Abstract
Pre-eclampsia is a hypertensive disorder in pregnancy, which accounts for 10–15% of the maternal and perinatal mortality worldwide. Abnormal placental development and tissue hypoxia are its main etiologic factors. The present diagnostic methods of blood pressure monitoring and renal function evaluation are insufficient in the early detection of pre-eclampsia. Since molecular events portent well ahead of the disease onset, biomarker research for the early diagnosis of pre-eclampsia has recently generated ambitious clinical targets. However, no clinically validated biomarker has so far been reported for the prediction of pre-eclampsia. Therefore, this review takes stock of the current understanding of pre-eclampsia from a molecular biology perspective and critically evaluates the following diagnostic potentials claimed for the biomarkers: placental proteins, angiogenic markers, and cell-free fetal DNA (cffDNA) in maternal circulation. Though the emerging evidences in favor of the fetal-specific epigenetic marker, hypermethylated RASSF1A of cffDNA, are highlighted, it pitches for a broader strategy of ‘combination biomarker approach’ for the reliable forecasting and triaging of pre-eclampsia.
Keywords: Cell-free fetal DNA, Hypermethylated RASSF1A, Molecular biomarker, Pre-eclampsia
Introduction
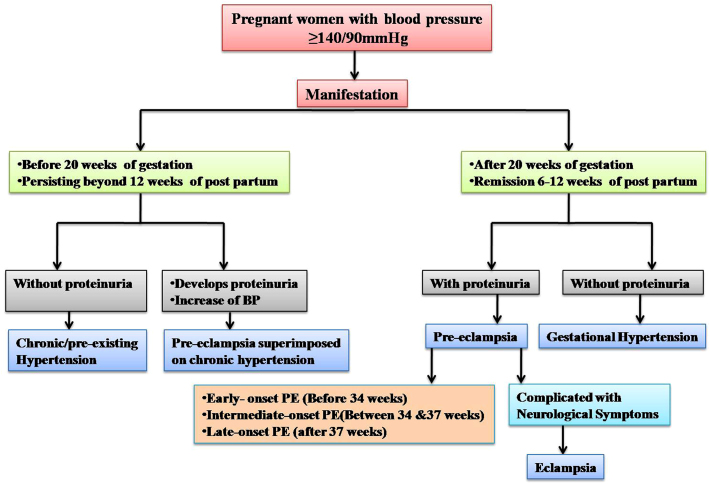
Hippocrates in 4th century BC described hypertensive disorders of pregnancy as a ‘worse state with headache, heaviness and convulsions.’1 Based on the etiology and symptoms, it later came to be called as toxemia, gestosis, pregnancy-induced hypertension, or pre-eclampsia. A consensus on the classification and definition of hypertensive disorders of pregnancy emerged in the 12th world congress of International Society for the Study of hypertension (ISSHP) in Paris in 2000. Accordingly, the disease entity has now been categorized as the following: pre-eclampsia/eclampsia, gestational hypertension, chronic (pre-existing) hypertension, and pre-eclampsia superimposed on chronic hypertension.2 The classification of hypertensive disorders is in Fig. 1.
Fig. 1.
Classification of hypertensive disorders.
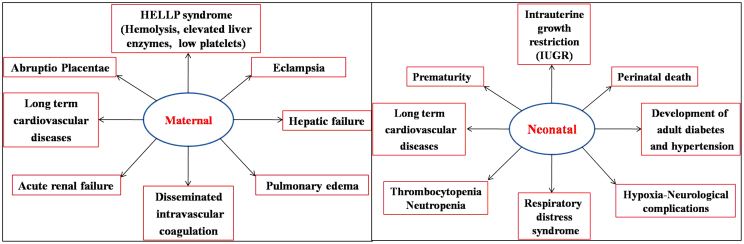
Pre-eclampsia is a hypertensive complication affecting 2–8% of the pregnancies. It is thereby a major contributing factor for the high maternal/perinatal mortality and morbidity seen worldwide.3 Pre-eclampsia patients with high blood pressure can go to an extreme of HELLP syndrome. Hemolysis with elevated liver enzymes and low platelets and further onset of neurological symptoms lead them to eclampsia. Eclampsia is characterized by the following multiple organ involvement: hepatic/renal failure, disseminated intravascular coagulation, pulmonary/cerebral edema, and hemorrhage. The complications occur not only to the mother but also to the fetus – increased risk of respiratory ailments, intrauterine growth restriction (IUGR), and preterm birth.4 Maternal and neonatal complications of pre-eclampsia are summarized in Fig. 2.
Fig. 2.
Maternal and neonatal complications of pre-eclampsia.
The risk factors for pre-eclampsia are the following: maternal age over 40 years, obesity, nullipary, family/previous history, renal or autoimmune disorders, and multiple pregnancies. Diagnosis of pre-eclampsia is usually done by determining blood pressure and proteinuria. However, these are insufficient in detecting the disease either in the early presymptomatic stages or in rightly assessing fetal status – time of delivery or preterm births.5 Admittedly, asymptomatic cases can end up in eclampsia.6 False or miss in diagnosis also occur.7 Early identification of pre-eclampsia and evaluation of fetal health through biomarkers therefore can tremendously improve antenatal care. Moreover, this would differentiate pre-eclampsia from already existing hypertension or glomerular diseases and also in stratifying risk groups.7
The recent past has witnessed a surge in the search for reliable molecular markers for the prediction of pre-eclampsia. A predictive biomarker is defined as a molecule that could clearly estimate the biological status of the disease with high sensitivity and specificity.8 It should preferably establish the therapeutic and prognostic factors of the disease also. With these objectives, many molecules have been investigated for the correct prediction of pre-eclampsia. Being noninvasive, most of them focused on maternal serum markers: placental proteins, angiogenic molecules, DNA markers, etc. Overall, these have helped in improving the current understanding of its pathogenesis. Nonetheless, clinically validated markers for routine screening of pre-eclampsia are yet to come.6, 7, 8 Herein, we review the major molecular events of pre-eclampsia toward determining predictable biomarkers.
Molecular basis of pre-eclampsia
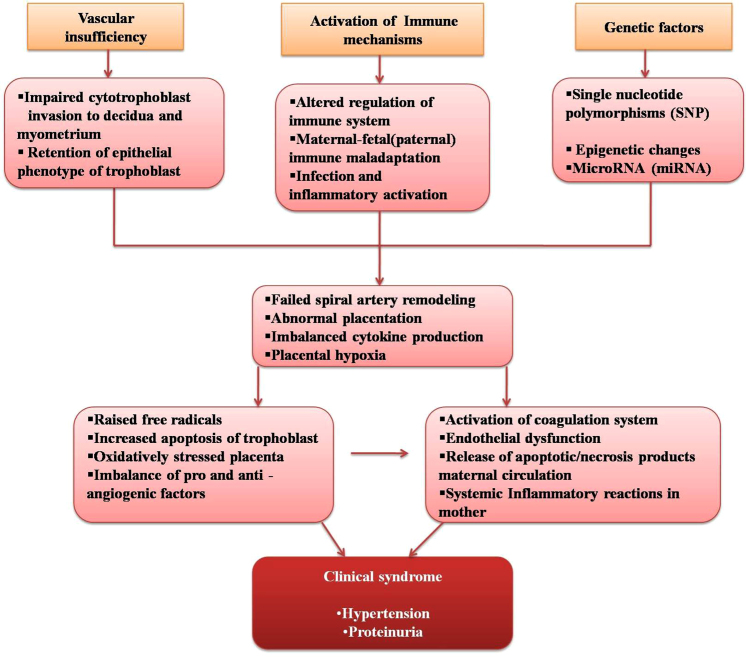
Pre-eclampsia is a maternal response to anomalous placentation. Vascular, immune, and genetic theories have been suggested for its cellular/molecular events (Fig. 3). Placenta is a vascular structure with a primary villous layer of cytotrophoblast (undifferentiated) and syncytiotrophoblast (differentiated) cells. Inner secondary villi are formed by mesenchymal cells, macrophages, fibroblasts, and fetal blood cells. Synchronized vascularization of the placenta is central to the exchange of nutrients, oxygen, and waste materials between mother and fetus. This is accomplished through several steps, vasculogenesis – formation of new blood vessels and angiogenesis – growth of new blood vessels. Alternately, the phenotype of cytotrophoblast can change from epithelial to endothelial type also, and is termed as pseudovasculogenesis.9 In addition to these, maternal spiral arteries undergo modifications to facilitate blood flow to the placenta.10 To this effect, cytotrophoblast cells of placenta sequentially differentiate into extra villous trophoblast (EVT) and invade the decidua, maternal spiral arteries, and then the myometrium. These have been related to the expression of cell surface molecules like chemokine receptor (CCR10), switchover of the expression of adhesion molecules from α6β4, αVβ6, E-cadherin/CD324 to αVβ3, α1β1, VE-cadherin/CD144, VCAM-1 (vascular cell adhesion molecule-1)/CD106, ECAM-1 (platelet endothelial cell adhesion molecule-1)/CD31, and secretion of proteolytic enzymes MMPs (matrix metalloproteinases 1, 2, 3, 9, and 14 and cathepsins), which aid in the degradation of extracellular matrix. At this point, urokinase plasminogen activator, a serine protease, activates MMPs and advances the migration of trophoblasts, during which the cell cycle is also arrested for preventing proliferation.11, 12
Fig. 3.
Molecular events in the progress and development of pre-eclampsia.
During the process of relocation, trophoblast interacts with several types of cells like uterine natural killer cells (uNK), stromal cells, lymphocytes, immune cells, etc.11, 12 The physiological process of artery modification is dependent on the balanced interaction between several cytokines produced by these cells and concomitant expression of activator and inhibitor receptors on trophoblasts. uNK cells secrete cytokines like, IL10, IL8, granulocyte macrophage colony-stimulating factor (G-CSF1 and 2), and tumor necrosis factor α (α-TNF).13 Besides, uNK cells express ‘pro’ and ‘anti’ angiogenic factors, transforming growth factor β (TGFβ), vascular endothelial growth factor (VEGF), and placental growth factor (PGF). uNK cells also assist invasion by interacting with the following receptors: killer immunoglobulin-like receptors (KIR), the C-type lectin heterodimer family (CD94/NKGs) receptors, the natural killer cytotoxicity receptors (NCR), and the immunoglobulin-like transcripts (ILT) with class I HLA (human leukocyte antigen) molecules expressed on EVT as HLA-G, HLA-C, and HLA-E. Impairment in all these cause altered cytokine production, decreased MMP secretion, and retention of proliferative trophoblast instead of invasive phenotype. Spiral artery remodeling is seriously affected and eventually a disordered placentation results in pre-eclampsia. Scarce blood supply to the placenta causes chronic hypoxia within the intervillous space, which generates oxidative stress leading to placental cellular apoptosis and exaggerated inflammatory responses.6
Pre-eclampsia is a two-stage disease. The first phase of trophoblast invasion and artery modification are clinically asymptomatic. Placental hypoxia and oxidative stress activate intracellular signaling cascades, secretion of growth factors, antiangiogenic factors, vasoconstrictors, and cytokines. These, along with products of placental necrosis/apoptosis, are released to the maternal circulation. This leads to increased systemic vascular resistance, enhanced platelet aggregation, activation of the coagulation system, and endothelial cell dysfunction. Imbalance of vasodilators and vasoconstrictors results in muscular spasm. Acute atherosis and spiral artery thrombosis have also been implicated in causing severe placental ischemia and infarction.14 All of the above lead to the second phase of clinical signs of pre-eclampsia.
Pre-eclampsia also has a genetic background. The disease shows a familial susceptibility and variance with respect to ethnic and socioeconomic factors. Interaction of many single nucleotide polymorphisms (SNPs) in the genes involved in the pathogenic processes has also been noted. However, genome-wide association studies done so far have not been able to pinpoint on a single candidate gene. Genetic imprinting is the expression of either the maternal or paternal allele, where the other one is silenced and epigenetic changes have also been attributed to trophoblast invasion and fetal development.15, 16 The following miRNAs, the nonprotein coding RNAs, have also been implicated: miR-16, miR-29b, miR-181a, miR-335, and miR-222, which regulate angiogenesis and apoptosis,.17 Needless to mention, insight into the molecular landscape of pre-eclampsia lays the foundation for prudent biomarker research.
Molecular markers for pre-eclampsia prediction
From the foregoing, it is obvious that pathological mechanisms of pre-eclampsia are initiated in the early weeks of pregnancy, where the changes are predominant in the molecular plane. As the placenta plays a pivotal role in the development and progression of the disorder, molecular groups from maternal blood may have the potential for early prediction. Its importance is evident from the fact that delivery of fetus and placenta is the ultimate cure of pre-eclampsia.8 Markers may be proteins, hormones, metabolic products, or DNA seen in the maternal circulation. Table 1 describes the important predictive markers identified so far with their functions in pregnancy and the gestation age at which the prediction is possible. However, the clinical validity of these markers for an early prediction/screening is still to be established.
Table 1.
Important protein markers for prediction of pre-eclampsia.
| Marker | Type of protein | Function | Gestational age | Combinations of markers for prediction |
|---|---|---|---|---|
| PAPP-A | Metalloproteases | Cleaves insulin growth factor from its binding proteins | 11–13 weeks | pp-13, uterine artery Doppler, PIGF, sEng, ADAM12 |
| ADAM12 | Metalloproteases | Modulates the activity of insulin growth factors | 11–14 weeks | PAPP-A, uterine artery Doppler, PIGF, MAP |
| Cystatin C | Cysteine protease inhibitor | Inhibits cysteine proteases cathepsins B and L and involved in trophoblast invasion | 15–20 weeks | β2-Macroglobulin |
| pp-13 | Galectin family of proteins | Placental implantation and maternal vascular remodeling | 9–12 weeks 11–13 weeks |
Individual predictor, PIGF, PAPP-A, uterine artery Doppler, P-selectin |
| Pentraxin-3 | Humoral immune system component | Prevents maternal alloimmunization against the fetus | 11–14 weeks | Individual predictor, uterine artery Doppler |
| P-selectin | Cell surface adhesion molecule | Role in inflammatory reactions and recruitment of leucocytes | 10–14 weeks | Individual predictor, activin A, inhibin A, PTX3, PIGF, pp-13 |
| ADMA | Inhibitor of NO synthase | Reversibly inhibits release of NO | 23–25 weeks | Individual predictor |
| hCG | Hormone | Maintains corpus luteum, promotes progesterone production, and differentiation of cytotrophoblast | Second trimester | Individual predictor, uterine artery Doppler |
| Inhibin A and activin A | Hormone | Development of placenta, trophoblast cell differentiation and regulation of gonadotrophins | Second trimester | PIGF, uterine artery Doppler, PP-13, P-selectin, PTX3, PIGF |
| CRH | Hormone | Stimulates ACTH release | Second trimester | CRH-binding protein |
| VEGF | Proangiogenic factor | Angiogenesis and maintaining placental vasculature | Second trimester | PIGF, sFlt-1, sEng |
| PIGF | Proangiogenic factor | Angiogenesis, maintaining placental vasculature | Second trimester | sFlt-1, VEGF, sEng, PAPP-A, pp -13, activin A, inhibin A, PTX3 |
| sFlt-1 | Antiangiogenic factor | Blood vessel homeostasis, trophoblast cell function | Second trimester | PIGF, VEGF, sEng |
| sEng | Antiangiogenic factor | Angiogenesis, regulates NO synthesis, vasodilation, capillary formation | Second trimester | PIGF, VEGF, sFlt-1, PAPP-A, pp -13 activin A, inhibin A, PTX3 |
ACTH, adrenocorticotropicohormone; ADAM12, A disintegrin and metalloprotease12; ADMA, asymmetric dimethyl arginine; CRH, corticotropin-releasing hormone; hCG, human chorionic gonadotropin; MAP, mean arterial pressure; NO, nitric oxide; pp-13, placental protein-13; PIGF, placental growth factor; PAPP-A, pregnancy-associated plasma protein A; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase 1; TGF β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Placental proteins
The role of proteases in the remodeling process of placental development is important. Two zinc-dependant metalloproteases, pregnancy-associated placental protein A (PAPP-A) and ADAM12 (A disintegrin and metalloprotease 12), cleave the insulin-like growth factor (IGF) bound to its binding protein (IGFBP) and increase its bioavailability. IGF regulates the transport of amino acids and glucose to the placenta and aids in trophoblast invasion. PAPP-A is secreted by syncytiotrophoblast and its concentrations continuously increase with the advent of pregnancy.18 Currently, it is used as a first trimester screening marker for Down syndrome. ADAM12 exists in a long membrane-bound form (ADAM12-L) and a short secreted form (ADAM12-S). Therefore, low values of both PAPP-A and ADAM12 in 11–14 weeks of gestation have been cited to have predictive potential for pre-eclampsia though there are opposing reports also.8, 19 On the other hand, in combination with other placental proteins like pp-13/PIGF and uterine artery Doppler, they have shown better prediction. Low values of PAPP-A in early trimesters are also related to pregnancy complications like spontaneous miscarriage, placental abruption, chromosomal abnormalities, and IUGR. For ADAM12, the alterations were seen in IUGR and aneuploidy.8
A synchronous act of cysteine proteases and its inhibitors is essential in matrix degradation, a crucial step in trophoblast invasion. Cystatin C, an inhibitor of cysteine proteases (cathepsins B and L), is expressed in trophoblasts and decidual macrophages. Serum values of cystatin C are mentioned to be high in pre-eclampsia, presumably due to the underlying endothelial changes. Along with β2-macroglobulin, cystatin C could reliably forecast pre-eclampsia in a group of 30 patients. High values have been observed in 15–20 weeks of gestation, and these individuals subsequently developed pre-eclampsia.20, 21
Placental-specific protein, pp-13, from the galectin family, has been widely studied for predicting pre-eclampsia. pp-13 is present in the syncytiotrophoblast of fetomaternal interference. Due to the carbohydrate-binding domain and β-galactoside affinity, pp-13 mediates immunological functions and supports placental development/vascular remodeling.22 Therefore, pp-13 has been highly correlated with pre-eclampsia even at 9–12 weeks of gestation. In combination with uterine artery Doppler, it had 90% detection rate, and with PAPP-A, it demonstrated 91% sensitivity and specificity at first and second trimesters for pre-eclampsia.22, 23
Inflammatory reactions and endothelial disturbances predominate in pre-eclampsia. Pentraxin-3 (PTX3) is an acute-phase response protein expressed in the amniotic epithelium, chorionic mesoderm, perivascular stroma, decidual cells, and terminal villi of placenta.24 Increased levels of PTX3 in pre-eclampsia are attributed to the inflammatory responses of the disease. PTX3 binds to the apoptotic cells and lessens the immune reactions against the fetus. High values of PTX3 at 11–14 weeks have been stated in early-onset cases of pre-eclampsia but not for the late-onset/gestational hypertension groups and in fetal growth restriction (FGR). Conversely, the hike was not correlated to placental perfusion impairment assessed by Doppler.23, 24 P-selectin is a cell adhesion molecule expressed in the endothelial cells and platelets, which modulates inflammatory reactions. It acts as a receptor for binding leucocytes to the activated platelets and endothelium. High concentration of this protein has been found in 10–14 weeks of gestation, which later developed into pre-eclampsia.25 This is attributed to the increased platelet activation and neutrophil–endothelial adhesion, which occurs in the early weeks of pre-eclampsia. Together with activin-A and vascular endothelial growth factor receptor (VEGFR), however, the detection rate was only 59%.23 The harmonious action of vasodilators and vasoconstrictors is disturbed in placental pathologies. Nitric oxide (NO) is the major vasodilator of placenta. Asymmetric dimethyl arginine (ADMA) reversibly inhibits nitrous oxide synthase and release of NO induces endothelial dysfunction. Normal pregnancy therefore shows reduced levels of circulating ADMA in maternal plasma. On the reverse, elevated levels in pre-eclampsia may be due to the impaired formation of vascular components of placenta.26
Placental hormones are another probable set of predictors for pre-eclampsia. The hormonal role in pregnancy complications has been well reviewed by Reis et al. Vascular insufficiency of spiral arteries lead to hypoxia and subsequent hyperplasia of trophoblast.27 This releases placental hormones into maternal circulation. Human chorionic gonadotropin (hCG) is the main glycoprotein hormone secreted by syncytiotrophoblast. It helps in maintaining corpus luteum, promotes progesterone production, induces angiogenesis, brings out differentiation of cytotrophoblast, and also helps in protecting the migrating trophoblast. A high concentration of hCG (>2.0 MoM) in maternal plasma has been reported in the second trimester of pre-eclampsia cases. However, this association was seen only in multiparous women in another study. The increase of hCG may be because of developmental anomalies of placenta and subsequent reactive hyperplasia of syncytiotrophoblast. It may also be due to the hypersecretory state of trophoblasts in response to hypoxia in pre-eclampsia.27
Inhibin-A and activin-A are two transforming growth factor family members released from the placenta, deciduas, and fetal membranes. These are involved in the development of placenta, trophoblast cell differentiation, and in the regulation of gonadotrophins.8, 27 Both are simultaneously increased in the circulation; activin stimulates hCG synthesis, which in turn causes inhibin secretion. Elevated levels were found in second trimester also, which subsequently developed as pre-eclampsia. At 15–19 weeks, inhibin appeared to be more sensitive than activin A, while at 21–25 weeks, sensitivity was more for activin A. There are some other reports suggesting the predictive as well as prognostic capability of these proteins in the first and second trimesters. Another neuropeptide, corticotropin-releasing hormone (CRH), is synthesized by placenta that stimulates adrenocorticotropicohormone (ACTH) release.27 CRH has endocrine, paracrine, and autocrine functions. It acts as a vasodilator and regulates maternal and fetal pituitary functions. Increase of CRH has been found in the second trimester in pre-eclampsia cases. CRH along with CRH-binding protein (CRH-BP) improved the predictive and prognostic values.
Protein molecules, which are directly involved in the pathological processes, may predict the disease with good sensitivity and specificity. However, there is disparity in results with different studies. Differences in study protocol and procedures might have contributed to it.
Angiogenic markers
Angiogenesis is the formation of new blood vessels from the pre-existing ones and is a major step in placental vascularization. It is mediated through the synergistic action of pro- and antiangiogenic factors. VEGF and placental growth factors (PIGF) are from the former, while receptors of VEGFR-1 and 2 belong to the latter. VEGF interacts with both the antiangiogenic receptors whereas PIGF binds only to VEGFR-1. They assist in blood vessel formation and regulate trophoblast functions. VEGFR-2 is also known as kinase insert domain receptor (KDR) and Flk-1 (fetal liver kinase 1), whereas VEGFR-1 is an fms-like tyrosine kinase 1 receptor (Flt-1). Soluble endoglin (sEng), another antiangiogenic factor is a truncated form of endoglin. This is expressed on syncytiotrophoblast and endothelial cells and forms the co-receptor in transforming growth factor (TGF) β1 and β3. It regulates the production of NO, the vasodilator. A soluble form of Flt-1 (sFlt-1), secreted from placenta, binds to VEGF and PIGF, and is a splice variant of Flt-1. Placental antiangiogenic factors released into the maternal circulation cause disturbances in maternal endothelium, which ends up in hypertension.7, 8, 22
In pre-eclampsia pregnancies, low values of VEGF and PIGF and increased levels of sFlt-1 and sEng in maternal circulation have been observed. These antiangiogenic proteins in the placenta bind and neutralize VEGF and PIGF, which affects endothelial cell homeostasis and elicits clinical symptoms of pre-eclampsia. Alteration of these proteins has been noticed prior to the onset of clinical symptoms also. Several reports support PIGF as an early forecaster, whereas many studies could not prove this relationship. Low values of PIGF were seen at 15–19 and 17–21 weeks of pregnancy, and these individuals later developed pre-eclampsia. Dissimilar reports regarding VEGF in prediction of pre-eclampsia have also been published. Studies reported a 2nd trimester prediction of the disease, whereas some of the studies could foresee correctly only within 5 weeks of disease development. Yet other groups were unable to detect the VEGF concentrations below <30 pg/ml.8 sFlt-1 and sEng are two antiangiogenic factors, which have shown high circulating levels prior to the onset of clinical symptoms. Rise of sFlt-1 was detectable even before 5–6 weeks of clinical phase, whereas sEng surfaced from 2 to 3 months onwards. Alteration of both the peptides was correlated with the severity of the disease. Levine et al. suggested that sFlt1: PIGF ratio and (sFlt 1 + sEng): PIGF could be a better predictor for pre-eclampsia.8, 23
Cell-free fetal DNA
Usually, placentation involves apoptosis of trophoblast cells. During this process, fetal DNA is released into maternal circulation. Cell-free fetal (cffDNA) was introduced as a marker in prenatal diagnosis by Lo et al. in 1997.28 Thereafter, many investigations have gone to define its source, characters, and diagnostic/prognostic potential. CffDNA has been cited to be present from 9 weeks of gestation, which showed a constant increase with the progress of pregnancy. cffDNA in the plasma of pregnant women forms around 3–6% of the total cell-free DNA in maternal circulation.28 It is rapidly cleared from maternal circulation and is not detectable after 2 h postpartum. Hence, results are not affected by previous pregnancy complications. As of now, cffDNA is expansively evaluated for the prenatal screening of aneuploidy, single gene disorders, chromosomal aberrations, placental-associated disorders, and Rh factor. Few studies have also demonstrated the increase in fetal DNA from women with symptomatic pre-eclampsia.
The predictive status of cffDNA in pre-eclampsia is now enthusiastically explored. The experiments of quantitation/detection of cffDNA were initially done by Y chromosome-specific markers. For this reason, the studies were limited for women with male fetus only. Discovery of fetal-specific epigenetic markers has enhanced its feasibility in noninvasive prenatal diagnosis. Promoter of RASSF1A gene, which is hypermethylated in placenta and hypomethylated in maternal blood cells, is a prospective marker for cffDNA analysis. This allows the use of methyl-sensitive restriction enzyme digestion for detecting the placental-derived hypermethylated RASSF1A sequences in maternal plasma.29 In our study using hypermethylated RASSF1A to quantify cffDNA, we observed the gestational age dependant increase of hypermethylated RASSF1A in plasma of normal pregnant women.30 Consistent cffDNA values were obtained from early second trimester to the development of pre-eclampsia. cffDNA quantitation with fetal-specific gene marker seems to be a promising approach for early diagnosis of pre-eclampsia. Important studies are summarized in Table 2.
Table 2.
Studies on cell-free fetal DNA (cffDNA) in prediction of pre-eclampsia.
| Study | Marker to quantify cffDNA | Gestational age (in weeks) | Results in prediction of pre-eclampsia |
|---|---|---|---|
| Salvianti et al. (2015) | Hypermethylated RASSF1A | 8–17 | Cutoff value of 7.49 GE/ml in cffDNA concentration with 100% sensitivity and 50% specificity |
| Papantoniou et al. (2013) | Hypermethylated RASSF1A | 11–13 | Cutoff value of 512 GE/ml in cffDNA values with specificity and sensitivity of 100% |
| Poon et al. (2013) | Chromosome selective sequencing of single nucleotide polymorphic and nonpolymorphic loci | 11–13 | No significant difference in values between pre-eclampsia cases and normal |
| Yu et al. (2013) | SRY | 15–20 (18) | Cutoff value (logged) of 2.62 with a sensitivity of 90% and specificity of 85% for early-onset pre-eclampsia |
| Kim et al. (2012) | Hypermethylated RASSF1A | 15–28 | 3.3-fold increase in cffDNA concentration |
| Sifakis et al. (2009) | DYS | 11–13 | Significant increase in cffDNA only in early-onset pre-eclampsia (95.5 vs 51.5 GE/ml) and not in late-onset group |
| Gozdziewicz et al. (2009) | SRY | 15–22 (18) | Significant increase in cffDNA values (3.2 vs 0.595 GE/ml) |
| Crowley et al. (2007) | SRY | 10–20 (13.5) | No significant increase in cffDNA before 20 weeks (30.5 vs 27.5 GE/ml) |
| Cotter et al. (2005) | RhD | 9–22 (15.3) | Fourfold increase risk of disease development for tenfold increase of copies/ml |
| Levine et al. (2004) | DYS | 17–28 | Increase but not significant in 17–24 weeks and significant rise from 24 to 28 weeks |
| Cotter et al. (2004) | SRY | 15.7 ± 3.6 | Increase in cffDNA is associated with 8-fold risk of disease development |
| Farina et al. (2004) | DYS | 20 ± 2.08 | 2.39-fold increase of cffDNA in low-risk population |
| Zhong et al. (2002) | SRY | 19–24 (21.0) | Significant increase with 422.9 vs 128.5 copies/ml |
| Leung et al. (2001) | SRY | 11–22 (17) | Significant increase with 41.9 vs 22.0 GE/ml |
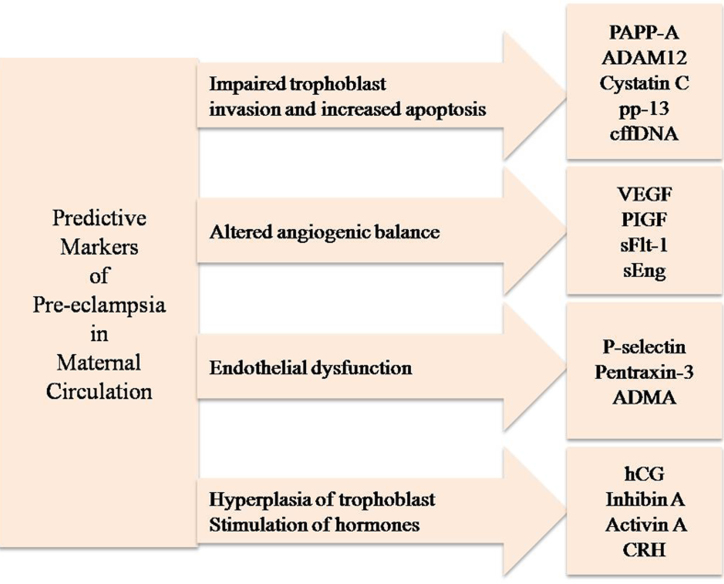
With the entire preceding information as a base logic, we propose a set of predictive markers from maternal circulation for pre-eclampsia as shown in Fig. 4. It is expected that the classification would streamline and consolidate future research in the early diagnosis of pre-eclampsia.
Fig. 4.
Summary of molecular markers for pre-eclampsia.
Conclusions
Protein markers, angiogenic factors, and cffDNA appear to have the potential for early diagnosis of pre-eclampsia. Invariably, these molecules represent different key phases in disease progression. Though good amount of reliability has been ascribed to many of them in predicting pre-eclampsia, further validation is warranted from a clinical point of view. This is because several of the proposed molecules are noticed in other placental pathologies also. Among all, cell-free DNA with its specific marker for fetus appears to have better credentials for early diagnosis/screening of pre-eclampsia. Notwithstanding the emerging evidences in favor of cell-free DNA, its verification in large population subsets is highly desired to ascertain reproducibility and cost effectiveness within a standardized uniform protocol. Including ‘biomarker combinations’ corroborating to critical molecular pathways, such as apoptosis of trophoblast cells of placenta, endothelial dysfunction, hyperplasia of trophoblasts, and angiogenic imbalance, may also be a prudent approach in this direction.
Conflicts of interest
The authors have none to declare.
References
- 1.Bell M.J. A historical overview of preeclampsia–eclampsia. J Obstet Gynecol Neonatal Nurs. 2010;39(5):510–518. doi: 10.1111/j.1552-6909.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown M.A., Lindheimer M.D., de Swiet M., Van Assche A., Moutquin J.M. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20(1):IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 3.Ghulmiyyah L., Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36(1):56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Backes C.H., Markham K., Moorehead P., Cordero L., Nankervis C.A., Giannone P.J. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. doi: 10.1155/2011/214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sibai B.M. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):181–192. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 6.Sibai B., Dekker G., Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 7.Polsani S., Phipps E., Jim B. Emerging new biomarkers of pre-eclampsia. Adv Chronic Kidney Dis. 2013;20(3):271–279. doi: 10.1053/j.ackd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Staff A.C. Circulating predictive biomarkers in pre-eclampsia. Pregnancy Hypertens. 2011;1:28–42. doi: 10.1016/j.preghy.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Mutter W.P., Karumanchi S.A. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75(1):1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitley G.S., Cartwright J.E. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31(6):465–474. doi: 10.1016/j.placenta.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laresgoiti-Servitje E., Gomez-Lopez N., Olson D.M. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16(5):510–524. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright J.E., Fraser R., Leslie K., Wallace A.E., James J.L. Remodelling at the maternal–fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140(6):803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- 13.Hanna J., Goldman-Wohl D., Hamani Y. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 14.Powe C.E., Levine R.J., Karumanchi S.A. Preeclampsia, a disease of the maternal endothelium: the role of angiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts J.M., Cooper D.W. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 16.Williams P.J., Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):405–417. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013;94(2):247–257. doi: 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- 18.Christians J.K., Beristain A.G. ADAM12 and PAPP-A: candidate regulators of trophoblast invasion and first trimester markers of healthy trophoblasts. Cell Adh Migr. 2016;10(1–2):147–153. doi: 10.1080/19336918.2015.1083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzinger K.R., Zhong Y., Cahill A.G., Odibo L., Macones G.A., Odibo A.O. Efficiency of first-trimester uterine artery Doppler, a-disintegrin and metalloprotease 12, pregnancy-associated plasma protein A and maternal characteristics in the prediction of preeclampsia. J Ultrasound Med. 2013;32(9):1593–1600. doi: 10.7863/ultra.32.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen K., Wide-Swensson D., Schmidt C. Cystatin C, beta 2-microglobulin and beta-trace protein in pre-eclampsia. Acta Obstet Gynecol Scand. 2007;86(8):921–926. doi: 10.1080/00016340701318133. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen K., Larsson I., Hansson S.R. Increased cystatin C expression in the pre-eclamptic placenta. Mol Hum Reprod. 2007;13(3):189–195. doi: 10.1093/molehr/gal111. [DOI] [PubMed] [Google Scholar]
- 22.Carty D.M., Delles C., Dominiczak A.F. Novel markers predicting preeclampsia. Trends Cardiovasc Med. 2008;18(5):186–194. doi: 10.1016/j.tcm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grill S., Rusterholz C., Zanetti-Dallenbach R. Potential markers of pre-eclampsia – a review. Reprod Biol Endocrinol. 2009;7:70–84. doi: 10.1186/1477-7827-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akolekar R., Casagrandi D., Livanos P., Tetteh A., Nicolaides K.H. Maternal plasma pentraxin 3 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat Diagn. 2009;29(10):934–938. doi: 10.1002/pd.2311. [DOI] [PubMed] [Google Scholar]
- 25.Bosio P.M., Cannon S., McKenna P.J., O’Herlihy C., Conroy R., Brady H. Plasma P-Selectin is elevated in the first trimester in women who subsequently develop pre-eclampsia. BJOG. 2001;108(7):709–715. doi: 10.1111/j.1471-0528.2001.00170.x. [DOI] [PubMed] [Google Scholar]
- 26.Braekke K., Ueland P.M., Harsem N.K., Staff A.C. Asymmetric dimethylarginine in the maternal and fetal circulation in pre-eclampsia. Pediatr Res. 2009;66(4):411–415. doi: 10.1203/PDR.0b013e3181b33392. [DOI] [PubMed] [Google Scholar]
- 27.Reis F.M., D’Antona D., Petraglia F. Predictive value of hormone measurements in maternal and fetal complications of pregnancy. Endocr Rev. 2002;23(2):230–257. doi: 10.1210/edrv.23.2.0459. [DOI] [PubMed] [Google Scholar]
- 28.Lo Y.M., Corbetta N., Chamberlain P.F. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 29.Chan K.C., Ding C., Gerovassili A. Hypermethylated RASSF1A in maternal plasma: a universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem. 2006;52:2211–2218. doi: 10.1373/clinchem.2006.074997. [DOI] [PubMed] [Google Scholar]
- 30.Seema S., Sahai K., Arora D. Fetal specific hypermethylated RASSF1A quantification in pregnancy. J Matern Fetal Neonatal Med. 2016 doi: 10.1080/14767058.2016.1188917. early online 1–5. [DOI] [PubMed] [Google Scholar]