−1·04 to 2·06) |
NA | County-level smoke-free laws were associated with a one percentage point decrease in discharge rates among children admitted for asthma; state smoke-free laws were not associated with significant changes in discharge rates among children admitted for asthma, besides the effect of county laws |
| Mackay (2010)51 | Public places and workplaces (including restaurants and bars) | NR | 21 415 | 4·4% (3·3 to 5·5) | NA | −19·5% (−22·4 to −16·5) | National smoke-free public places and workplaces were associated with a gradual 20% decrease per year in paediatric emergency asthma admissions |
| Millett (2013)55 | Public places and workplaces (including restaurants and bars) | NR | 217 381 | 2·2% (2 to 3) | −8·9% (−11 to −7) | −3·4% (−4 to −2) | National smoke-free public places and workplaces were associated with an immediate 9% decrease in emergency admissions to hospital for paediatric asthma, and a subsequent gradual 3% decrease per year |
| Rayens (2008)59 | Most businesses open to the public (including restaurants and bars)§§ | 395 116 | 5322 | 12·7% | −18·0% (−29·0 to −4·0) | NA | The county-wide smoke-free law in most public places was associated with an 18% decrease in emergency department visits for paediatric asthma |
| Shetty (2011)61 | All workplaces except restaurants and bars: 100% smoke-free Any smoke-free workplace, restaurant, or bar law |
NR | NR | NR | 100% smoke-free workplaces: 14·6% (3·7 to 25·5) Any smoke-free law: 9·0 (−1 to 19·1) |
NA | State-wide or region-wide 100% smoke-free workplaces were associated with a 15% increase in hospital admissions for children with asthma; there was no evidence for an association between any state-wide or region-wide smoke-free legislation and asthma admissions |
| RTI admissions (upper and lower) | |||||||
| Been, Millett (2015)16 | Public places and workplace (including restaurants and bars) | NR | 1 651 675 | NR | −3·5% (−4·7 to −2·3) | −0·5% (−0·9 to −0·1) | National smoke-free legislation was associated with an immediate 4% reduction and an additional 0·5% annual reduction in childhood acute RTI hospital admissions |
| Vicedo-Cabrera (2017)64 | Public places and workplaces (including restaurants and bars), with several exceptions in the hospitality sector†† | NR | 29 345 | NR | 2·7% (–9·7 to 16·7) | NA | Federal smoke-free legislation was not associated with a significant change in RTI hospital admissions |
| Upper RTI admissions | |||||||
| Been, Millett (2015)16 | Public places and workplaces (including restaurants and bars) | NR | 979 370 | NR | 1·9% (0·5 to 3·2) | −1·9 (−2·3 to −1·5) | National smoke-free legislation was associated with an initial immediate 2% increase in childhood upper RTI hospital admissions, followed by a gradual decrease of 2% per year |
| Hawkins (2016)18 | State or local 100% smoke-free workplaces or restaurants, or both | NR | 410 686 | NR | State: −2% (−6 to 2) Local: 6% (−2 to 14) |
NA | State or local smoke-free workplaces or restaurants were not associated with significant changes in emergency department visits for upper RTIs |
| Lower RTI admissions | |||||||
| Been, Millett (2015)16 | Public places and workplaces (including restaurants and bars) | NR | 672 305 | NR | −13·8% (−15·6 to −12·0) | 0·2% (−0·6 to 0·9) | National smoke-free legislation was associated with an immediate 14% reduction in childhood lower RTI hospital admissions |
| Hawkins (2016)18 | State or local 100% smoke-free workplaces or restaurants, or both | NR | 139 239 | NR | State: −8% (−13 to −4) Local: 3% (−6 to 12) |
NA | State-wide smoke-free workplaces or restaurants were associated with an 8% decrease in emergency department visits for lower RTIs |
| Lee (2016)19 | Public places and workplaces (including restaurants) | 691 480 | 75 870 | NR | −33·5% (−36·4 to −30·5) | −13·9% (−16·0 to −11·7) | Comprehensive smoke-free legislation was associated with an immediate 34% reduction in hospital admissions for childhood lower RTIs, and a subsequent gradual decrease of 14% per year |
NA=not applicable. NR=not reported. RTI=respiratory tract infection.
Both smoke-free laws were accompanied by a tobacco tax increase and mass-media campaign.
Exceptions to this smoke-free law were: hotels, bars and restaurants, sports, arts and culture venues, amusement arcades, tobacconist shops, international passenger transport systems, private spaces, open air, and designated areas for smoking within each facility.
The smoke-free law now included hospitality venues: hotels, bars and restaurants, sports, art and culture venues, amusement arcades, tobacconist shops, and international passenger transport systems. Designated smoking areas within each facility were still allowed.
Percentage point change.
The single smoke-free law model includes either the step or slope change of a single smoke-free law into the model.
The final was obtained by including all three step changes and all three slope changes in one model and removing the least significant factors one at a time.
No association measures were reported.
Authorised smoking in establishments smaller than 80 m2 and designated smoking areas in larger establishments.
Different states passed different 100% smoke-free laws: workplaces, restaurants, and bars (eight states); restaurants and bars (two states); workplaces and restaurants (one state); and workplaces (one state).
Including, but not limited to restaurants, bars, bowling alleys, bingo halls, convenience stores, laundromats, and other business open to the public.
Table 2.
Association between implementation of smoking cessation services and primary outcomes
| Details of intervention | Population at risk (n) | Events (n) | Slope before intervention (% change in events per year) | Direct change in events (step change, %; 95% CI) | Sustained change in events per year (slope change, %; 95% CI) | Summary of findings | |
|---|---|---|---|---|---|---|---|
| Preterm birth | |||||||
| Jarlenski (2014)47 | State adoption of one of two optional Medicaid enrolment policies, allowing more low-income pregnant women to receive prenatal care, including smoking cessation services (presumptive eligibility and the unborn child option)* | 24 544 | NR | NR | Overall: −1·4%§ (−4·7 to 2·0)Comprehensive: −2·2%§ (−5·9 to 1·5)Non-comprehensive: 1·3%§ (−2·4 to 5·1) | NA | Neither optional Medicaid enrolment policy was associated with significant changes in preterm birth |
| Asthma exacerbations requiring hospital attendance | |||||||
| Hawkins (2016)18 | Health reform legislation that provided counselling for smoking cessation and tobacco cessation treatment to Medicaid recipients | NR | 112 808 | NR | 2% (−4 to 8) | NA | The state-wide health reform legislation in MA, USA, was not associated with significant changes in emergency department visits for asthma |
| Upper RTI admissions | |||||||
| Hawkins (2016)18 | Health reform legislation that provided counselling for smoking cessation and tobacco cessation treatment to Medicaid recipients | NR | 337 628 | NR | −6% (−10 to −1) | NA | The state-wide health reform legislation in MA, USA, was associated with a 6% decrease in emergency department visits for upper RTIs |
| Lower RTI admissions | |||||||
| Hawkins (2016)18 | Health reform legislation that provided counselling for smoking cessation and tobacco cessation treatment to Medicaid recipients | NR | 113 137 | NR | 0% (−6 to 6) | NA | The state-wide health reform legislation in MA, USA, was not associated with significant changes in emergency department visits for lower RTIs |
NR=not reported. NA=not applicable. RTI=respiratory tract infection.
Presumptive eligibility: low-income pregnant women are presumed to be eligible for Medicaid, so they can receive care (including smoking cessation services) while their Medicaid applications are still pending. The unborn-child option: the state can consider a fetus a “targeted low-income child”, allowing coverage of prenatal care (including smoking cessation services) and delivery to low-income pregnant women, even if they cannot provide documentation of citizenship or residency.
Table 3.
Association between implementation of tobacco taxation and primary outcomes
| Details of intervention | Population at risk (n) | Events (n) | Slope before intervention (% change in events per year) | Direct change in events (step change, %; 95% CI) | Sustained change in events per year (slope change, %; 95% CI) | Summary of findings | |
|---|---|---|---|---|---|---|---|
| Preterm birth | |||||||
| Hawkins (2014)46 | Effect of cigarette excise tax increase (in USD$; December 2010 rates) on mothers, by years of maternal education | 9 981 855 | NR | NR | White mothers: 0–11 years, −0·07%§ (−0·11 to −0·02); 12 years, −0·02%§ (−0·05 to 0·01); 13–15 years, −0·01%§ (−0·03 to 0·00); ≥16 years, −0·00%§ (−0·01 to 0·01) per USD$ increase in tax | NA | Cigarette taxes were associated with a decrease in preterm birth among white mothers with the least amount of education |
| Hawkins (2014)46 | Effect of cigarette excise tax increase (in USD$; December 2010 rates) on mothers, by years of maternal education | 2 722 846 | NR | NR | Black mothers: 0–11 years, −0·08%§ (−0·14 to −0·03); 12 years, −0·04%§ (−0·07 to −0·01); 13–15 years, −0·03%§ (−0·05 to −0·01); ≥16 years, −0·01%§ (−0·01 to −0·00) per USD$ increase in tax | NA | Cigarette taxes were associated with a decrease in preterm births among black mothers with any level of education; among black mothers, there was a gradient across maternal education levels, with the largest decreases among mothers with the least amount of education |
| Hawkins (2014)46 | Effect of cigarette excise tax increase (in USD$; December 2010 rates) on mothers, by years of maternal education | 2 444 673 | NR | NR | Hispanic mothers: 0–11 years, 0·01%§ (−0·00 to 0·02); 12 years, −0·00%§ (−0·01 to 0·00); 13–15 years, −0·01%§ (−0·02 to 0·00); ≥16 years, −0·00%§ (−0·00 to 0·00) per USD$ increase in tax | NA | Cigarette taxes were not associated with significant changes in preterm births among Hispanic mothers with any level of education |
| Hawkins (2014)46 | Effect of cigarette excise tax increase (in USD$; December 2010 rates) on mothers, by years of maternal education | 804 447 | NR | NR | Asian/Pacific Islander mothers: 0–11 years, 0·01%§ (−0·01 to 0·04); 12 years, −0·01%§ (−0·01 to 0·00); 13–15 years, −0·00%§ (−0·01 to 0·01); ≥16 years, 0·00%§ (−0·00 to 0·00) per USD$ increase in tax | NA | Cigarette taxes were not associated with significant changes in preterm births among Asian/Pacific Islander mothers with any level of education |
| Hawkins (2014)46 | Effect of cigarette excise tax increase (in USD$; December, 2010, rates) on mothers, by years of maternal education | 244 823 | NR | NR | Native American/Alaska Native mothers: 0–11 years, −0·02%§ (−0·08 to 0·04); 12 years, 0·01%§ (−0·02 to 0·03);13–15 years, 0·00%§ (−0·03 to 0·03); ≥16 years, −0·01%§ (−0·02 to 0·01) per USD$ increase in tax | NA | Cigarette taxes were not associated with significant changes in preterm births among Native American/Alaska Native mothers with any level of education |
| Markowitz (2013)53 | Cigarette excise tax increase (in 2008 USD$)Cigarette price increase (in 2008 USD$) | Maternal age <20 years: 54 132Maternal age 20–24 years: 101 723Maternal age 25–34 years: 183 763Maternal age >34 years: 53 109 | Maternal age <20 years: 5413Maternal age 20–24 years: 7120Maternal age 25–34 years: 11 026Maternal age >34 years: 3718 | NR | Cigarette excise tax: maternal age <20 years, −2·0%§ (−4·0 to 0·0) per USD$ increase in tax;maternal age 20–24 years, −0·7%§ (−1·4 to −0·0) per USD$ increase in tax; maternal age 25–34 years, −0·2%§ (−1·0 to 0·6) per USD$ increase in tax; maternal age >34 years, −1·0%§ (−1·9 to −0·1) per USD$ increase in taxCigarette price: NR | NA | State-wide increases in cigarette excise tax were associated with a 0·7 percentage point decrease in preterm births among women aged 20–24 years, and a 1·0 percentage point decrease among women aged >34 years |
| Asthma exacerbations requiring hospital attendance | |||||||
| Hawkins (2016)18 | Cigarette excise tax increase in USD$ | NR | 128 807 | NR | −5% (−11 to 1) per USD$ increase in tax | NA | State-wide increase in cigarette excise tax was not associated with significant changes in emergency department visits for paediatric asthma |
| Landers (2014)49 | Cigarette excise tax increase in USD$ | NR | NR | Mean rate across all states and years: 9·02 per 10 000 (SD 9·66; range 0·00– 144·47) | −0·53%§ (−0·99 to −0·06) per USD$ increase in tax | NA | State-wide increase in cigarette excise tax was associated with a 0·5 percentage point decrease in asthma discharge rates |
| Ma (2013)50 | USD$0·69 cigarette excise tax increase;USD$0·35 cigarette excise tax increase | 28 498 070 | 702 771 | 0·04 | USD$0·69 cigarette excise tax increase: −11·01% (−24·71 to 2·77);USD$0·35 cigarette excise tax increase: −22·02% (−33·46 to −9·95) | USD$0·69 cigarette excise tax increase: 4·88% (1·29 to 8·59)USD$0·35 cigarette excise tax increase: −4·72% (−8·01 to −1·44) | The first cigarette excise tax increase (USD$0·69) was not associated with significant immediate changes, but was associated with a significant, gradual increase in asthma-related hospital admissions of 0·5% per year; the second cigarette excise tax increase (USD$0·35) was associated with both a 22% immediate decrease as well as a gradual 5% decrease in asthma-related hospital admissions per year |
| Upper RTI admissions | |||||||
| Hawkins (2016)18 | Cigarette excise tax increase in USD$ | NR | 410 686 | NR | −2% (−6% to 2%) per USD$ increase in tax | NA | State-wide increase in cigarette excise tax was not associated with significant changes in emergency department visits for upper RTIs |
| Lower RTI admissions | |||||||
| Hawkins (2016)18 | Cigarette excise tax increase in USD$ | NR | 139 239 | NR | −9% (−16 to −2) per USD$ increase in tax | NA | State-wide increase in cigarette excise tax was associated with a 9% decrease in emergency department visits for lower RTIs |
NA=not applicable. NR=not reported. RTI=respiratory tract infection.
A national study from the Netherlands, comprising 1 980 727 births, found no change in perinatal mortality following a law to prohibit smoking in workplaces and on public transport, or following expansion of the law to include restaurants and bars.58 In a study from England, comprising 10 291 113 births, comprehensive smoke-free legislation in public places and workplaces (including restaurants and bars) was associated with a reduction in stillbirths (–7·8%; 95% CI −18·0 to −3·5) and neonatal deaths (–7·6%; −11·7 to −3·4).32 The overall effect on perinatal mortality (ie, stillbirths and early neonatal deaths combined) was not reported in this study. Therefore, no meta-analysis was possible with these two studies.
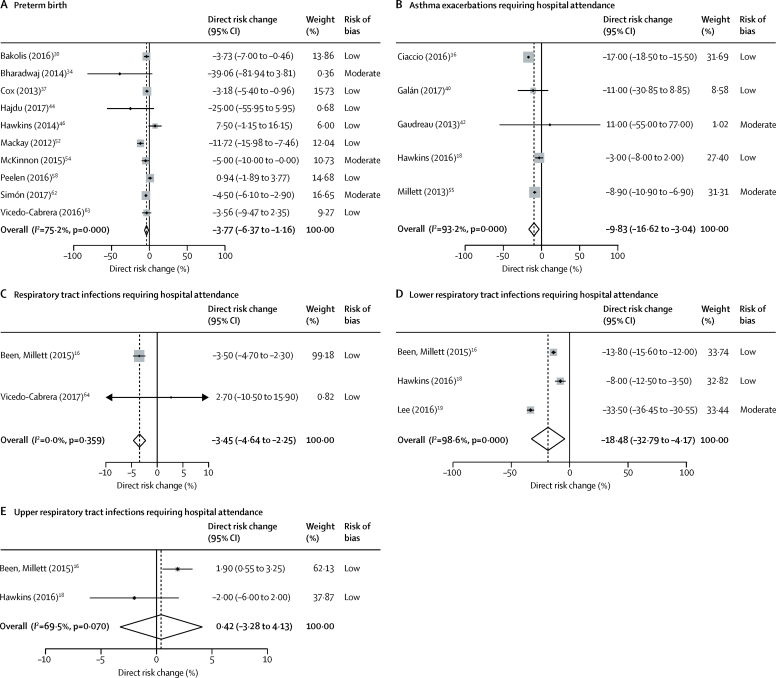
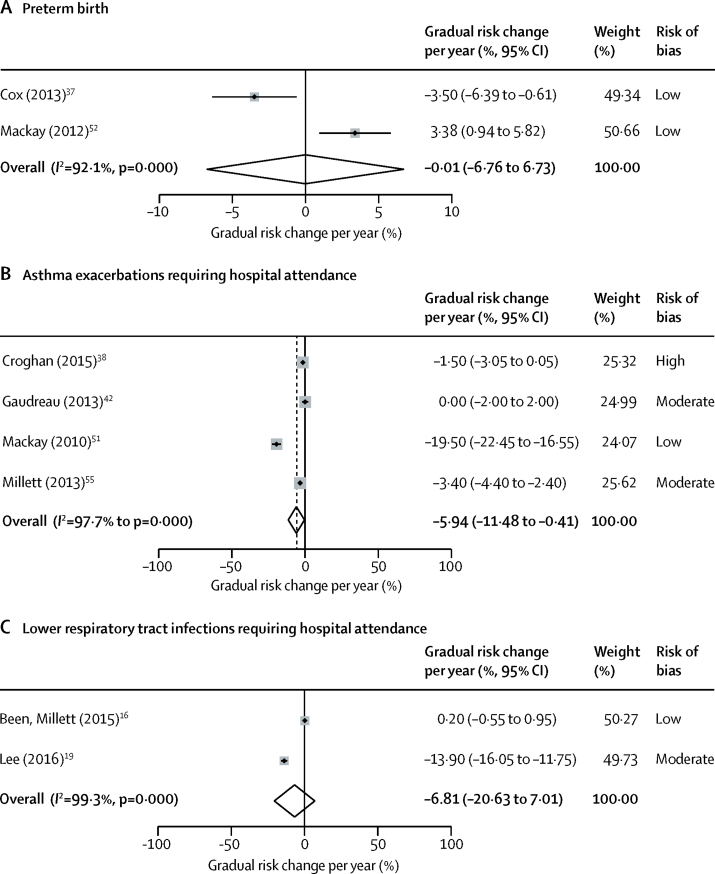
15 studies investigated the association between smoke-free legislation and preterm births.30, 31, 34, 37, 43, 44, 45, 46, 52, 53, 54, 56, 58, 62, 63 In the meta-analysis, smoke-free legislation was associated with a significant immediate reduction in preterm births (ten studies, 27 530 183 individuals; −3·77% [95% CI −6·37 to −1·16]; figure 2A). Two studies caused some funnel plot asymmetry suggestive of publication bias, but this asymmetry was unlikely to have affected our findings (appendix p 34). No additional gradual change in preterm births was evident (two studies, 1 316 633 individuals; −0·01% per year [95% CI −6·76 to 6·73]; figure 3A). One study47 examined the association between provision of smoking cessation services and preterm births. Medicaid enrolment policies permitting low-income pregnant women to receive smoking cessation services were not associated with a change in preterm births (table 2).47 Reductions in preterm birth were observed after tobacco tax increases among women in specific population subgroups in two studies.46, 53 One study reported tobacco taxation to be associated with reduced rates of preterm birth among white mothers with low levels of education and among black mothers irrespective of level of education (table 3).46 The other study reported a 0·7 percentage point decrease in preterm births per USD$ increase in tax among women aged 20–24 years, and a 1·0 percentage point decrease per USD$ increase in tax among women older than 34 years (table 3).53
Figure 2.
Meta-analysis of immediate changes in primary outcomes after implementation of smoke-free legislation
(A) Preterm birth. (B) Asthma exacerbations requiring hospital attendance. (C) Respiratory tract infections requiring hospital attendance. (D) Lower respiratory tract infections requiring hospital attendance. (E) Upper respiratory tract infections requiring hospital attendance.
Figure 3.
Meta-analysis of gradual changes in primary outcomes after implementation of smoke-free legislation
(A) Preterm birth. (B) Asthma exacerbations requiring hospital attendance. (C) Lower respiratory tract infections requiring hospital attendance.
Associations between smoke-free legislation and the incidence of hospital attendances for childhood asthma were reported in ten studies (table 1).18, 36, 38, 40, 42, 49, 51, 55, 59, 61 In the meta-analysis, both an immediate reduction in asthma exacerbations requiring hospital attendance (five studies, 684 826 events; −9·83% [95% CI −16·62 to −3·04]; figure 2B) and an additional gradual reduction were seen (four studies, 243 377 events; −5·94% per year [95% CI −11·48 to −0·41]; figure 3B). No change in asthma admissions was seen following a health reform legislation that provided smoking cessation services for Medicaid recipients in one study.18 Among three US studies18, 49, 50 with overlapping populations evaluating tobacco taxation and asthma exacerbations requiring hospital attendance, the study with the lowest risk of bias found no significant reductions following state-wide increases in cigarette excise tax (table 3).18
The association between smoke-free legislation and the incidence of hospital admissions for acute respiratory tract infections was reported in four studies (table 1).16, 18, 19, 64 In the meta-analysis, an immediate reduction was seen in respiratory tract infections (upper and lower respiratory tract infections combined) requiring hospital attendance (two studies, 1 681 020 events; −3·45% [95% CI −4·64 to −2·25]; figure 2C). For the studies that reported specifically on lower respiratory tract infections, the meta-analysis showed an immediate reduction in admissions for lower respiratory tract infections following smoke-free legislation (three studies, 887 414 events; −18·48% [95% CI −32·79 to −4·17]; figure 2D). No additional gradual reduction in lower respiratory tract infections was observed (two studies; 748 175 events: −6·81% per year [95% CI −20·63 to 7·01]; figure 3C). No significant association between smoke-free legislation and admissions for upper respiratory tract infections was seen in the meta-analysis (two studies; 1 390 056 events; 0·42% [95% CI −3·28 to 4·13]; figure 2E). One study18 reported that a health reform legislation that provided smoking cessation services for Medicaid recipients was associated with an immediate −6% (95% CI −10 to −1) decrease in hospital admissions for childhood upper respiratory tract infection, but not in admissions for lower respiratory tract infection (table 2). The same study18 evaluated the effect of tobacco taxation, showing a −9% decrease (95% CI −16 to −2) in lower respiratory tract infections requiring admission to hospital per USD$ increase in cigarette excise tax at the state level (table 3).
We did not identify any studies assessing the effect of other MPOWER policies on child health.
In sensitivity analyses, inclusion of non-EPOC studies in the meta-analyses or restriction of the primary analyses to studies with low to moderate risk of bias did not materially change the effect estimates for smoke-free legislation and our primary outcomes (appendix pp 35–39).
Point estimates for the association between smoke-free legislation and our primary outcomes were generally much larger when subgroup analyses were restricted to studies assessing comprehensive smoke-free laws than when studies assessing partial smoke-free laws were analysed (preterm birth: seven studies, 9 355 359 individuals, −5·12% [95% CI −7·24 to −2·99]; hospital attendances for asthma: four studies, 556 019 events, −12·49% [–19·78 to −5·20]; appendix, pp 40–44).
11 studies assessed whether the association between implementation of tobacco control policies and child health varied according to indicators of socioeconomic status (appendix pp 45, 46).16, 29, 30, 33, 44, 46, 51, 54, 55, 62, 63 One study16 showed that the most deprived children experienced the largest gradual reduction in hospital admissions for respiratory tract infection following smoke-free legislation (–1·5% per year [95% CI −2·1 to −1·0]). In two studies,44, 46 improvements in perinatal outcomes were greater among babies born to parents with low levels of education following smoke-free legislation than among those born to parents with high levels of education,44 and among babies born to black mothers with any level of education and to white mothers with low levels of education following tobacco tax increases.46 Other studies did not identify a clear socioeconomic gradient in the association between tobacco control policies and child health.
27 studies assessed the association between tobacco control policies and secondary outcomes (appendix, pp 47–76). In the meta-analyses (appendix pp 77–85), smoke-free legislation was associated with immediate reductions in very preterm birth (five studies; 3 354 636 individuals; −9·99% [95% CI −15·74 to −4·24]), low birthweight (nine studies; 35 206 918 individuals, −2·77% [–4·36 to −1·19]), and small for gestational age births (eight studies; 27 649 380 individuals; −1·84% [–3·21 to −0·47]), a gradual reduction in very small for gestational age births (two studies; 1 298 276 individuals; −0·60% per year [–0·60 to −0·60]), and a small increase in birthweight (seven studies; 3 238 575 individuals; 12·45 g [95% CI 2·09–22·81]). No significant changes in other secondary outcomes were seen following smoke-free legislation. Legislation to promote prenatal care, including smoking cessation services for low-income pregnant women, was not associated with a change in small for gestational age births in one US study.47 In another US study,28 although such legislation was associated with increased duration of gestation, depending on time of enrolment (308 521 participants; 0·063 weeks [95% CI 0·008–0·118] among women who enrolled in the Medicaid insurance programme before or during pregnancy and 0·086 weeks [0·004–0·168] among women who enrolled during pregnancy), it was not associated with a change in birthweight (appendix pp 66, 67). One study showed reductions in extremely and very preterm births following tobacco tax increases,53 with two others also showing an increase in gestation.28, 35 Among five studies assessing the link between tobacco tax and birthweight, two showed a positive effect,39, 46 albeit of very small magnitude. Accordingly, only one of these five studies showed a reduction in low birthweight following tobacco tax increases.46 This study also found reductions in small for gestational age births; both associations were confined to low socioeconomic groups.46 In two studies assessing very low birthweight, no changes were seen following tobacco tax increases.39, 53 Tobacco taxes were associated with a decreased risk of infant mortality in two studies assessing this association.57, 60 In one of these studies, however, an increase in fetal deaths was also observed.60 One study showed significant reductions in paediatric asthma prevalence following tobacco tax increases.33
Discussion
This systematic review and meta-analysis provides considerable evidence indicating child health benefits associated with implementation of MPOWER policies. By pooling data of 27·5 million births, 685 000 hospital admissions for asthma, and 2·3 million hospital admissions for respiratory tract infections, we found a 3·7% reduction in preterm births, a 9·8% reduction in childhood hospital admissions for asthma, and an 18·5% reduction in hospital admissions for lower respiratory tract infections following implementation of smoke-free legislation. Subgroup analyses suggested that health benefits were increased when the most comprehensive laws were applied. We also identified several studies indicating that tobacco tax increases and governmental support for smoking cessation services could benefit child health. Taken together with substantial existing evidence on the effectiveness of tobacco control policies in improving adult health, these findings provide strong support for implementation of such policies comprehensively across the world.
This study is, to our knowledge, the most comprehensive assessment done to date of the effect of tobacco control policies on perinatal and child health outcomes. On the basis of our previous work,15 and the challenges of evaluating governmental policies through randomised trials,69, 70 we anticipated that most eligible studies would be of quasi-experimental design. We therefore followed EPOC guidelines to restrict our primary analyses to study types that were considered to be at lowest risk of bias. We confirmed the robustness of our findings via a number of prespecified sensitivity analyses, which indicated that our findings were not sensitive to exclusion of studies with a high risk of bias or inclusion of purely observational studies. Our work builds on existing evidence since it focuses on all available evidence on the effect of tobacco control policies on perinatal and child health. The consistency of this evidence, in our view, supports the validity of our findings.
However, our study has some limitations. The risks of residual confounding and bias in quasi-experimental studies—due to non-random allocation of the intervention and the absence of a control group—need to be considered when interpreting the results.71 Additional limitations include between-study heterogeneity in methodology, differences in follow-up duration and diagnosis ascertainment, the absence of assessment of the likely causal pathways between the policies and their health effects in several studies, and the low number of studies in each meta-analysis, which precluded assessment of publication bias for most outcomes and the use of meta-regression.
This study adds to our previous work.15 We identified an additional 24 studies on the effect of smoke-free legislation on child health, comprising additional data from more than 10 million births, 4·6 million GP diagnoses, and 2·2 million hospital admissions. These additional studies allowed us, for the first time, to identify the association between smoke-free legislation and reductions in severe respiratory tract infections, which is particularly relevant since respiratory tract infections account for the vast majority of the global burden of disease resulting from second-hand smoke exposure in children.1 We also broadened the scope of this study to include all MPOWER policies, identifying several studies on the effect of tobacco tax increases and smoking cessation services on child health. We also identified one study evaluating a tobacco control policy that could not be classified according to WHO's MPOWER Framework. Following an increase in the minimum legal age to purchase cigarettes from 18 years to 21 years in the US state of Pennsylvania, a 1·4 (95% CI −2·6 to −0·2) percentage point reduction in low birthweight was observed, which was largest among smoking mothers and associated with a significant reduction in prenatal cigarette consumption.72
Socioeconomic disparities in smoking and related morbidity are widely documented and affect both adults and children. For example, such disparities were estimated to account for 38% of the inequality in stillbirths and 31% of the inequality in infant deaths in Scotland.73 Previous systematic reviews74, 75, 76 showed that, among MPOWER measures, tobacco taxation has the greatest potential to reduce socioeconomic disparities associated with smoking in both young people and adults. We identified some evidence suggesting a pro-equity effect of both tobacco taxation and smoke-free legislation on early-life health. Since smokers are over-represented among deprived communities, such relative benefits of tobacco control policies translate into larger absolute effects in children from low socioeconomic groups than in children from high socioeconomic groups.
Given the inherent restrictions in attributing causality from quasi-experimental studies, it is important to interpret the findings in light of circumstantial evidence supporting the link between tobacco control policies and child health benefits. We have previously described the main likely causal pathways.23 Tobacco smoke exposure during fetal stages and childhood is associated with various adverse perinatal and child health outcomes.4, 5, 6, 8, 77, 78, 79, 80, 81, 82, 83 Several studies have shown substantial reductions in maternal smoking31, 34, 52, 56, 84, 85 and in second-hand smoke exposure among adults (including pregnant women) and children after implementation of tobacco control policies (appendix pp 86–89).12, 13, 86, 87, 88, 89, 90 Whereas smoke-free laws specifically target public spaces, various studies have shown subsequent increases in smoking cessation and reduced initiation,12, 91, 92 as well as changes in social norms leading to decreased smoking in the home environment,93, 94, 95, 96, 97 which is probably the primary source of second-hand smoke exposure among children. Our study provides further support for a causal association, since we found the largest decreases in our outcomes of interest when comprehensive smoke-free legislation was considered. This observation is suggestive of a dose–response association, which has previously also been identified for adult studies.98 Because of the low number of studies in individual meta-analyses we did not formally test for this interaction, and future efforts to do so might strengthen our findings as more evidence becomes available.
The global health burden of tobacco use is tremendous and its total global economic cost is estimated to be around USD$1·4 trillion.99 Despite global progress in tobacco control, over a third of the world's population remains unprotected by any MPOWER policy at the recommended level.2, 11 This issue is important because 40–50% of children worldwide are regularly exposed to tobacco smoke, and tobacco control policies have substantial potential to reduce the associated burden of death and disease.1 This global burden is acknowledged by the prioritisation within SDG 3 of more effective FCTC implementation and its aim to reduce early-life mortality; our data now show that these initiatives can act synergistically. Because our effect estimates are expressed as relative changes, background prevalence of smoking and second-hand smoke exposure, and of the health outcomes evaluated, should be considered when extrapolating our findings to local contexts. We did not formally assess the comparative effectiveness or cost-effectiveness of different MPOWER policies. Tax increases are considered to be the most effective measure to reduce smoking prevalence,2 and although our review indicates that tobacco taxation is likely to be associated with child health benefits, the evidence was particularly strong for smoke-free legislation. Smoke-free laws are the tobacco control policy most strongly supported by the public and appear to be the most straightforward measure to protect child health, particularly when implemented comprehensively.12 The synergistic effect of various policies implemented at the highest recommended levels in reducing smoking prevalence should be considered when planning policy changes,14 which, when implemented as part of a strong tobacco control programme, can be highly cost-effective.100, 101 Ongoing monitoring is needed to continue to evaluate the effectiveness of policies aimed at reducing the impact of tobacco, in particular the effectiveness of novel endgame strategies targeted at ending rather than controlling the global tobacco epidemic.102
Reports indicate that at least two of five people living in low-income and middle-income countries remain unprotected by any MPOWER policy measure,2 and that wide variations in implementation and compliance are present across these countries.103 This finding is of concern, since these countries have the largest burden of tobacco-related illness and death, and harbour nearly 80% of the world's smokers.2 We highlight an important gap in the literature as more research is required in low-income and middle-income countries to understand the effect of tobacco control policies in these regions. Modelling approaches are increasingly being used to estimate the effect of tobacco control policies in low-income and middle-income countries, and original studies are now becoming available.104, 105 Efforts are underway to address the current absence of a child health focus in this area, which will be essential to inform the global policy agenda. Furthermore, we found no studies specifically evaluating early-life health outcomes in relation to legislation to prohibit tobacco advertising and sponsorship, or warnings against the dangers of tobacco. Priority should be given to establishing a core set of outcomes related to perinatal and child health, alongside adult health, for all future studies examining the effect of tobacco control policies.
In conclusion, given the positive findings of this systematic review it is crucial that the uptake of comprehensive tobacco control policies is accelerated worldwide to further protect children from the health hazards of tobacco smoke exposure,106 in parallel with efforts to evaluate the effectiveness of novel policy initiatives.
Acknowledgments
Acknowledgments
This study was funded by the Scottish Government Chief Scientist Office (CSO), and by personal fellowships to JVB from the Netherlands Lung Foundation (4.2.14.063JO) and the Erasmus MC. AS is supported by the Farr Institute. CM is funded by an NIHR Research Professorship award. We thank Wichor Bramer for assistance in preparing the search strategy; Amanda Amos, Anna Gilmore, Stanton Glantz, Summer Hawkins, Zubair Kabir, David Levy, Daniel Mackay, and Sara Markowitz for providing advice as members of the expert panel; Ioannis Bakolis, Bianca Cox, Summer Hawkins, Julian Johnsen, Glenn Landers, Britt McKinnon, and Yelena Tarasenko for providing additional information or data upon request on behalf of all authors of their respective articles; and Daan Nieboer and Chris Weir for providing assistance in the statistical analyses.
Contributors
JVB and AS secured funding for this work. JVB, JPM, CM, SB, and AS designed the study and wrote the protocol. TF and AK did the study search, study selection, data extraction, and risk of bias assessment. JVB supervised all the steps in the review process. TF did the data analysis and created the figures. All authors interpreted the findings. TF, AK, and JVB drafted the manuscript and appendix. AS supervised the writing, and JPM, CM, and SB provided feedback.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 2.WHO . WHO report on the global tobacco epidemic, 2017. Monitoring tobacco use and prevention policies. World Health Organization; Geneva: 2017. [Google Scholar]
- 3.Blencowe H, Cousens S, Oestergaard MZ. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 4.Flenady V, Koopmans L, Middleton P. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics. 2011;127:734–741. doi: 10.1542/peds.2010-3041. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93:f351–f361. doi: 10.1136/adc.2007.133553. [DOI] [PubMed] [Google Scholar]
- 7.Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184:87–97. doi: 10.1093/aje/kwv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang K, Wang X. Maternal smoking and increased risk of sudden infant death syndrome: a meta-analysis. Leg Med. 2013;15:115–121. doi: 10.1016/j.legalmed.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Simons E, To T, Moineddin R, Stieb D, Dell SD. Maternal second-hand smoke exposure in pregnancy is associated with childhood asthma development. J Allergy Clin Immunol Pract. 2014;2:201–207. doi: 10.1016/j.jaip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 10.WHO . Guidelines for implementation of Article 13 of the WHO Framework Convention on Tobacco Control (tobacco advertising, promotion, and sponsorship) World Health Organization; Geneva: 2008. [Google Scholar]
- 11.GBD 2015 Tobacco Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman SJ, Tan C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health. 2015;15:744. doi: 10.1186/s12889-015-2041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frazer K, Callinan JE, McHugh J. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev. 2016;2 doi: 10.1002/14651858.CD005992.pub3. cd005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravely S, Glovino GA, Craig L. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. Lancet Public Health. 2017;2:e166–e174. doi: 10.1016/S2468-2667(17)30045-2. [DOI] [PubMed] [Google Scholar]
- 15.Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet. 2014;383:1549–1560. doi: 10.1016/S0140-6736(14)60082-9. [DOI] [PubMed] [Google Scholar]
- 16.Been JV, Millett C, Lee JT, van Schayck CP, Sheikh A. Smoke-free legislation and childhood hospitalisations for respiratory tract infections. Eur Respir J. 2015;46:697–706. doi: 10.1183/09031936.00014615. [DOI] [PubMed] [Google Scholar]
- 17.Been JV, Szatkowski L, van Staa TP. Smoke-free legislation and the incidence of paediatric respiratory infections and wheezing/asthma: interrupted time series analyses in the four UK nations. Sci Rep. 2015;5:15246. doi: 10.1038/srep15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins SS, Hristakeva S, Gottlieb M, Baum CF. Reduction in emergency department visits for children's asthma, ear infections, and respiratory infections after the introduction of state smoke-free legislation. Prev Med. 2016;89:278–285. doi: 10.1016/j.ypmed.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SL, Wong WH, Lau YL. Smoke-free legislation reduces hospital admissions for childhood lower respiratory tract infection. Tob Control. 2016;25:e90–e94. doi: 10.1136/tobaccocontrol-2015-052541. [DOI] [PubMed] [Google Scholar]
- 20.Been JV, Mackenbach JP, Millett C, Basu S, Sheikh A. Tobacco control policies and perinatal and child health: a systematic review and meta-analysis protocol. BMJ Open. 2015;5:e008398. doi: 10.1136/bmjopen-2015-008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: including non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011) The Cochrane Collaboration; London: 2011. [Google Scholar]
- 23.Faber T, Been JV, Reiss IK, Mackenbach JP, Sheikh A. Smoke-free legislation and child health. NPJ Prim Care Respir Med. 2016;26:16067. doi: 10.1038/npjpcrm.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagijo MA, Sheikh A, Duijts L, Been JV. Reducing tobacco smoking and smoke exposure to prevent preterm birth and its complications. Paediatr Respir Rev. 2017;22:3–10. doi: 10.1016/j.prrv.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 25.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cochrane Effective Practice and Organisation of Care Review Group Suggested risk of bias criteria for EPOC reviews. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.docx (accessed Aug 3, 2017).
- 27.Effective Public Health Practice Project Quality assessment tool for quantitative studies. http://www.ephpp.ca/PDF/Quality%20Assessment%20Tool_2010_2.pdf (accessed July 13, 2017).
- 28.Adams EK, Markowitz S, Dietz PM, Tong VT. Expansion of Medicaid covered smoking cessation services: maternal smoking and birth outcomes. Medicare Medicaid Res Rev. 2013;3:e1–23. doi: 10.5600/mmrr.003.03.a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral M. The effect of local smoking ordinances on fetal development: evidence from California. Working paper. University of the Pacific; 2009. pp. 1–27. [Google Scholar]
- 30.Bakolis I, Kelly R, Fecht D. Protective effects of smoke-free legislation on birth outcomes in England—a regression discontinuity design. Epidemiology. 2016;27:810–818. doi: 10.1097/EDE.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartholomew KS, Abouk R. The effect of local smokefree regulations on birth outcomes and prenatal smoking. Matern Child Health J. 2016;20:1526–1538. doi: 10.1007/s10995-016-1952-x. [DOI] [PubMed] [Google Scholar]
- 32.Been JV, Mackay DF, Millett C, Pell JP, van Schayck OCP, Sheikh A. Impact of smoke-free legislation on perinatal and infant mortality: a national quasi-experimental study. Sci Rep. 2015;5:13020. doi: 10.1038/srep13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhai M. Understanding the gradient in childhood asthma: the effect of cigarette taxes on inequality. PhD thesis. University of Illinois at Chicago; 2014. [Google Scholar]
- 34.Bharadwaj P, Johnsen JV, Loken KV. Smoking bans, maternal smoking and birth outcomes. J Public Econ. 2014;115:72–93. [Google Scholar]
- 35.Briggs RJ. Essays on the economics of indoor and outdoor environments. PhD thesis. The University of Texas at Austin; 2009. The impact of smoking bans on birth weight: is less more? pp. 64–96. [Google Scholar]
- 36.Ciaccio CE, Gurley-Calvez T, Shireman TI. Indoor tobacco legislation is associated with fewer emergency department visits for asthma exacerbation in children. Ann Allergy Asthma Immunol. 2016;117:641–645. doi: 10.1016/j.anai.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox B, Martens E, Nemery B, Vangronsveld J, Nawrot TS. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. BMJ. 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croghan IT, Ebbert JO, Hays JT. Impact of a countywide smoke-free workplace law on emergency department visits for respiratory diseases: a retrospective cohort study. BMC Pulm Med. 2015;15:1–8. doi: 10.1186/1471-2466-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans WN, Ringel JS. Can higher cigarette taxes improve birth outcomes? J Public Econ. 1999;72:135–154. [Google Scholar]
- 40.Galán I, Simón L, Boldo E. Changes in hospitalizations for chronic respiratory diseases after two successive smoking bans in Spain. PLoS One. 2017;12:e0177979. doi: 10.1371/journal.pone.0177979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Baughman RA. Do smoking bans improve infant health? Evidence from U.S. births: 1995–2009. East Econ J. 2017;43:472–495. [Google Scholar]
- 42.Gaudreau K, Sanford CJ, Cheverie C, McClure C. The effect of a smoking ban on hospitalization rates for cardiovascular and respiratory conditions in Prince Edward Island, Canada. PLoS One. 2013;8:e56102. doi: 10.1371/journal.pone.0056102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hade E. Analyses of the impact of the Ohio smoke-free workplace act. Ohio Department of Health; Columbus, OH: 2011. Analysis of the association between birth outcomes and the Ohio tobacco ban; pp. 24–29.http://www.rosscountyhealth.com/brochures/ODHAnalysis.pdf (accessed July 13, 2017). [Google Scholar]
- 44.Hajdu T, Hajdu G. Smoking ban and health at birth [preliminary discussion paper] Institute of Economics, Centre for Economic and Regional Studies, Hungarian Academy of Sciences; Budapest, Hungary: 2017. http://real.mtak.hu/49950/1/MTDP1706.pdf (accessed July 13, 2017). [Google Scholar]
- 45.Hankins S, Tarasenko Y. Do smoking bans improve neonatal health? Health Serv Res. 2016;51:1858–1878. doi: 10.1111/1475-6773.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkins SS, Baum CF, Oken E, Gillman MW. Associations of tobacco control policies with birth outcomes. JAMA Pediatr. 2014;168:186–196. doi: 10.1001/jamapediatrics.2014.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarlenski M, Bleich SN, Bennett WL, Stuart EA, Barry CL. Medicaid enrollment policy increased smoking cessation among pregnant women but had no impact on birth outcomes. Health Aff. 2014;33:997–1005. doi: 10.1377/hlthaff.2013.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabir Z, Daly S, Clarke V, Keogan S, Clancy L. Smoking ban and small-for-gestational age births in Ireland. PLoS One. 2013;8:e57441. doi: 10.1371/journal.pone.0057441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landers G. The impact of smoke-free laws on asthma discharges: a multistate analysis. Am J Public Health. 2014;104:e74–e79. doi: 10.2105/AJPH.2013.301697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma ZQ, Kuller LH, Fisher MA, Ostroff SM. Use of interrupted time-series method to evaluate the impact of cigarette excise tax increases in Pennsylvania, 2000–2009. Prev Chronic Dis. 2013;10:e169. doi: 10.5888/pcd10.120268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackay D, Haw S, Ayres JG, Fischbacher C, Pell JP. Smoke-free legislation and hospitalizations for childhood asthma. N Engl J Med. 2010;363:1139–1145. doi: 10.1056/NEJMoa1002861. [DOI] [PubMed] [Google Scholar]
- 52.Mackay DF, Nelson SM, Haw SJ, Pell JP. Impact of Scotland's smoke-free legislation on pregnancy complications: retrospective cohort study. PLoS Med. 2012;9:e1001175. doi: 10.1371/journal.pmed.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markowitz S, Adams EK, Dietz PM, Kannan V, Tong VT. Tobacco control policies, birth outcomes, and maternal human capital. J Hum Cap. 2013;7:130–160. [Google Scholar]
- 54.McKinnon B, Auger N, Kaufman JS. The impact of smoke-free legislation on educational differences in birth outcomes. J Epidemiol Community Health. 2015;69:937–943. doi: 10.1136/jech-2015-205779. [DOI] [PubMed] [Google Scholar]
- 55.Millett C, Lee JT, Laverty AA, Glantz SA, Majeed A. Hospital admissions for childhood asthma after smoke-free legislation in England. Pediatrics. 2013;131:e495–e501. doi: 10.1542/peds.2012-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page RL, Slejko JF, Libby AM. A citywide smoking ban reduced maternal smoking and risk for preterm births: a colorado natural experiment. J Women's Health. 2012;21:621–627. doi: 10.1089/jwh.2011.3305. [DOI] [PubMed] [Google Scholar]
- 57.Patrick SW, Warner KE, Pordes E, Davis MM. Cigarette tax increase and infant mortality. Pediatrics. 2016;137:1–8. doi: 10.1542/peds.2015-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peelen MJ, Sheikh A, Kok M. Tobacco control policies and perinatal health: a national quasi-experimental study. Sci Rep. 2016;6:23907. doi: 10.1038/srep23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rayens MK, Burkhart PV, Zhang M. Reduction in asthma-related emergency department visits after implementation of a smoke-free law. J Allergy Clin Immunol. 2008;122:537–541. doi: 10.1016/j.jaci.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 60.Sen A, Pierard E. Estimating the effects of cigarette taxes on birth outcomes. Can Public Policy. 2011;37:257–276. doi: 10.3138/cpp.37.2.257. [DOI] [PubMed] [Google Scholar]
- 61.Shetty KD, DeLeire T, White C, Bhattacharya J. Changes in U.S. hospitalization and mortality rates following smoking bans. J Pol Anal Manag. 2011;30:6–28. doi: 10.1002/pam.20548. [DOI] [PubMed] [Google Scholar]
- 62.Simón L, Pastor-Barriuso R, Boldo E. Smoke-free legislation in Spain and prematurity. Pediatrics. 2017;139:e20162068. doi: 10.1542/peds.2016-2068. [DOI] [PubMed] [Google Scholar]
- 63.Vicedo-Cabrera AM, Schindler C, Radovanovic D. Benefits of smoking bans on preterm and early-term births: a natural experimental design in Switzerland. Tob Control. 2016;25:e135–e141. doi: 10.1136/tobaccocontrol-2015-052739. [DOI] [PubMed] [Google Scholar]
- 64.Vicedo-Cabrera AM, Röösli M, Radovanovic D. Cardiorespiratory hospitalisation and mortality reductions after smoking bans in Switzerland. Swiss Med Wkly. 2017;146:w14381. doi: 10.4414/smw.2016.14381. [DOI] [PubMed] [Google Scholar]
- 65.Bianchi M, Campi R, Bonati M. Smoke-free legislation and asthma. N Eng J Med. 2011;364:87–88. doi: 10.1056/NEJMc1011724. [DOI] [PubMed] [Google Scholar]
- 66.Dove MS, Dockery DW, Connolly GN. Smoke-free air laws and asthma prevalence, symptoms, and severity among nonsmoking youth. Pediatrics. 2011;127:102–109. doi: 10.1542/peds.2010-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabir Z, Clarke V, Conroy R, McNamee E, Daly S, Clancy L. Low birthweight and preterm birth rates 1 year before and after the Irish workplace smoking ban. BJOG. 2009;116:1782–1787. doi: 10.1111/j.1471-0528.2009.02374.x. [DOI] [PubMed] [Google Scholar]
- 68.Venkataramani AS, Bor J, Jena AB. Regression discontinuity designs in healthcare research. BMJ. 2016;352:i1216. doi: 10.1136/bmj.i1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci. 2000;1:31–49. doi: 10.1023/a:1010024016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350:h2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Been JV, Sheikh A. Investigating the link between smoke-free legislation and stillbirths. Expert Rev Respir Med. 2016;10:109–112. doi: 10.1586/17476348.2016.1125784. [DOI] [PubMed] [Google Scholar]
- 72.Yan J. The effects of a minimum cigarette purchase age of 21 on prenatal smoking and infant health. East Econ J. 2014;40:289–308. [Google Scholar]
- 73.Gray R, Bonellie SR, Chalmers J. Contribution of smoking during pregnancy to inequalities in stillbirth and infant death in Scotland 1994–2003: retrospective population based study using hospital maternity records. BMJ. 2009;339:b3754. doi: 10.1136/bmj.b3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown T, Platt S, Amos A. Equity impact of interventions and policies to reduce smoking in youth: systematic review. Tob Control. 2014;23:e98–105. doi: 10.1136/tobaccocontrol-2013-051451. [DOI] [PubMed] [Google Scholar]
- 75.Brown T, Platt S, Amos A. Equity impact of population-level interventions and policies to reduce smoking in adults: a systematic review. Drug Alcohol Depend. 2014;138:7–16. doi: 10.1016/j.drugalcdep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Hill S, Amos A, Clifford D, Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control. 2014;23:e89–e97. doi: 10.1136/tobaccocontrol-2013-051110. [DOI] [PubMed] [Google Scholar]
- 77.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ko TJ, Tsai LY, Chu LC. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol. 2014;55:20–27. doi: 10.1016/j.pedneo.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 80.US Department of Health and Human Services . The health consequences of smoking—50 years of progress. A report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- 81.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182:465–472. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silvestri M, Franchi S, Pistorio A, Petecchia L, Rusconi F. Smoke exposure, wheezing, and asthma development: a systematic review and meta-analysis in unselected birth cohorts. Pediatr Pulmonol. 2015;50:353–362. doi: 10.1002/ppul.23037. [DOI] [PubMed] [Google Scholar]
- 83.Vardavas CI, Hohmann C, Patelarou E. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur Respir J. 2016;48:115–124. doi: 10.1183/13993003.01016-2015. [DOI] [PubMed] [Google Scholar]
- 84.Adams EK, Markowitz S, Kannan V, Dietz PM, Tong VT, Malarcher AM. Reducing prenatal smoking: the role of state policies. Am J Prev Med. 2012;43:34–40. doi: 10.1016/j.amepre.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen KH, Wright RJ, Sorensen G, Subramanian SV. Association between local indoor smoking ordinances in Massachusetts and cigarette smoking during pregnancy: a multilevel analysis. Tob Control. 2013;22:184–189. doi: 10.1136/tobaccocontrol-2011-050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akhtar PC, Currie DB, Currie CE, Haw SJ. Changes in child exposure to environmental tobacco smoke (CHETS) study after implementation of smoke-free legislation in Scotland: national cross sectional survey. BMJ. 2007;335:545. doi: 10.1136/bmj.39311.550197.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bell R, Glinianaia SV, Waal ZV. Evaluation of a complex healthcare intervention to increase smoking cessation in pregnant women: interrupted time series analysis with economic evaluation. Tob Control. 2017 doi: 10.1136/tobaccocontrol-2016-053476. DOI:10.1136/tobaccocontrol-2016-053476 published online Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holliday JC, Moore GF, Moore LA. Changes in child exposure to secondhand smoke after implementation of smoke-free legislation in Wales: a repeated cross-sectional study. BMC Public Health. 2009;9:430. doi: 10.1186/1471-2458-9-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jarvis MJ, Sims M, Gilmore A, Mindell J. Impact of smoke-free legislation on children's exposure to secondhand smoke: cotinine data from the Health Survey for England. Tob Control. 2012;21:18–23. doi: 10.1136/tc.2010.041608. [DOI] [PubMed] [Google Scholar]
- 90.Moore GF, Currie D, Gilmore G, Holliday JC, Moore L. Socioeconomic inequalities in childhood exposure to secondhand smoke before and after smoke-free legislation in three UK countries. J Public Health. 2012;34:599–608. doi: 10.1093/pubmed/fds025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katikireddi SV, Der G, Roberts C, Haw S. Has childhood smoking reduced following smoke-free public places legislation? A segmented regression analysis of cross-sectional UK school-based surveys. Nicotine Tob Res. 2016;18:1670–1674. doi: 10.1093/ntr/ntw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shang C. The effect of smoke-free air law in bars on smoking initiation and relapse among teenagers and young adults. Int J Environ Res Public Health. 2015;12:504–520. doi: 10.3390/ijerph120100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng KW, Glantz SA, Lightwood JM. Association between smokefree laws and voluntary smokefree-home rules. Am J Prev Med. 2011;41:566–572. doi: 10.1016/j.amepre.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JT, Glantz SA, Millett C. Effect of smoke-free legislation on adult smoking behaviour in England in the 18 months following implementation. PLoS One. 2011;6:e20933. doi: 10.1371/journal.pone.0020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mons U, Nagelhout GE, Allwright S. Impact of national smoke-free legislation on home smoking bans: findings from the International Tobacco Control Policy Evaluation Project Europe Surveys. Tob Control. 2013;22:e2–e9. doi: 10.1136/tobaccocontrol-2011-050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore GF, Moore L, Littlecott HJ. Prevalence of smoking restrictions and child exposure to secondhand smoke in cars and homes: a repeated cross-sectional survey of children aged 10–11 years in Wales. BMJ Open. 2015;5:e006914. doi: 10.1136/bmjopen-2014-006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nazar GP, Lee JT, Glantz SA, Arora M, Pearce N, Millett C. Association between being employed in a smoke-free workplace and living in a smoke-free home: evidence from 15 low and middle income countries. Prev Med. 2014;59:47–53. doi: 10.1016/j.ypmed.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan CE, Glantz SA. Association between smokefree legislation and hospitalizations for cardiac, cerebrovascular and respiratory diseases: a meta-analysis. Circulation. 2012;126:2177–2183. doi: 10.1161/CIRCULATIONAHA.112.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.US National Cancer Institute. WHO . The economics of tobacco and tobacco control. National Cancer Institute Tobacco Control Monograph 21. NIH Publication No. 16-CA-8029A. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2016. and Geneva: World Health Organization. [Google Scholar]
- 100.Kalkhoran S, Glantz SA. Smoke-free policies: cleaning the air with money to spare. Lancet. 2014;383:1526–1528. doi: 10.1016/S0140-6736(14)60224-5. [DOI] [PubMed] [Google Scholar]
- 101.Lightwood J, Glantz SA. The effect of the California tobacco control program on smoking prevalence, cigarette consumption, and healthcare costs: 1989–2008. PLoS One. 2013;8:e47145. doi: 10.1371/journal.pone.0047145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McDaniel PA, Smith EA, Malone RE. The tobacco endgame: a qualitative review and synthesis. Tob Control. 2016;25:594–604. doi: 10.1136/tobaccocontrol-2015-052356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Husain MJ, English LM, Ramanandraibe N. An overview of tobacco control and prevention policy status in Africa. Prev Med. 2016;91s:s16–s22. doi: 10.1016/j.ypmed.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Basu S, Glantz S, Bitton A, Millett C. The effect of tobacco control measures during a period of rising cardiovascular disease risk in India: a mathematical model of myocardial infarction and stroke. PLoS Med. 2013;10:e1001480. doi: 10.1371/journal.pmed.1001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalkhoran S, Sebrié EM, Sandoya E, Glantz SA. Effect of Uruguay's national 100% smokefree law on emergency visits for bronchospasm. Am J Prev Med. 2015;49:85–88. doi: 10.1016/j.amepre.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toebes B, Gispen ME, Been JV, Sheikh A. A missing voice: the human rights of children to a tobacco-free environment. Tob Control. 2017 doi: 10.1136/tobaccocontrol-2017-053657. DOI:10.1136/tobaccocontrol-2017-053657 published online July 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.