Abstract
The Associative Deficit Hypothesis (ADH) posits that age-related differences in recognition of associations are disproportionately larger than age differences in item recognition because of age-related difficulty in binding and retrieval of two or more pieces of information in a memory episode. This proposition rests on the observation of disproportionately greater age differences in memory for associations than in recognition of individual items. Although ADH has been supported in experiments with verbal and nonverbal stimuli, the effects of task or stimulus characteristics on its generalizability remain unclear. In a series of experiments, we examined how salience and variability of face stimuli presented in face–name pairs affect age differences in recognition of items and associations. We found that a disproportionate age-related deficit in the recognition of face–name associations emerges when face stimuli are more complex, salient, variable, and distinctive, but not when standardized faces appear within minimal visual context. These findings indicate that age-related associative memory deficits may stem at least in part from age differences in use of stimulus characteristics for contextual support.
Keywords: aging, memory, associative deficit, face recognition, salience
Older adults perform worse than their younger counterparts on tests of episodic memory (Verhaeghen, Marcoen, & Goosens, 1993), including memory for associations between words and nonverbal stimuli. Forming associations seems particularly difficult for older people, even when the individual items are remembered well. This apparent discrepancy between age differences in memory for items and associations inspired the Associative Deficit Hypothesis (ADH; Naveh-Benjamin, 2000), which has been upheld in multiple experiments with various stimuli: pairs of words (e.g., Bender, Naveh-Benjamin, & Raz, 2010; Castel & Craik, 2003; Light, Patterson, Chung, & Healy, 2004; Naveh-Benjamin, 2000); objects (Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003); faces (Rhodes, Castel, & Jacoby, 2008); faces paired with spatial locations (Bastin & Van der Linden, 2006); persons paired with activities (Old & Naveh-Benjamin, 2008b); and face–name pairs (James, Fogler, & Tauber, 2008; Naveh-Benjamin, Guez, Kilb, & Reedy, 2004; Naveh-Benjamin, Shing, Kilb, Werkle-Bergner, Lindenberger, & Li, 2009; see Old & Naveh-Benjamin, 2008a for a meta-analysis). Although these findings suggest that ADH may be a universal phenomenon of aging, it is important to examine its boundaries of generalizability and to examine the conditions under which it may not hold.
Such an examination draws attention to stimulus characteristics that may differentially influence item and associative memory performance in younger and older adults. For associative binding involving visual stimuli, higher visual complexity of the items, or greater variability therein, may serve as a vehicle for environmental support of associative binding by enhancing item distinctiveness and saliency. That is, as increasing environmental support can improve memory (Craik, 1986), adding external contextual cues to enhance episodic memory by enriching the stimuli is a plausible way to mitigate associative deficits. Unfortunately, age-related deficits in memory for contextual information exceed those in memory for specific items (Spencer & Raz, 1995), and failure to bind content to contextual features surrounding the stimuli or embedded therein has been advanced as an explanation for age-related memory deficits (Chalfonte & Johnson, 1996; Light, 1991; Mitchell, Johnson, Raye, & D’Esposito, 2000; Naveh-Benjamin, 2000). These studies, however, have primarily focused on age-related decrements in memory for contextual and source-related information over memory for content or contextual feature binding. Incorporating visual stimuli as contextual support necessitates feature binding and unitization, and visual complexity may tax age-limited cognitive resources such as working memory and attention, which are essential for effective associative binding and retrieval. To the best of our knowledge, the differential contributions of visual complexity that give rise to stimulus salience and to emergence of age-related inter-item binding deficits has not been examined.
Recognizing that a given name belongs to a given face is a difficult task for older adults (James, 2004). Moreover, differences in stimulus properties, sample characteristics, and intentionality of study may modify the extent of the age-related face–name associative memory deficits (Naveh-Benjamin et al., 2009). Although face stimuli in studies of associative memory span a wide range from ecologically valid and contextually detailed (Naveh-Benjamin, Guez, Kilb, & Reedy, 2004) to standardized and contextually impoverished (James, 2004), the influence of stimulus complexity and differences in item saliency and distinctiveness on the magnitude of age-related differences in memory for face–name associations has not previously been systematically investigated. Similarly, the saliency and distinctiveness of face stimuli arising from inter-stimulus differences in auxiliary visual dimensions including lighting, affect, eye and skin color, camera angle, and background composition might contribute to the production of such deficits. Thus, it is unclear how stimulus properties affect relational binding, as the influences of image salience, complexity, and variability on generation of the age-related associative deficit are poorly understood. Accordingly, the effect of stimulus characteristics on the magnitude of age differences in associative memory merits further evaluation.
This study (Experiment 1) was conceived as an attempt to replicate the ADH effect (Naveh-Benjamin, 2000) previously observed in typical older participants for face–name pairs (Naveh-Benjamin et al., 2004), in a sample of adults selected for optimal health (Bender et al., 2010). Importantly, the aim of Experiment 1 was to examine the generalizability of the ADH effect by replicating it in a sample that supported ADH for word pairs (Bender et al., 2010). The recognition task in Experiment 1 was adapted from Naveh-Benjamin et al. (2004), with minor modifications in the task design, but with a major alteration of the stimuli. The original color face stimuli sampled from Internet sources were replaced by standardized, grayscale face images from a normed database (Kennedy Hope, & Raz, 2009). However, as we were unable to replicate the finding by Naveh-Benjamin and colleagues in Experiment 1, we proceeded to examine the possible reasons for this replication failure in Experiments 2a/b, and 3. That is, driven by the failure to replicate an ostensibly robust cognitive phenomenon in Experiment 1, we investigated the impact of stimulus characteristics, and specifically differences in stimulus complexity and salience, on age differences in binding of verbal and nonverbal stimuli.
Experiment 1
Method
Participants
Participants were recruited from the greater Detroit metropolitan community through advertisements in local media, flyers, and word of mouth, and all signed an informed consent form approved by the University Institutional Review Board (IRB). Prior to enrollment, potential participants completed a self-report health questionnaire to screen for a history of health problems including cardiovascular, neurological, or psychiatric disease, cancer, head trauma with loss of consciousness for more than five minutes, thyroid disorder, diabetes, and treatment for drug and alcohol abuse. Persons who reported drinking more than three alcoholic beverages per day, or taking anticonvulsive, anxiolytic, antipsychotic, or antidepressant medications were not enrolled in the study. All participants were native English speakers and had normal near, far, and color vision (Optec 2000 Vision Tester, Stereo Optical Co., Inc., Chicago, IL) and adequate speech-range hearing (MA27 Screening Audiometer, Maico Diagnostics, Eden Prairie, MN). Participants were screened for depressed mood with a depression questionnaire (CES-D; Radloff, 1977; cut-off score of 15), and for cognitive impairment with the Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975; cut-off score of 26).
The sample consisted of 236 adults (18 to 85 years of age), including 156 women and 80 men, who had at least a high school diploma or equivalency, with mean education approaching four years of college (15.9 ± 2.5 years). This sample was a subset of the 278 participants for whom an associative deficit has been observed for word pairs (Bender et al., 2010). These data were acquired in the first wave of a longitudinal study, with the word pair task reported by Bender et al. (2010) and the face–name task administered on the consecutive days of testing. From the 278 participants, 37 withdrew from the study before completing the face–name task. An additional five participants completed the face–name task, but their data were unusable due to experimenter errors in counterbalancing assignments. The 42 participants, whose data could not be included in the present study, did not differ from the rest of the sample on any screening criteria. In the 236 participants retained for analysis, men and women did not differ in mean age, years of education, or MMSE (see Table 1).
Table 1.
Sample Descriptors for Men and Women in Experiment 1
| Variable | Men Mean ± SD |
Women Mean ± SD |
t | p |
|---|---|---|---|---|
| Age (in years) | 51.8 ± 16.2 | 52.3 ± 15.3 | 0.27 | .79 |
| Education (in years) | 16.0 ± 2.5 | 15.6 ± 2.5 | 0.82 | .41 |
| MMSE | 28.8 ± 1.1 | 28.9 ± 1.0 | 0.95 | .34 |
Note. MMSE = Mini-Mental State Examination.
Design and Procedure
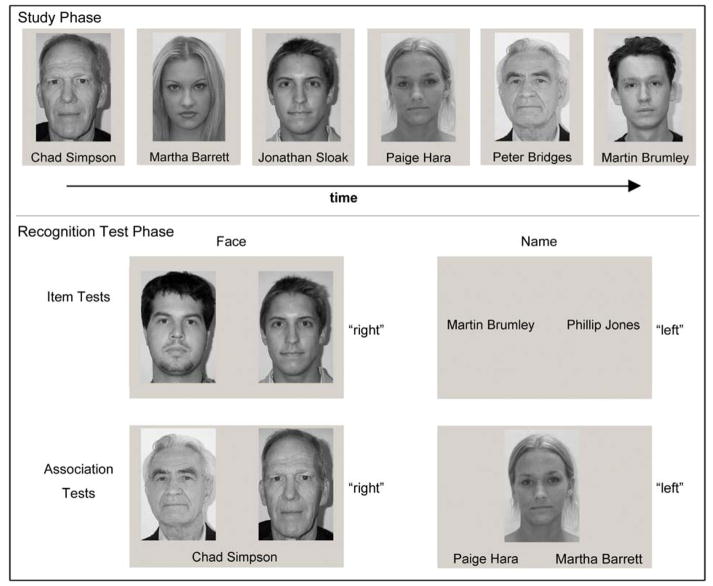
To test differential recognition of items and associations, we employed a two-alternative, forced-choice (2-AFC) paradigm to test participants’ ability to remember faces, names, and face–name pairings (Naveh-Benjamin et al., 2004). The experimental procedure was divided into three blocks, each of which included separate phases for study of stimulus pairs, a brief interpolated activity task, and four separate recognition tasks. After performance on the first block, the procedure was repeated twice using new stimulus lists for study and recognition testing. Each of the three blocks involved a study phase that included 48 unique, gender-matched face–name pairings. The combinations of first and last names (an equal number of masculine and feminine) were sampled randomly from a phone directory (Naveh-Benjamin et al., 2004). Face stimuli rendered in gray scale were sampled from the Minear and Park (2004) database that was modified and normed in our lab on samples drawn from the population that also served as the source for all samples used in the present studies (Kennedy et al., 2009). Half of the face stimuli were sampled from the younger (ages 18–49) and half from the older (ages 50–94) age category. Face stimuli were balanced for sex and ethnic diversity across the age categories. Any given name or face appeared on only one of the tests. Examples of the face–name stimuli and the task design are shown in Figure 1.
Figure 1.
Examples of stimuli from the face–name recognition task in Experiment 1. In the study phase, participants saw 48 face–name pairs for 3 s each with 200 ms inter-trial interval. Separate, two-alternative forced-choice recognition tasks tested participants’ recognition memory for items and associations for faces and names, in 12 trials per test. The item task had participants indicate which face or name (left or right) had been presented at study. In the association task, participants responded by indicating which face or name (left or right) had been paired with the target item during the study phase.
In the study phase of each block, one of three lists of 48 face–name pairs was presented, in which each face–name pair was displayed on the computer screen for 3 s with the face appearing above the name with a 200 ms inter-pair interval. The order of the three stimulus lists was counterbalanced in a Latin square. Because age-related differences are not observed on incidental learning of face–name associations (Naveh-Benjamin et al., 2009), we informed the participants before the first block that their memory for the names, the faces, and their pairings would be tested and that they were to pay attention to each face, each name, and each face–name pair.
To minimize rehearsal following study, participants counted backwards by threes from a random number between 900 and 999 for 60 s. Following study and interpolated activity phases, participants performed recognition tests for four types of stimuli: face-item, name-item, face-association, and name-association. The item recognition tests required participants to distinguish previously studied targets from unstudied foil stimuli. In contrast, all test stimuli on the two associative recognition tests were presented at study phase, and participants were required to choose the correct pairings from two faces presented with a single name (face-association) or two names shown with one face (name-association). The four 2-AFC recognition tests were administered in a counterbalanced order for each participant (24 possible presentation orders).
During the test phase, participants indicated recognition by pressing one of two keys on a 104-key Dell computer keyboard (Dell Inc., Round Rock, TX). Response labels were placed on keys to facilitate response; a “Left” label was on the ‘D’ key and a “Right” label was on the ‘K’ key. Target / foil screen position [left, right] was randomized during tests. To prevent using mismatches as recognition decision cues (Naveh-Benjamin et al., 2004), the faces, names, and combinations were matched on age and gender. Participants were told that all stimuli had appeared in the study phase and that their task was to choose the appropriate pairing. All tests were self-paced with a response initiating the subsequent trial, following a 250 ms inter-trial interval. Prior to the study session, participants received a one-trial familiarization for each test.
Forced-Choice Item (Face/Name) Recognition tests
Participants performed 12 trials per stimulus type. In each trial, two faces (two names) appeared side-by-side; one – a previously studied (“old”) target, the other – new (i.e., a foil). Target and foil faces were matched on age, sex, and ethnicity, and target and foil first names were matched on sex. Participants pressed a key to indicate whether the left or right stimulus was recognized as “old.”
Forced-Choice Name/Face Associative Recognition tests
In each of 12 trials, a single face (name) appeared above two, side-by-side, names (faces) with similar characteristics. All were among the members of the originally studied pairs, but one item was originally paired with the target, whereas the other was a foil previously paired with a different item at study. Participants indicated via key press whether the left or right stimulus was presented with the target item during study. Following completion of study and test phases for one stimulus list, the procedure was repeated with the remaining two lists.
Data Conditioning
Responses under 300 ms or longer than 10 s were considered errors and excluded from analyses. Proportion correct (PC, number of correct responses / total number of trials), the most sensitive index of discriminability in 2-AFC tasks (Green & Swets, 1966; Stanislaw & Todorov, 1999), was used as in previous associative memory studies (Naveh-Benjamin et al., 2004). The PC scores were arcsine-transformed to minimize skewness. Due to experimenter error, five participants were administered list presentations that were inconsistent with the Latin square counter-balancing, and their data were excluded from the analyses.
Statistical Analyses
The general linear modeling (GLM) framework was employed to analyze recognition performance, with PC as the dependent variable, age, centered at the sample mean, as a continuous independent variable, and sex and list order (1, 2, 3) as categorical covariates. Test (items, associations), stimulus types (faces and names), and presentation list (1, 2, 3) were repeated measures. The second-order interactions were tested, but removed from the final model if found nonsignificant; the reduced model was re-evaluated. Huynh-Feldt correction was used to account for sphericity violations in tests of interactions. Post-hoc tests of simple effects were performed using Newman-Keuls tests (critical p < .05). We hypothesized that in accord with ADH, older adults would perform disproportionately worse on tests of associative memory compared to item recognition. In other words, we hypothesized a significant Age × Test interaction. Although the list and list order factors as well as their interactions with other variables were included as nuisance covariates to test for potential differences across those dimensions, we had no a priori hypotheses regarding those effects.
Results
Recognition accuracy
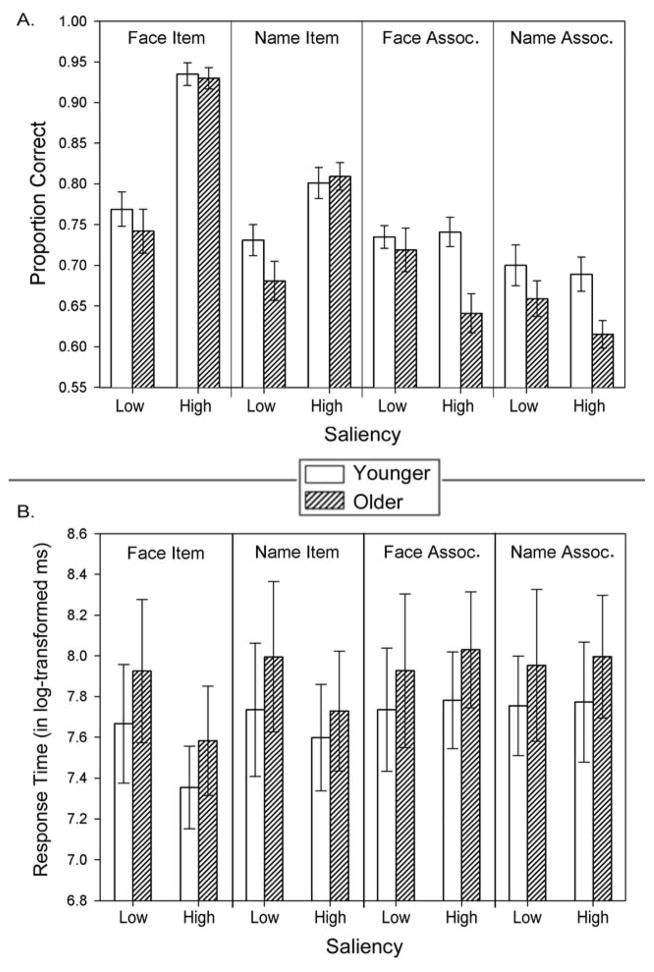
Main effects and interactions, including nonsignificant trends (p < .15), are shown in Table 2. Advanced age was associated with lower PC across tests, but there were no other significant main effects (all p > .1). Most notably, there was no Test × Age interaction, (F < 1) as age-related differences of equivalent magnitude were observed for recognition of items and associations (Fig. 2). In addition, there were several significant interactions with the repeated measure factors, list and list order that were unrelated to aging or tests of an age-related associative memory deficit. The differences among various combinations of stimulus type, list, and order on post-hoc tests were significant but small and revealed no meaningful patterns.
Table 2.
Experiment 1: Main Effects and Significant Interactions
| Effect or Interaction | F | p | ηp2 |
|---|---|---|---|
| Accuracy | |||
| Age | 47.1 | 0.000 * | 0.169 |
| Sex | 2.2 | 0.142 | 0.009 |
| List Order | 0.1 | 0.929 | 0.001 |
| Test | 0.2 | 0.668 | 0.002 |
| List | 18.6 | 0.000 * | 0.074 |
| List × List Order | 27.3 | 0.000 * | 0.191 |
| List × Sex | 4.7 | 0.010 * | 0.020 |
| Stimulus × List × Age | 2.9 | 0.057 | 0.012 |
| Response Time | |||
| Age | 29.585 | 0.000 * | 0.114 |
| Sex | 0.097 | 0.756 | 0.000 |
| List Order | 3.151 | 0.045 * | 0.027 |
| Test | 0.602 | 0.439 | 0.003 |
| Stimulus | 0.181 | 0.671 | 0.001 |
| List | 7.828 | 0.000 * | 0.033 |
| List × Age | 2.317 | 0.100 | 0.010 |
| List × List Order | 12.711 | 0.000 * | 0.099 |
| Test × List × Sex | 2.472 | 0.086 | 0.011 |
Notes. Accuracy models reflect arcsine-transformed proportions of correct scores; a log-transformation was applied to modeled response times;
p < 0.050.
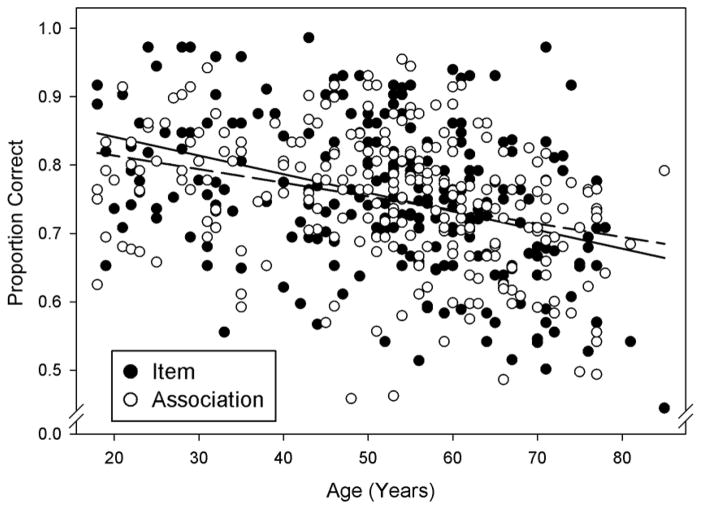
Figure 2.
Scatterplot depicting mean proportions of correct responses on the face–name recognition tasks for items and associations (Y-axis) and participant age (X-axis) in Experiment 1 in a continuous lifespan sample of healthy adults. Solid black circles and the solid regression line depict scores on item tasks (averaged across face and name item recognition subtasks), and open circles and the broken regression line indicates scores on associative recognition tasks (averaged across face and name associative recognition subtasks).
To ensure that the testing conditions did not produce scores with differential unreliability, we evaluated the internal consistency of the four tasks, for all lists. Cronbach’s α for face recognition ranged from .53 to .68, and for name recognition from .59 to .64, for the three lists of face–associative recognition from .57 to .60, and from .56 to .62 for name–associative recognition. Thus, comparable reliability was obtained for all recognition tests.
Speed of processing
Response times (RT) recorded for all tasks, trial-by-trial, were averaged across hit trials for each test and stimulus list. To correct for RT distribution skewness, we applied a log transformation. Log-transformed RT (logRT) was the dependent variable in a GLM similar to the one employed in the PC analyses above. The analyses showed a main effect of age on logRT but no interactions with Test (see Table 2).
In addition, we re-analyzed the previously reported word pair task data (Bender et al., 2010) for 236 participants included in the present study. Briefly, using a general linear modeling (GLM) framework we evaluated the differential contributions of age (centered at the sample mean) on arcsine transformed A’ scores calculated from the hit rate and false alarm rates for recognition of items (studied targets vs. unstudied foils) and associations (intact pairs vs. rearranged pairs), which were treated as repeated measures. Results of the GLM analysis showed a significant Age× Task interaction, F(1, 234) = 16.36, p < 0.001, ηp2 = 0.065, in addition to significant main effects of Age F(1, 234) = 19.282, p < 0.001, ηp2 = 0.076, and Task F(1, 234) = 91.71, p < 0.001 ηp2 = 0.282. The Age × Task interaction reflects greater negative associations between age and associative recognition (r = –0.308, p < 0.001) than between age and item recognition (r = –0.174, p < 0.01; Steiger’s Z* = 2.64, p < 0.01).
Discussion
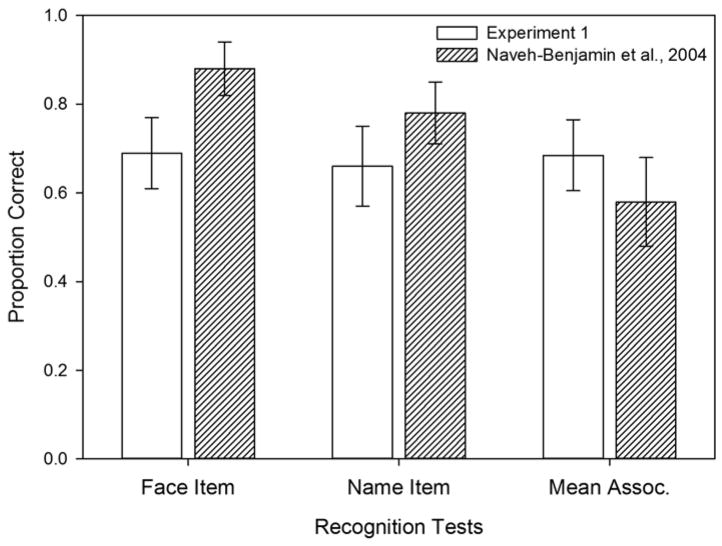
The results in the Experiment 1 were obtained from the same sample that had shown a reliable age-related associative deficit with unrelated word pairs (Bender et al., 2010), yet we found no such deficit for face–name associations. To compare the results of this experiment to those reported in the study on which Experiment 1 of the present study was based (Naveh-Benjamin et al., 2004), we computed the descriptive statistics for the older adults aged at least 60 years (n = 50, mean age = 71.0, sd = 4.5 years) in our Experiment 1. Although recognition performance by older adults in Experiment 1 did not differ across tests and stimulus types, they performed considerably better on recognition of associations than was reported for older participants in the Naveh-Benjamin et al. (2004) study (Fig. 3). However, older participants in Experiment 1 performed worse on name recognition and substantially worse on face recognition than the older group reported by Naveh-Benjamin and colleagues (2004). Thus, although recognizing faces and names in the current task was more difficult for older participants, associations were better recognized than in the Naveh-Benjamin et al. (2004) study.
Figure 3.
Grouped bar graphs comparing mean recognition performance in Experiment 1 (open bars) with performance reported by Naveh-Benjamin et al. (2004; diagonally-patterned bars). Bar heights reflect mean proportion correct on tests of face- and name-item recognition. Associative recognition is averaged across face- and name-associative recognition tests. Error bars reflect 95% confidence intervals.
Differences in stimuli, task parameters, and sample selection could have accounted for the discrepancy between Naveh-Benjamin et al. (2004) and the results of Experiment 1. First, the face stimuli employed by Naveh-Benjamin and colleagues were color photographs of variable quality taken from yearbooks and other sources on the Internet. In comparison with the uniform, normed, grayscale stimuli used in our Experiment 1, those face stimuli contained a variety of distinct details (e.g., color, clothing accessories, affective expressions, and background items such as cars, trees, and houses). Color and visual contextual details facilitate object recognition (Berry, 1991). Notably, Naveh-Benjamin and colleagues (2009) also found no age-related associative deficit using identical face stimuli from the same Minear and Park (2004) grayscale set, but under an incidental memory paradigm. Thus, it is possible that various manipulations of stimulus saliency account for the discrepancy between the studies in the age-related associative deficit. Such deficit may be restricted to more distinct, complex, and explicitly encoded visual stimuli. Second, the discrepancies in the magnitude of the associative deficit could stem from differences in the sample composition, as inclusion of persons with common age-related conditions such as diabetes and cardiovascular disease in the previous study (Naveh-Benjamin et al., 2004) could have exacerbated deficits in cognitive performance. However, because the ADH was upheld for word pairs, but not for face–name pairs in the present study, it is more likely differences in test materials and not altered sample composition are responsible for this discrepancy.
We conducted Experiment 2a to examine possible reasons for the discrepancies in the generation of the associative deficit between Experiment 1 and the prior study (Naveh-Benjamin et al., 2004). In Experiment 2a, we directly compared the capacity of the original and modified versions of the task, including two different types of face stimuli, to elicit the associative deficit. Importantly, whereas the original version of the task (Naveh-Benjamin et al. 2004) included four study lists, each with 32 pairs presented at study and eight trials for each of the four recognition tasks, the modified version reported in Experiment 1 differed in several aspects: three lists with 48 pairs were presented at study, and 12 trials were presented per recognition test. Two plausible reasons for the absence of an age-related associative deficit in Experiment 1 were sample and stimulus characteristics. Thus, in the comparison of the two tasks, we adhered to the less rigorous eligibility criteria used by Naveh-Benjamin and colleagues (2004) and replicated their extreme age groups design. As in Experiment 1, one set of faces consisted of monochromatic, grayscale face stimuli from the normed pool (Kennedy et al., 2009), whereas the second set consisted of items from the original pool (Naveh-Benjamin et al., 2004), face images with higher and more variable visual complexity that were more distinctive and salient than stimuli used in the Experiment 1. The latter contained more visual detail and complexity than the faces used in Experiment 1. We reasoned that although enriched stimuli may help item recognition (Naveh-Benjamin & Craik, 1995; Oliva & Torralba, 2007), processing of extra details may serve to distract older participants from face–name binding, becoming costlier for older adults to form associations, thus reducing their performance differentially.
Experiment 2a
Method
Participants
Participants were 32 younger (mean age = 21.59, SD = 3.01 years) and 32 older (mean age = 71.97, SD = 4.86 years) adults, evenly divided by sex. The younger participants were recruited from psychology classes, and the older participants came from the greater Detroit metropolitan community. Participants signed an informed consent form approved by the University IRB. Younger participants received course research credit, and older participants earned $25. All participants were native English speakers, had a minimum of a high school diploma or equivalency, and scored at least 26 on the MMSE (Folstein et al., 1975).
Participants were excluded if they reported any of the following: current substance abuse or dependency treatment, consuming more than 3 alcoholic beverages on more than 2 nights a week, seeing a physician or other health professional for cognitive problems, unconsciousness for more than 5 minutes during the last 15 years, head injury resulting in hospitalization for more than 24 hours in the last 15 years, history of stroke, electroconvulsive treatment, epilepsy, brain surgery, encephalitis, meningitis, multiple sclerosis, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, schizophrenia, bipolar disorder, or currently taking any psychiatric medication other than selective serotonin reuptake inhibitors. In contrast to the sample employed in Experiment 1, however, persons with history of cardiovascular disease, diabetes, and thyroid disorder were allowed to participate. In both age groups, men and women did not differ in age, education, or MMSE (p > .05 for all; Table 3).
Table 3.
Sample Descriptors for Men and Women in Experiment 2a
| Variable | Men | Women | t | p |
|---|---|---|---|---|
| Younger Adults | ||||
| Age (in years) | 21.4 ± 1.8 | 21.8 ± 1.8 | 0.50 | .62 |
| Education (in years) | 15.5 ± 1.4 | 15.8 ± 1.2 | 0.68 | .50 |
| MMSE | 29.4 ± 0.9 | 28.7 ± 1.1 | 1.97 | .06 |
| Older Adults | ||||
| Age (in years) | 72.2 ± 5.1 | 71.8 ± 4.8 | 0.25 | .80 |
| Education (in years) | 16.7 ± 1.9 | 16.3 ± 3 | 0.42 | .68 |
| MMSE | 28.5 ± 1.2 | 28.8 ± 0.9 | 0.67 | .51 |
Notes: Mean values ± standard deviations are presented. MMSE = Mini-Mental State Examination.
Design and Procedure
As in Experiment 1, we employed a 2-AFC paradigm to test recognition for names, faces, and pairings of the two. The testing procedure employed in Experiment 2a included separate administrations of two different, but related memory tests, each with item and associative recognition tasks, but employing different types of face stimuli. The first task used the uniform grayscale stimuli with neutral background described in Experiment 1, and is referred to as the low-saliency (LS) test. The second task used the more visually complex face stimuli described in Naveh-Benjamin et al. (2004) and is referred to here as the high-saliency (HS) task. The order of presentation for the HS and LS tasks was counterbalanced across participants.
Task Design
The stimuli and procedure for the LS test were identical to those described in Experiment 1. In the study phase for the HS test, stimuli from one of four lists of 32 face–name stimuli pairs appeared on a computer screen, followed by the same interpolated activity as in Experiment 1. Participants then performed four 2-AFC recognition tests as in Experiment 1 and with LS test. The names (first and last; equal number of masculine and feminine names) were also taken from the Naveh-Benjamin et al. (2004) set, and were augmented with names sampled randomly from a phone directory to supplement those from the original task that were used as stimuli in Experiment 1 and the LS task; care was taken to avoid using any common first or last name with different face stimuli. In the absence of any a priori hypotheses regarding the name stimuli as the mnemonic element responsible for the discrepancy between the results from Experiment 1 and those reported by Naveh-Benjamin et al. (2004), we did not counterbalance names across the HS and LS tasks.
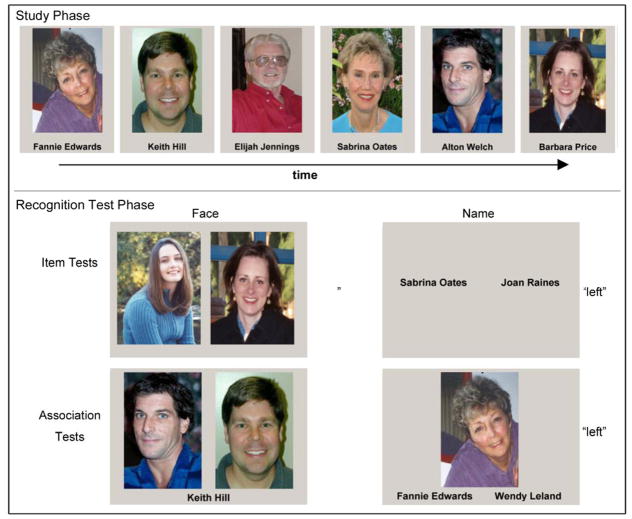
HS faces were in color, balanced for sex, age (18–25 vs. 65–80 years), and positive vs. neutral emotional expressions. Unlike the highly standardized LS faces that showed faces from the neck up, HS stimuli varied in size, proportion of body in the frame, background objects or scenery, extra-facial features or accessories (hats or sunglasses), camera and light source angle, and contrast, (see Figure 4 for examples of the HS face–name stimuli and the task design).
Figure 4.
Example study and test stimuli from the high-saliency (HS) face–name recognition task in Experiment 2a. In the HS study phase, participants saw 32 face–name pairs for 3 s each. Separate, two-alternative forced-choice recognition tasks with 8 trials each tested participants’ recognition memory for items and associations for faces and names. The item task had participants indicate which face or name (left or right) had been presented at study. Association recognition trials required participants to decide which face or name (left or right) had been paired with the target item during the study phase.
The HS stimulus lists were counterbalanced in a Latin square. From each list of names and faces, 32 face–name pairings were created with gender-matched face–name pairings. Based on the stimuli and procedure from Naveh-Benjamin et al. (2004), each list of 32 HS face–name pairs was further subdivided into three separate sub-lists, in an effort to minimize the likelihood of binding specific facial feature–name combinations across the sample. Each sub-list included the same names and faces, but the face–name pairings were counterbalanced such that names were paired with different faces for each sub-list. At study, each HS face was displayed at the top of the screen with the name below it for 3 s, without a pause between pairs of stimuli. The 2-AFC recognition tests were then administered in a random order for each participant (24 possible presentation orders). Any name or face appeared on only one of the tests. All tests were self-paced and required a response to one item for the next trial stimulus to appear. Following completion of study-test phases for each stimulus list, the procedure was repeated with the remaining stimulus lists, the order of which was counterbalanced across the sample.
Forced-Choice Recognition tests
The tests were identical to those described in Experiment 1, with minor modifications. The number of trials differed between the HS and LS tasks, with 12 trials in each test for the LS set, and (for consistency with prior studies) 8 trials in each test on the HS set. To prevent using mismatches in response selection (Naveh-Benjamin et al., 2004) on recognition tests, the two faces (names) in each display were matched for age and gender. At associative recognition tasks, participants were told that they had already seen all components and their task was to choose the appropriate pairings. Although the LS testing procedure was identical to Experiment 1, the HS tasks required participants to respond by pressing the ‘Z’ or ‘/’ keys for left–right responses, respectively, as in the original Naveh-Benjamin et al (2004) task. Similarly, a 250 ms delay followed each response before the next item appeared in the LS test, but there was no delay between items in the HS test. For both the HS and LS tasks, participants received the same intentional study instructions for items and pairings as in Experiment 1. Prior to the study session, participants performed a practice task in which six face–name pairings were shown, followed by 60 s of interpolated activity and a familiarization trial on each test. To assess the potential impact of age differences in speed of processing on the results, trial RT were recorded, and mean RT for hit trials were calculated for each test and for all stimuli of the LS and HS sets.
Data Conditioning and Statistical Analyses
Data were screened to ensure statistical assumptions were met. To facilitate comparison between the two types of stimuli, mean scores for each test were generated by averaging the PC values across the lists, separately for tests of LS and HS stimuli. We used GLM with PC as the dependent variable, age group and sex as between-subject factors, and test (item vs. association), stimulus type (faces vs. names), and saliency (LS vs. HS) as within-subject factors. The second-order interactions were included in the full model, but if found nonsignificant, the model was re-evaluated with those terms omitted. Huynh-Feldt corrected values were used for interactions to address violations of the sphericity assumption. Post-hoc decomposition of higher order interactions was performed using Newman-Keuls tests of simple comparisons with critical ps < .05. In addition to assuming superior performance by younger adults, we also hypothesized that older participants would demonstrate an associative deficit for HS but not LS stimuli as evidenced by significant interactions of Test with both Age Group and Saliency, and a three-way interaction among those factors. Based on the findings of Naveh-Benjamin et al. (2004) we also hypothesized that faces would be better recognized than names, independent of age.
Results
Recognition Performance
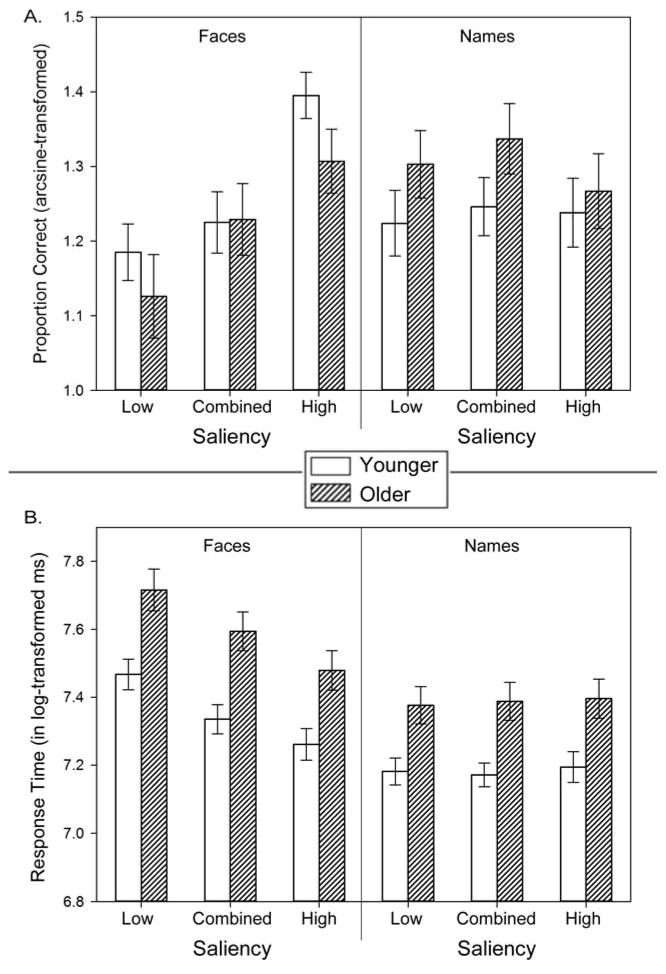
As shown in Table 4, the analyses yielded multiple significant interactions, including most notably a three-way Saliency × Test × Age Group interaction. To assess whether the Test × Age Group interaction was restricted to the HS stimuli, as hypothesized, this interaction was probed by subsidiary GLM analyses, separately for LS and HS tasks. Age group served as a between-subjects factor; item and association performance, averaged across stimulus types, were treated as repeated measures. The subsidiary analyses revealed a significant Test × Age Group interaction for the HS stimuli, F[1, 62] = 15.08, p < .001, ηp2 = 0.195, but not the LS stimuli, F[1, 62] = 0.07, p = .786, ηp2 = 0.002. In addition, post-hoc Newman-Keuls tests of simple effects revealed no age differences in item recognition for HS stimuli, which yielded the highest scores of all stimulus–task combinations among older participants. In contrast, on the HS association tasks, older adults attained their lowest mean scores – significantly below the levels observed on all other combinations of task, stimulus type, and age groups (details in Table 5 and Figure 5A).
Table 4.
Experiment 2a: Main Effects and Significant Interactions
| Effect or Interaction | F | p | ηp2 |
|---|---|---|---|
| Accuracy | |||
| Age Group | 8.429 | 0.005 * | 0.123 |
| Sex | 2.047 | 0.158 | 0.033 |
| Saliency | 39.753 | 0.000 * | 0.398 |
| Test | 116.433 | 0.000 * | 0.660 |
| Test × Age Group | 3.669 | 0.060 | 0.058 |
| Stimulus | 43.046 | 0.000 * | 0.418 |
| Saliency × Test | 67.348 | 0.000 * | 0.529 |
| Saliency × Test × Age Group | 5.628 | 0.021 * | 0.086 |
| Saliency × Stimulus | 2.904 | 0.094 | 0.046 |
| Test × Stimulus | 5.990 | 0.017 * | 0.091 |
| Test × Stimulus × Sex × Age Group | 3.245 | 0.077 | 0.052 |
| Saliency × Test × Stimulus | 5.000 | 0.029 * | 0.077 |
| Response Time | |||
| Age Group | 11.289 | 0.001 * | 0.158 |
| Sex | 1.374 | 0.246 | 0.022 |
| Saliency | 45.935 | 0.000 * | 0.434 |
| Test | 135.073 | 0.000 * | 0.692 |
| Stimulus | 22.554 | 0.000 * | 0.273 |
| Stimulus × Sex | 2.767 | 0.101 | 0.044 |
| Saliency × Test | 84.015 | 0.000 * | 0.583 |
| Saliency × Test × Age Group | 3.010 | 0.088 | 0.048 |
| Saliency × Stimulus | 2.313 | 0.134 | 0.037 |
| Test × Stimulus | 17.341 | 0.000 * | 0.224 |
| Saliency × Test × Stimulus | 10.620 | 0.002 * | 0.150 |
| Saliency × Test × Stimulus × Sex × Age Group | 3.365 | 0.072 | 0.053 |
Notes. Accuracy models reflect arcsine-transformed proportions of correct scores; a log-transformation was applied to modeled response times;
p < 0.050.
Table 5.
Experiment 2a: Recognition Memory Performance in Young and Older Adults
| LS | HS | |||
|---|---|---|---|---|
| Item | Associations | Item | Associations | |
| Proportion Correct | ||||
| Younger adults | .75 ± .08 | .72 ± .08 | .87 ± .08 | .72 ± .09 |
| Older adults | .71 ± .09 | .69 ± .10 | .87 ± .06 | .63 ± .09 |
| Response Times (msec) | ||||
| Younger adults | 2331 ± 627 | 2422 ± 642 | 1850 ± 421 | 2494 ± 640 |
| Older adults | 3083 ± 1092 | 3053 ± 1221 | 2234 ± 672 | 3205 ± 995 |
Notes. Mean values ± standard deviations are presented; HS = high-saliency stimuli; LS = low-saliency stimuli.
Figure 5.
Comparing recognition performance on two face–name recognition tasks for items and associations by younger and older adults in Experiment 2a. Open bars reflect younger adults and diagonally patterned bars represent older adults. Error bars reflect standard errors of the means. A. Proportion correct by younger and older adults on face–name recognition tasks using low saliency (LS) and high saliency (HS) face stimuli. B. Log-transformed response times of younger and older adults on the LS and HS face–name recognition tasks.
The analyses also yielded a Saliency × Test × Stimulus Type interaction, with Newman-Keuls tests revealing that old and young participants scored best on HS face recognition, followed by the HS name recognition, and LS face recognition; scores were similar on the LS name and face association tasks as well as on the HS face association task. Performance was worst on the HS name association task, and only slightly better on the LS name association task. In addition, a significant main effect of age group reflected superior recognition performance by younger adults, and a significant main effect of saliency was observed, as greater accuracy was noted in recognition of the HS than of the LS stimuli. A significant main effect of test was due to better recognition of items compared to associations. Similarly, a significant main effect of stimulus type resulted from superior recognition of faces than of names. Table 5 contains scores broken down by test, age group, and stimulus type.
We performed additional analyses to test whether a greater item distinctiveness advantage was associated with a greater disadvantage for associative memory. After calculating the differences between HS and LS item recognition and between HS and LS associative recognition, we computed zero-order correlations between the two difference scores for younger and older adults. No significant associations were noted for younger (r = –0.115, p = 0.542), or older participants (r = –0.320, p = 0.074). The association for older adults became significant, however, after excluding two participants with extremely high positive difference scores, both for items and associations: r = –0.411, p = 0.024. Sensitivity analysis (Hulley, Cummings, Browner, Grady, & Newman, 2013) indicates that rejecting a null hypothesis for a moderate correlation (i.e., r = –0.40) at α = 0.50, two-tailed, would require 47 participants per group, or roughly doubling the current sample size.
Speed of processing
Trial-by-trial RTs were recorded and averaged across hit trials for each test and stimulus list for tests of both HS and LS stimuli (see Table 5). To correct for RT distribution skewness, we applied a log transformation. LogRT was the dependent variable in a GLM like the one employed in the PC analyses, above. We hypothesized faster responses for recognition of items than of associations, shorter RTs for correct recognition of HS than of LS stimuli, and faster responses for recognition of individual faces than of names.
The RT analyses (see Table 5) revealed several significant interactions. Post-hoc Newman-Keuls tests of a significant Saliency × Test × Stimulus Type interaction showed that HS faces elicited the fastest RTs. There were no differences in RT among LS items and both LS and HS associations. Two second-order interactions indicated faster responses to HS items, and faster responses to face items. There was also a main effect of age group, as younger adults responded faster than older adults, but there were no significant interactions with age group. In addition, there was a main effect of saliency, with faster responses to HS than to LS stimuli. There was a main effect of test, with faster RT on tests of items than of associations. Finally, there was a main effect of stimulus type that reflected correct recognition of name stimuli taking longer than correct recognition of faces (see Fig. 5).
Discussion
This experiment produced two notable findings. First, the magnitude of age-related decrements in recognition memory varied across tasks and stimulus types. No age differences were observed in the accuracy of HS item recognition, which was the easiest task for the older adults. In contrast, on the HS association tasks, older adults attained their lowest scores. Thus, as hypothesized, greater saliency and visual complexity of face stimuli boosted recognition for both age groups (effect size, Cohen’s d with Hedges correction, d = 1.46 for the young and d = 2.08 for the old), but was linked to reduced recognition of associations in older adults (d = −0.62), without affecting that of the young (d = 0).
The observed facilitation of recognition by stimuli enrichment is in accord with reports suggesting that higher levels of implicitly encoded visual features enable faster, more accurate object recognition (Chun & Jiang, 1996), and that memory is better engendered by realistically colored images than by stimuli rendered in grayscale (Berry, 1991). Regardless of age, objects and surrounding congruent scenes are processed and encoded together (Fenske, Aminoff, Gronau, & Bar, 2006; Rémy et al., 2013). Moreover, spatial contextual information perceptually primes subsequent object recognition, and face recognition is enhanced by reinstatement and impeded by deletion of encoding context (Memon & Bruce, 1983; Watkins, Ho, & Tulving, 1976; Winograd & Rivers-Bulkeley, 1977).
According to Yonelinas (2002), associative familiarity is only possible with unitization of associated elements. Younger and older adults show equivalent memory for complex stimuli with a foreground object and background scene, and memory for such images may represent a special case of associative memory due to unitization of scenic elements (Gutchess & Park, 2009). The results of Experiment 2 are consistent with this interpretation, and support the view that highly correlated visual elements promote unitization (Mayes, Montaldi, & Migo, 2007). Cognitive and mnestic procedures deployed by older adults benefit from the use of external cues, and may attenuate concomitant age-related reductions in the efficacy of self-initiated processes. The increased reliance on environmental support may, however, further impair memory processes when such external cues do not support specific cognitive operations. The present findings suggest that although external cues may reliably enhance face recognition, for older adults this benefit may come at the expense of reduced associative binding with names. Thus, the negative effect of stimulus enrichment on associative memory contradicts the belief that salient context is universally beneficial for memory performance (see Naveh-Benjamin & Craik, 1995, for a similar position) and offers an important qualifier of that proposition.
The differences in task or stimulus properties between the two tasks may present a plausible explanation of the performance discrepancies between HS and LS tasks. Whereas the HS task consisted of 48 face–name pairs at study and 12 trials per test, in the LS task 32 pairs were presented at study and only 8 trials per test were used at the recognition stage. Accordingly, it is possible that differences in study and recognition loads were responsible for the disparity between HS and LS performance in Experiment 2a. To address this concern, we administered the LS recognition task to a new sample drawn from the same local community population and according to the same health and exclusion criteria as those employed in Experiment 2a. We did, however, vary the number of stimulus pairs presented during study as well as the number of recognition trials to test whether differences in study and recognition loads might be responsible for the observed discrepant results.
Experiment 2b
Method
Participants
Participants were 28 younger (14 men and 14 women, mean age = 21.86; SD = 2.94 years), and 24 older (11 men and 13 women, mean age = 71.25; SD = 7.22 years) adults. The young participants were recruited from psychology classes at Wayne State University. Older participants were recruited from the greater Detroit metropolitan community, as before. Twenty-six young participants received research participation credits for their course; two young and all older participants earned $15. All participants were native English speakers, had a minimum of a high school diploma or equivalency, and scored above 26 on the MMSE (Folstein et al., 1975) to rule out cognitive impairment. Participant eligibility was determined using the same criteria as in Experiment 2a.
Sample Characteristics
Men and women did not differ in education, or MMSE scores (see Table 6). Although there was no significant difference in MMSE scores (t[50] = –1.78, p > .05), older adults had, on average, one more year of education than did younger adults: t(50) = –2.02, p < .05. Among the older but not younger adults, men were significantly older than women: mean age = 76.2, SD = 6.8 years vs. 67.1, SD = 4.5 years, t(22) = –3.93, p < .001.
Table 6.
Sample Demographics for Men and Women in Experiment 2b
| Variable | Men | Women | t | p |
|---|---|---|---|---|
| Younger adults | ||||
| Age (in years) | 22.5 ± 3.9 | 21.2 ± 1.5 | 1.16 | .25 |
| Education (in years) | 15.4 ± 1.4 | 15.9 ± 1 | 1.05 | .30 |
| MMSE | 29.2 ± 0.8 | 28.8 ± 1.2 | 1.12 | .27 |
| Older adults | ||||
| Age (in years) | 76.2 ± 6.8 | 67.1 ± 4.5 | 3.93 | .00 |
| Education (in years) | 17.1 ± 2.1 | 16.4 ± 2.9 | 0.67 | .51 |
| MMSE | 27.9 ± 1.6 | 28.8 ± 1.4 | 1.43 | .17 |
Notes. MMSE = Mini-Mental State Examination. Mean values ± standard deviations are presented.
Design and Procedure
The stimuli were identical to those from Experiment 1 and to the LS stimuli of Experiment 2a. Here, each of the three study lists of face–name pairs were modified so that each list had 8, 10, or the original 12 items per test. To create the new lists, the original LS stimulus lists of 48 face–name pairs from Experiment 1 were used, and randomly selected face–name pairings were removed according to the scheme of one young male, one young female, one old male, one old female. Two such foursomes were removed to create the lists of 40 pairs (i.e., for the 10-item test lists) and an additional two of such groupings were removed to create the lists of 32 pairs (i.e., for the 8-item test lists). Recognition trials associated with the removed stimuli were omitted from the recognition tests to yield the intact and reduced lists. In the study phase, one of the three lists of stimuli (32, 40, or 48 pairs) counterbalanced across participants was displayed on a computer screen. Participants then counted backwards for 60 s to prevent rehearsal. Following the study phase and interpolated activity, participants performed four 2-AFC recognition tests (face–item, name–item, face–association, name–association). Following completion of testing, the process was repeated with the remaining stimulus lists until all participants had completed testing on all three list lengths.
Data Conditioning and Statistical Analyses
PC scores were computed for each recognition task by dividing the number of correct recognition judgments by the total number of trials in each test. Data were screened for violation of statistical assumptions. Six scores were generated for each participant, reflecting PC for the item and association sub-tasks for each of the three list lengths, collapsed across stimulus types. The GLM framework was employed to analyze recognition performance on the four 2-AFC tasks from 3 lists of varying length. Age group was a categorical predictor, and the two tests (item and association), and three list lengths (32, 40, and 48 studied pairs) were treated as repeated measures. Huynh-Feldt corrected values were used to address violations of sphericity assumption. We hypothesized that all participants would perform better on recognition of shorter lists of both items and associations. In addition, we hypothesized a three-way interaction of age, test, and list length, such that older adults would have better associative recognition on shorter lists than younger adults would.
Results and Discussion
The GLM analyses revealed a significant main effect of Age Group that reflected better performance by younger participants compared to their older counterparts, but there were no significant interactions with age (Table 7). Post-hoc tests of a significant Test × List Length interaction revealed superior memory for associations, but not for items, on a shorter list (32-item vs. 40 or 48 items). Notably, however, there was no List Length × Age Group interaction, nor any other significant effects. Furthermore, recognition performance on the longest lists was comparable to the results of Experiment 2a (Table 8). Next, the GLM analyses were re-run to evaluate differences in log-transformed hit RTs. Newman-Keuls post-hoc tests of a significant List Length × Age Group interaction showed slower responses by older than by younger participants. Although there were no differences among RTs of older adults to the different list lengths, younger participants’ responses were slower for the 12-item tests than for the shorter list lengths. Notably, there was no Age Group × Test × List Length interaction for RTs.
Table 7.
Experiment 2b: Main Effects and Significant Interactions
| Effect or Interaction | F | p | ηp2 |
|---|---|---|---|
| Accuracy | |||
| Age Group | 5.639 | 0.021 * | 0.101 |
| Test | 0.485 | 0.489 | 0.009 |
| List Length | 0.529 | 0.591 | 0.010 |
| Test × List Length | 3.494 | 0.041 * | 0.066 |
| Response Times | |||
| Age Group | 28.285 | 0.000 * | 0.361 |
| Test | 4.951 | 0.031 * | 0.018 |
| List Length | 6.167 | 0.004 * | 0.036 |
| List Length × Age Group | 4.244 | 0.021 * | 0.025 |
Notes. Accuracy models reflect arcsine-transformed proportions of correct scores; a log-transformation was applied to modeled response times;
p < 0.050.
Table 8.
Comparison of Low-Saliency (LS) Recognition in Experiments 2a and 2b
| Experiment 2a | Experiment 2b+ | |||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Younger Adults | ||||
| Items | 0.750 | 0.720 – 0.779 | 0.788 | 0.748 – 0.828 |
| Associations | 0.718 | 0.687 – 0.748 | 0.754 | 0.706 – 0.802 |
| Older Adults | ||||
| Items | 0.711 | 0.680 – 0.743 | 0.732 | 0.687 – 0.778 |
| Associations | 0.689 | 0.654 – 0.724 | 0.702 | 0.647 – 0.758 |
Notes. CI = Confidence interval;
+ Mean values only for performance on lists with 48 pairs and 12 trials per test, comparable to the list lengths in Experiments 1 and 2a.
The results of Experiment 2b demonstrated that memory and recognition loads do not affect item memory in older adults. The absence of a significant list length effect in Experiment 2b supports the proposition that variation in memory load cannot explain the findings of Experiments 1 and 2a. Because shorter study lists with fewer face–name pairs and fewer recognition trials did not differentially affect performance in older adults, these findings lend further support to the notion that the differences in associative deficits observed in those experiments are indeed due to mediating effects of stimulus saliency, and not to other differences between the two tasks in Experiment 2a.
Additional support for this proposition comes from the finding in Experiment 2a that the HS items produced the fastest RTs and the best item recognition. The results of Experiment 2a suggest that ADH is predicated, at least in the current study, on very high recognition of items, in addition to reduced associative recognition. Because the age-related associative deficit hinges on a difference between recognition of items and associations, differences in item recognition between the stimuli sets that differ in saliency may influence the likelihood of observing the phenomenon and thereby supporting ADH. Although Experiment 2b demonstrated that the generation of an associative deficit does not depend on length of encoding and retrieval lists, it did not explicitly compare item recognition for the two stimulus types. Thus, it is important to examine whether item recognition of HS faces is superior to that for LS faces, while holding other properties constant between the two. Furthermore, it is possible that differential item recognition between the two tasks may be affected by the explicit associative encoding of faces and names together during encoding. Therefore, in light of these concerns and because of the differential performance on the HS and LS saliency sets reported in Experiment 2a, we investigated whether item recognition differed between the HS and LS saliency sets, without an associative memory component. In addition, we hypothesized that greater visual complexity and variability of the HS faces would enhance item recognition, as reflected by higher accuracy and faster responses.
Experiment 3
Method
Participants
We recruited younger participants from psychology classes, and older participants from the greater Detroit metropolitan community. Younger and older participants were compensated with experiment participation credit and monetary remuneration of $25, respectively. Participants provided informed consent in accord with University IRB procedures. For purposes of consistency with the prior study, we replicated the participant eligibility criteria used in Experiments 2a and 2b.
Participants were 41 younger (18–30 years old; mean age = 22.34; SD = 1.74 years; 66% women) and 34 older (60–84 years old; mean age = 68.94; SD = 6.78 years; 56% women) adults. In both age groups, men and women did not differ with regard to age, self-reported years of education, or MMSE scores (see Table 9).
Table 9.
Sample Descriptors for Men and Women in Experiment 3
| Variable | Men | Women | t | p |
|---|---|---|---|---|
| Younger Adults | ||||
| Age (in years) | 22.8 ± 3.3 | 21.8 ± 2.5 | 1.10 | .28 |
| Education (in years) | 15.1 ± 1.0 | 15.2 ± 1.2 | 0.28 | .78 |
| MMSE | 28.9 ± 1.0 | 29.1 ± 0.9 | 0.70 | .49 |
| Older Adults | ||||
| Age (in years) | 66.8 ± 6.9 | 70.6 ± 6.4 | 1.68 | .103 |
| Education (in years) | 15.7 ± 2.7 | 16.9 ± 2.2 | 1.38 | .178 |
| MMSE | 27.9 ± 1.0 | 28.5 ± 1.1 | 1.58 | .123 |
Notes. MMSE = Mini-Mental State Examination; Mean values ± standard deviations are presented.
Design and Procedure
Experimenters administered the task using SuperLab 4.5 (Cedrus Corp., San Pedro, CA), which serially presented participants with visual stimuli – individual faces or names were presented separately in different administrations. In contrast to the previous experiments, participants studied lists composed exclusively of items; no associations were studied or tested in this experiment. Recognition of faces and names was tested in separate study-test administrations. In tests of face recognition, participants studied three separate lists consisting exclusively of face stimuli. One list included only LS faces, one list included only HS faces, and a third list included equal numbers of LS and HS faces. Stimuli for tests of name recognition were surnames taken from the sets used in Experiments 1 and 2a. As the names did not differ on any dimensions across studied lists, they served as controls for the tests of face recognition. Participants completed administrations for all three lists for one stimulus type (face or names) before administration of the three lists for the other type, and the order was counterbalanced across the sample. The HS and LS faces were stimuli from Experiment 2a supplemented by similar HS images from free online sources, and LS stimuli were added from the PAL face database (Minear & Park, 2004). Face stimuli were evenly divided by sex and age, with 40 items per list, and three versions of each list assigned randomly across the sample in a Latin square design. Recognition test stimuli were sampled from the study lists, excluding the first and last three items from each list to minimize primacy and recency effects. Target–foil combinations across lists were paired on the dimensions of gender, age, physical characteristics, and ethnicity.
Item recognition task
Recognition tests for faces and names were administered separately, in a randomized order. For each stimulus list (HS, LS, combined) participants studied 40 items, and the order of list presentation was randomized in pseudo-Latin square design. A custom-designed task implemented in SuperLab version 4.5 (Cedrus Corp., San Pedro, CA) serially presented 40 faces (names), one face (name) at a time, for 3 s each followed by a 200 ms inter-stimulus interval. Immediately following study, the program instructed participants to count backwards by threes from a random number between 900 and 999 for one minute. Following this interpolated activity, participants completed a 2-AFC recognition task similar to the item recognition tasks in Experiments 1 and 2. To limit the encoding and recognition loads and maintain some similarity in the number of recognition trials with the previous experiments, participants completed 20 name or face recognition trials per list, rather than test recognition on all studied items. Previously presented targets and unstudied foils were presented side-by-side and remained on the screen until the participant responded. For the combined list trials, targets and foils originated from the same stimulus type (LS or HS). Experimenters instructed participants to keep their fingers over the keyboard ‘D’ and ‘J’ keys labeled “LEFT” and “RIGHT,” and to indicate as quickly and carefully as possible which of the items they recognized as “old.”
Data Conditioning and Analysis
To correct skewness an arcsine transformation was applied to PC scores and a log transformation was applied to the RT data. Name and face recognition were analyzed separately using a GLM analytic framework with PC as a dependent variable, saliency (3 levels: LS, combined, and HS) as repeated measure, age group (young, old), sex (men, women), and their interactions – as independent variables. We hypothesized that higher levels of saliency in face stimuli would yield better recognition performance with faster responses. We expected better performance by younger than older participants, overall. However, because saliency appears to affect inter-item associative binding differentially, we did not expect an interaction of age and saliency on item recognition performance without an explicit associative component. However, we did expect that older adults would show a particular benefit of saliency on response timing. For all models, if the interaction between Sex and Age Group was nonsignificant (p > .1), the model was re-evaluated with the interaction omitted. Huynh-Feldt correction was used to address violations of the sphericity assumption.
Results and Discussion
The GLM evaluating face recognition accuracy revealed neither a main effect of age group, nor of sex (Table 10). Our hypothesized main effect of saliency was decomposed with post-hoc comparisons: independent of age group, face recognition performance on the HS face stimuli (unadjusted mean = 0.953, SD = 0.062) was superior to performance on the LS (unadjusted mean = 0.881, SD = 0.130) and on the combined stimulus condition (unadjusted mean = 0.915, SD = 0.097; p < .001 for both); Bonferroni correction rendered the difference between the LS and combined stimuli nonsignificant (corrected p = .109). In a subsidiary GLM analysis comparing recognition performance of HS and LS items included in the combined list, age group was an independent variable and the arcsine transformed responses for HS and LS items were repeated measures. The analysis of responses in the combined list showed a main effect of saliency, F(1, 72) = 16.349, p < .001, ηp2 = 0.185. In the combined list, HS faces (unadjusted mean = 0.942, SD = 0.102) were better recognized than LS faces (unadjusted mean = 0.899, SD = 0.105). We carried out additional post-hoc t-tests to compare recognition of HS and LS faces in the combined list to performance on the lists that included only HS or LS faces. Recognition performance for HS items in the combined list was similar to performance on the list that included only HS items (t[73] = 0.687, p > .05), and recognition of HS faces on the combined list did not statistically differ from recognition performance on the list including only HS faces (t[73] = 1.465, p > .05). As expected there were neither significant main effects nor interactions for names (for all, p > .15), demonstrating no differences in recognition of individual surnames by age, sex, or across lists, because name stimuli served as controls for face recognition and did not differ across lists.
Table 10.
Experiment 3: Main Effects and Significant Interactions
| Effect or Interaction | F | p | ηp2 |
|---|---|---|---|
| Face Recognition Accuracy | |||
| Age Group | 0.695 | 0.407 | 0.010 |
| Sex | 2.181 | 0.144 | 0.029 |
| Saliency | 17.052 | 0.000 * | 0.192 |
| Name Recognition Accuracy | |||
| Age Group | 1.307 | 0.257 | 0.018 |
| Sex | 0.017 | 0.896 | 0.000 |
| Saliency | 0.392 | 0.676 | 0.005 |
| Face Recognition Response Time (RT) | |||
| Age Group | 10.418 | 0.002 * | 0.128 |
| Sex | 2.664 | 0.107 | 0.036 |
| Sex × Age Group | 6.802 | 0.011 * | 0.087 |
| Saliency | 53.846 | 0.000 * | 0.431 |
| Name Recognition RT | |||
| Age Group | 10.67 | 0.002 * | 0.131 |
| Sex | 2.939 | 0.091 | 0.040 |
| Sex × Age Group | 2.482 | 0.120 | 0.034 |
| Saliency | 0.129 | 0.879 | 0.002 |
Notes. Accuracy models reflect arcsine-transformed proportions of correct scores; a log-transformation was applied to modeled response times (RT);
p < 0.050.
The analyses of log-transformed RT for correct face recognition revealed a significant Sex × Age Group interaction. Post-hoc comparisons showed significantly slower responses by the older women in the sample (unadjusted mean = 2347.724, SD = 850.588 ms) in comparison to those by older men (unadjusted mean = 1768.151, SD = 467.924 ms; t[32] = 2.752, p < .01). In contrast, the logRTs for younger adults did not differ by sex (women’s unadjusted mean = 1604.480, SD = 488.189 ms; men’s unadjusted mean = 1682.445, SD = 374.430 ms; t[38] = 0.786, p > .4). Moreover, older women had significantly longer face recognition logRT than younger women (t[44] = 4.305, p < .001), whereas men’s logRT were unrelated to age (t[26] = 0.387, p > .7). A main effect of saliency was also followed up by post-hoc t-tests for repeated measures with Bonferroni correction. Independent of age, HS face recognition logRTs (unadjusted mean = 1655.501, SD = 658.944 ms) were significantly shorter than responses to both LS faces (unadjusted mean = 2055.832, SD = 765.984 ms) and combined stimuli (unadjusted mean = 1788.254, SD = 599.088 ms; corrected p < .001 for both; Fig. 6), possibly reflecting lower task difficulty for recognition of HS faces. Furthermore, mean logRT for the combined stimulus list was significantly faster than for the LS faces (corrected p < .001). Analyses of logRT for correct name recognition showed only a main effect of age group (F[72, 1] = 10.464, p < .001, ηp2 = 0.127) with slower responses by older (unadjusted mean = 1667.598, SD = 566.129) than by younger adults (unadjusted mean = 1350.040, SD = 310.188 ms).
Figure 6.
Comparing item recognition performance in Experiment 3 on three separate lists of faces and names by younger and older adults. Open bars represent younger adults and diagonally patterned bars, older adults. Error bars are standard errors of the means. A. Proportion correct by younger and older adults on face–name recognition tasks using low- (LS), and high-saliency (HS) face stimuli and combinations of the two. B. Response times of younger and older adults on the LS and HS face–name recognition tasks and a list combining LS and HS stimuli.
In line with the hypothesis generated following Experiment 2a, Experiment 3 demonstrated superior accuracy and faster responses in correct recognition of faces when images were more salient due to inclusion of non-facial contextual information and greater inter-item variability, independent of age. No differences were noted in name recognition, thus suggesting that the effect observed in Experiment 2a did not reflect an influence of additional lexical or visual elements requiring binding. It is apparent that recognition of single items is equally easy for adults of all ages. Thus, it provides a good benchmark for comparison with memory for face–name associations.
General Discussion
The main outcome of this series of experiments is establishing an important boundary condition of ADH. We demonstrated that the differential age-related deficit in recognition of face–name associations is not a universal phenomenon and that its generalization may depend on the properties of face stimuli. While replicating age-related differences in associative memory for pairs of names and highly salient faces, we observed no such effect for standardized face stimuli devoid of contextual cues and background details.
The effect of stimulus saliency and visual complexity was notable. Regardless of the observer’s age, highly distinctive face stimuli rendered in color and accompanied by multiple non-facial details as well as salient characteristics such as eye color and head rotation were easier to recognize and required shorter processing time than less distinctive, grayscale face stimuli did. In contrast, only for older adults was the association of names and faces clearly easier when fewer contextual details accompanied the face. The reasons for this finding are unclear but several candidate mechanisms can be contemplated.
Enriching stimuli by increasing the number of salient features (i.e., first and last names, visually complex faces) may put additional load on binding processes for intra-item unitization as well as inter-item associations. Thus, while enhancing item memory, it may contribute to disproportional escalation of the binding costs in older adults. In short, for older persons, stimulus complexity, salience, and embedded context, a friend of item recognition, turns into a foe of associative memory.
This phenomenon can be related to the “dark side of support” hypotheses recently proposed to explain age differences in memory (Lindenberger & Mayr, 2014). In their review, Lindenberger and Mayr noted that even when internal contextual cues benefit performance, older adults fail to capitalize on them and revert to less efficient external support (outsourcing). As older adults are progressively driven to “outsource” support to the external cues, increased reliance on added features and extra-stimulus context may hinder cognitive performance. It is likely that an age-related reduction in general resources rather than specific deficit is to blame for the emergence of this deficit (Benjamin, 2010; Spencer & Raz, 1995).
Although adding extraneous context improves face recognition (Goshen-Gottstein & Ganel, 2000), contextual cues need to be processed simultaneously with the stimulus to act as a supporting factor (Gibson, 1940). The transformation of stimulus complexity and saliency from a friend of memory to its foe may reflect both weakening of internal representations with age and a tendency to seek extra-stimulus cues in the climate of dwindling general cognitive resources (Lindenberger & Mayr, 2014; Neider & Kramer, 2011; Verhaeghen & Cerella, 2002).
Age-related declines in attentional control may also play a role in turning helpful cues to the “dark side.” In older adults, a rich, perceptually complex context may become a distractor diverting limited attentional resources needed for inter-item associative binding (e.g., of a face and a name) to intra-item unitization (e.g., of a face). Given the tendency of older participants to disengage from burdensome information by refraining from self-initiating access (Craik & Byrd, 1982), this may account for the disproportionate drop in performance. In addition, whereas intra-item unitization is processed by visual and ventral temporal cortical regions, processes that show relatively small age-related declines, item memory and associative binding are supported by medial temporal lobe regions that are more negatively affected by aging (Raz et al., 2005; Staresina & Davachi, 2010). The observed effect of stimulus complexity is in accord with a recently reported finding of improvement in item memory and decline in memory for associations in young adults under the condition of sensory degradation of the stimuli (Naveh-Benjamin & Kilb, 2014). Thus, recognition of noisier stimuli by younger adults appears to simulate the magnitude of the associative deficit by older adults, and suggests that greater perceptual and attentional resources required for item encoding may increase the cost of associative binding. Whatever the driving forces behind it, the shift from efficient utilization of internal cues and self-initiated strategies to less-than-optimal reliance on external support occurs gradually and its age trajectory remains poorly understood; understanding this phenomenon is a worthy goal for future investigations.
Several design characteristics could have contributed to the observed failure to uphold ADH with a particular kind of stimuli. Because ADH rests on the contrast between item and associative recognition, the observed stimulus saliency or visual complexity effect may reflect either side of this comparison. First, it is possible that ADH reflects a ceiling effect stemming from enhanced stimulus saliency and making item recognition too easy for many participants. If this were correct across studies, ADH would have appeared as an artifact of ceiling effects. The results of studies cited in the introduction and of our own findings do not support this proposition. In Experiment 2a, we indeed observed face recognition performance near ceiling for HS but not for LS face stimuli. However, such ceiling effects for item recognition were not apparent in the study by Bender and colleagues (2010) that included all the participants from Experiment 1 here, and upheld ADH in the recognition of word pairs. Furthermore, whereas a few studies supporting ADH have reported item recognition performance near ceiling across the age groups (Bastin & Van der Linden, 2006; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003, Experiment 1), the majority of equally supportive outcomes came from studies with no ceiling effects (Kilb & Naveh-Benjamin, 2007; Naveh-Benjamin, 2000; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003, Experiment 2; Naveh-Benjamin et al., 2004; Naveh-Benjamin et al., 2009). Thus, ceiling effects, per se, do not affect the likelihood of ADH rejection. Nonetheless, the possible contribution of a ceiling effect in specific studies cannot be ruled out.
Second, we cannot exclude the possibility that in previous studies, differential reliability was at play, with item recognition being less reliable than memory for associations. As we demonstrate here, this was not the case in the reported experiments as moderately high and uniform reliability was attained across tasks. However, in conducting future experiments that hinge on comparing multiple conditions or tasks, uniform reliability must be established.
A third possible reason for the observed disparities in the likelihood of finding the age-related associative deficit may reflect age differences in the processing of items as well as associations. As a rule, older adults benefit from enrichment of stimuli presented in recognition memory experiments, and indeed, our HS face stimuli elicited higher recognition rates than LS faces. It is possible that this is due to the propensity of contextual cues to reward reliance on familiarity over recollection in face recognition by older people (Bartlett, Strater, & Fulton, 1991; Yonelinas, 2002). Conditions that foster unitization of content and context may promote familiarity-based responses in older adults (Bastin et al., 2013). Moreover, names associated with less distinct faces are more difficult to recognize (Fig. 5a; Pantelis, van Vugt, Sekuler, Wilson, & Kahana, 2008), and the presence of multiple visual elements requiring associative binding or unitization may further facilitate item memory for names (Old & Naveh-Benjamin, 2012a). Furthermore, the support for ADH in word pairs and face–name pairs with highly salient, visually complex faces, but not in face–name pairs with standardized grayscale faces also suggests that semantic links for lexical stimuli can serve as contextual support for item memory at the expense of associative binding.
Finally, age-related deficits in using strategies, deploying attention and encoding contextual cues may all reflect fundamental properties of the aging brain, which has long been viewed as an information processing system with progressively decreasing signal-to-noise ratio (Birren, 1958; Crossman & Szafran, 1956; Layton, 1975; Li, Lindenberger, & Sikström,, 2001). Information theory (Shannon & Weaver, 1949) has been applied to explain age differences in diverse experimental phenomena, such as visual search (Hoyer & Familant, 1987), fragmented pictures identification (Cremer & Zeef, 1987), and auditory stimulus persistence (Raz, Millman, & Moberg, 1990). Increasing processing redundancy may be an optimal way of improving representational signal-to-noise ratio and minimizing transmission errors in a system composed of noisy elements. There are several ways to accomplish that goal. Improvement of signal-to-noise ratio results from increasing the time allocated for processing and allowing repeated runs for noise averaging (time redundancy). With a limited presentation time of three seconds to learn each pair, this was unlikely in the present study. Another way to improve processing of noisy stimuli is recruiting extra processing elements dedicated to the task (hardware redundancy). The latter approach corresponds to expansion of task-related activation in older adults reported by functional neuroimaging studies (Cabeza, Anderson, Locantore, & McIntosh, 2002; Grady et al., 1994). The same goal can be attained by making the stimuli more redundant, i.e., loading them with multiple correlated features (stimulus redundancy). It is the latter that may be at play in the experiments presented here.
In the outlined information-processing context, highly salient HS face stimuli with their multiple, distinctive contextual features are easier to recognize than LS stimuli. In encoding HS stimuli, binding and unitization of context and face occurs automatically (Gutchess & Park, 2009), and results in reduced difficulty of face recognition as observed in the Experiment 3. However, associating salient, visually complex face stimuli with names likely requires additional binding, and in a noisy processor, such additional binding may increase the likelihood of error in recognition of the association. Thus, greater visual complexity that is helpful in encoding and recognition of individual items may become detrimental in recognition of associations. Indeed, there are examples showing that use of context may be a costly proposition for an increasingly noisy aging information processor (Chee et al., 2006; Gazzaley, Cooney, Rissman, & D’Esposito, 2005; Hasher & Zacks, 1988; Neider & Kramer, 2011).
Limitations and Future Directions
The findings reported here should be interpreted in the context of several limitations. The first and the most obvious one is the cross-sectional design employed throughout this series of experiments. Therefore, the results, while being relevant to the questions about age differences, reveal nothing about the temporal dynamics of aging that lead to the observed differences (Hofer & Sliwinski, 2001; Lindenberger, von Oertzen, Ghisletta, & Hertzog, 2011).
Second, a coarse distinction between high and low saliency of the stimuli is only a first step in investigating the possible “dark side” of stimulus complexity, and saliency in age-related associative binding deficits. Future investigations of ADH should parametrize this dimension of the stimuli and establish the conditions under which the costs of context use become greater than the gains it promises in the enhancement of associative memory and how this cost–benefit balance may be altered by aging. The relative importance and salience of contextual cues embedded in the HS stimuli (e.g., indoor and outdoor background scenes, incidental objects displayed behind the respective faces, accessories, size, lighting, contrast, image quality, camera angle, proportion of body included in the image, and distinct emotional expressions signaling positive, negative or neutral affect) needs to be determined. Some of these cues may enhance facial memory in younger (Shimamura, Ross, & Bennett, 2006) but not older adults (Leigland, Schulz, & Janowsky, 2004), and increase false alarm responses in older but not younger participants (Werheid et al., 2010). The relative importance of each of these specific contextual cues and their role in modifying age-related patterns of mnemonic performance is unclear. The present findings do not clarify specific mechanisms that may distinguish between stimulus complexity, saliency, variability, or distinctiveness as the source of the differential results observed between the two types of face stimuli. Nonetheless, one conclusion stands: Whereas different types of visual features improve item memory regardless of age, they have the opposite effects on associative memory in younger and older adults. This demonstrates that under some conditions, older adults do not exhibit an associative memory deficit. One or all of these mechanisms of complexity, salience, variability, and distinctiveness may contribute to this, and future research could look more into the specific factors responsible for these effects.
Third, selection of participants might have systematically influenced the results, because, as in Naveh-Benjamin et al. (2004), the younger participants Experiments 2a, 2b, and 3 were undergraduate psychology students. This group might have had greater and more recent experience with mnemonic strategies than older adults did, or than the younger participants in Experiment 1, who were not drawn from undergraduate research participant pools. Such differences in strategy use and meta-memory may conceivably influence associative recognition performance (Bender & Raz, 2012). Future studies should evaluate optimally healthy aging samples as reported in Experiment 1 to exclude the influence of common age-associated pathologies in the examination of age differences for item and associative memory as a function of item complexity and saliency.
Fourth, because the present study originated as an attempt to replicate the findings reported by Naveh-Benjamin et al. (2004), we employed the same experimental task design. However, the interpretation of our findings may be limited by use of a 2-AFC recognition task, which precluded comparison of hit rate and false alarm rates. Thus, future studies applying single-item, yes/no recognition tasks may be better positioned to elucidate which aspect of associative recognition performance is more vulnerable to the effects of saliency and visual distinctiveness. Similarly, whereas item recognition tests required participants to distinguish studied targets from unstudied lures, the tests of associative recognition only included previously studied stimuli; future studies should consider also including novel stimuli in associative trials.
Finally, it is worth noting that not all contextual information may be equally burdensome to older people, and in some cases, context may improve recall and recognition. For example, adding self-relevant (Cassidy & Gutchess, 2012), or emotional contextual details (May, Rahhal, Berry, & Leighton, 2005), or incorporating autobiographic episodic information (Moberg & Raz, 1997) and other semantically enriching cues (Murphy, Cain, Gilmore, & Skinner, 1991) into otherwise spare stimuli benefits the memory performance of older adults and may even abolish age differences altogether. Investigating the disparate impact of various types of context on age differences in memory performance may help to further delineate the border conditions for ADH and elucidate its cognitive mechanisms.
In sum, we observed that enrichment of face stimuli significantly affects the magnitude of differential age effects on binding of face–name pairs. The age-related associative deficit is observed with highly distinct face stimuli that are accompanied by contextual details but not with more sparingly contextualized faces. The reason for this appearing and disappearing effect may be both the context-related benefit for item recognition and the cost of associative binding that stems from the same source.
Acknowledgments
This study was supported in part by a grant from the National Institutes of Health R37-AG-11230 to N. Raz. We thank Yvonne Brehmer for helpful commentary, Julia Delius for proofreading, and Cheryl Dahle, Cohen Carlisle, Kristen Kennedy, Angela Kilb, Sergejs Stepanovs, and Peng Yuan for assistance in various aspects of this study.
Contributor Information
Andrew R. Bender, Department of Psychology & Institute of Gerontology, Wayne State University; Center for Lifespan Psychology, Max Planck Institute for Human Development, Berlin, Germany
Moshe Naveh-Benjamin, Department of Psychological Sciences, University of Missouri.
Katheryn Amann, Institute of Gerontology, Wayne State University.
Naftali Raz, Department of Psychology & Institute of Gerontology, Wayne State University; Center for Lifespan Psychology, Max Planck Institute for Human Development, Berlin, Germany.
References
- Bartlett JC, Strater L, Fulton A. False recency and false fame of faces in young adulthood and old age. Memory & Cognition. 1991;19(2):177–188. doi: 10.3758/bf03197115. doi: http://dx.doi.org/10.3758/BF03197115. [DOI] [PubMed] [Google Scholar]
- Bastin C, Diana RA, Simon J, Collette F, Yonelinas AP, Salmon E. Associative memory in aging: the effect of unitization on source memory. Psychology and Aging. 2013;28(1):275–283. doi: 10.1037/a0031566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The effects of aging on the recognition of different types of associations. Experimental Aging Research. 2005;32(1):61–77. doi: 10.1080/03610730500326291. [DOI] [PubMed] [Google Scholar]
- Bender AR, Naveh-Benjamin M, Raz N. Associative deficit in recognition memory in a lifespan sample of healthy adults. Psychology and Aging. 2010;25(4):940–948. doi: 10.1037/a0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in recognition memory for items and associations: Contribution of individual differences in working memory and metamemory. Psychology and Aging. 2012;27(3):691–700. doi: 10.1037/a0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AS. Representational explanations of “process” dissociations in recognition: The DRYAD theory of aging and memory judgments. Psychological Review. 2010;177:1055–1079. doi: 10.1037/a0020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry LH. Visual complexity and pictorial memory: A fifteen year research perspective. In: Simonson MR, Treimer M, editors. Proceedings of selected research presentations at the Annual Convention of the Association for Educational Communications and Technology; Ames, IA: Iowa State University Press; 1991. pp. 92–102. [Google Scholar]
- Birren JE. Aging and psychological adjustment. Review of Educational Research. 1958;28:475–490. doi: 10.2307/1168974. [DOI] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cassidy BS, Gutchess AH. Social relevance enhances memory for impressions in older adults. Memory. 2012;20(4):332–345. doi: 10.1080/09658211.2012.660956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Craik FIM. The effects of aging and divided attention on memory for item and associative information. Psychology and Aging. 2003;18(4):873–885. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory and Cognition. 1996;24(4):403–416. doi: 10.3758/BF03200930. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Venkatraman V, Tan JC, Gutchess A, Sutton, … Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of Cognitive Neuroscience. 2006;18(4):495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cuing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology. 1998;36(1):28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Amsterdam: Elsevier Science; 1986. pp. 409–422. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub SE, editors. Aging and cognitive processes. New York: Plenum; 1982. pp. 191–211. [Google Scholar]
- Cremer R, Zeef EJ. What kind of noise increases with age? Journal of Gerontology. 1987;42(5):515–518. doi: 10.1093/geronj/42.5.515. [DOI] [PubMed] [Google Scholar]
- Crossman ERFW, Szafran J. Changes with age in the speed of information-intake and discrimination. Experientia Supplementum. 1956;4:128–135. [PubMed] [Google Scholar]
- Fenske MJ, Aminoff E, Gronau N, Bar M. Top-down facilitation of visual object recognition: Object-based and context-based contributions. Progress in Brain Research. 2006;155:3–21. doi: 10.1016/S0079-6123(06)55001-0. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. A systematic application of the concepts of generalization and differentiation to verbal learning. Psychological Review. 1940;47(3):196–229. doi: 10.1037/h0060582. [DOI] [Google Scholar]
- Goshen-Gottstein Y, Ganel T. Repetition priming for familiar and unfamiliar faces in a sex-judgment task: Evidence for a common route for the processing of sex and identity. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26(5):1198–1214. doi: 10.1037//0278-7393.26.5.1198. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, … Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Gutchess AH, Park DC. Effects of aging on associative memory for related and unrelated pictures. European Journal of Cognitive Psychology. 2009;21:235–254. doi: 10.1080/09541440802257274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding ageing: An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47(6):341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Familant ME. Adult age differences in the rate of processing expectancy information. Cognitive Development. 1987;2:59–70. doi: 10.1016/S0885-2014(87)90035-9. [DOI] [Google Scholar]
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- James LE. Meeting Mr. Farmer versus meeting a farmer: Specific effects of aging on learning proper names. Psychology and Aging. 2004;19(3):515–522. doi: 10.1037/0882-7974.19.3.515. [DOI] [PubMed] [Google Scholar]
- James LE, Fogler KA, Tauber SK. Recognition memory measures yield disproportionate effects of aging on learning face–name associations. Psychology and Aging. 2008;23(3):657–664. doi: 10.1037/a0013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Hope K, Raz N. Lifespan adult faces: Norms for age, familiarity, memorability, mood, and picture quality. Experimental Aging Research. 2009;35:268–275. doi: 10.1080/03610730902720638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb A, Naveh-Benjamin M. Paying attention to binding: Further studies assessing the role of reduced attentional resources in the associative deficit of older adults. Memory and Cognition. 2007;35(5):1162–1174. doi: 10.3758/BF03193486. [DOI] [PubMed] [Google Scholar]
- Layton B. Perceptual noise and aging. Psychological Bulletin. 1975;82(6):875–883. doi: 10.1037/0033-2909.82.6.875. [DOI] [PubMed] [Google Scholar]
- Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiology of Aging. 2004;25(8):1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation to cognition. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/S1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;43:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL, Patterson MM, Chung C, Healy MR. Effects of repetition and response deadline on associative recognition in young and older adults. Memory & Cognition. 2004;32(7):1182–1193. doi: 10.1016/S0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Mayr U. Cognitive aging: Is there a dark side to environmental support? Trends in Cognitive Science. 2014;18(1):7–15. doi: 10.1016/j.tics.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: What’s change got to do with it? Psychology and Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- May CP, Rahhal T, Berry EM, Leighton EA. Aging, source memory, and emotion. Psychology and Aging. 2005;20(4):571–578. doi: 10.1037/0882-7974.20.4.571. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Memon A, Bruce V. The effects of encoding strategy and context change on face recognition. Human Learning. 1983;2:313–326. [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, and Computers. 2004;36(4):630–633. doi: 10.3758/BF03206543. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10:197–206. doi: 10.1016/S0926-6410(00)00029-X. [DOI] [PubMed] [Google Scholar]
- Moberg PA, Raz N. Aging and olfactory recognition memory: Effect of encoding strategies and cognitive abilities. International Journal of Neuroscience. 1997;90:277–292. doi: 10.3109/00207459709000644. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: Elderly versus young persons. American Journal of Psychology. 1991;104(2):161–192. doi: 10.2307/1423153. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:1170–1188. doi: 10.1037/0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FIM. Memory for context and its use in item memory: Comparisons of younger and older persons. Psychology and Aging. 1995;10:284–293. doi: 10.1037/0882-7974.10.2.284. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: Further support using face-name associations. Psychology and Aging. 2004;19(3):541–546. doi: 10.1037/0882-7974.19.3.541. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Marom M. The effects of divided attention at encoding on item and associative memory. Memory & Cognition. 2003;31(7):1021–1035. doi: 10.3758/BF03196123. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, Bar-On M. Adult age differences in episodic memory: Further support for an associative-deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2003;29:826–837. doi: 10.1037/0278-7393.29.5.826. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Kilb A. Age-related differences in associative memory: The role of sensory decline. Psychology and Aging. 2014;29(3):672–683. doi: 10.1037/a0037138. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Shing YL, Kilb A, Werkle-Bergner M, Lindenberger U, Li SC. Adult age differences in memory for name–face associations: The effects of intentional and incidental learning. Memory. 2009;17(2):220–232. doi: 10.1080/09658210802222183. [DOI] [PubMed] [Google Scholar]
- Neider MB, Kramer AF. Older adults capitalize on contextual information to guide search. Experimental Aging Research. 2011;37(5):539–571. doi: 10.1080/0361073X.2011.619864. [DOI] [PubMed] [Google Scholar]
- Old S, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008a;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Old S, Naveh-Benjamin M. Memory for people and their actions: Further evidence for an age-related associative deficit. Psychology and Aging. 2008b;23:467–472. doi: 10.1037/0882-7974.23.2.467. [DOI] [PubMed] [Google Scholar]
- Oliva A, Torralba A. The role of context in object recognition. Trends in Cognitive Sciences. 2007;11(12):520–527. doi: 10.1016/j.tics.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Pantelis PC, van Vugt MK, Sekuler R, Wilson HR, Kahana MJ. Why are some people’s names easier to learn than others? The effects of face similarity on memory for face-name association. Memory & Cognition. 2008;36:1182–1195. doi: 10.3758/MC.36.6.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Millman D, Moberg PJ. On mechanisms of age-related differences in frequency discrimination with backward masking: Speed of processing versus stimulus persistence. Psychology and Aging. 1990;5:475–481. doi: 10.1037/0882-7974.5.4.475. [DOI] [PubMed] [Google Scholar]
- Rémy F, Saint-Aubert L, Bacon-Macé N, Vayssière N, Barbeau E, Fabre-Thorpe M. Object recognition in congruent and incongruent natural scenes: A life-span study. Vision Research. 2013;91:36–44. doi: 10.1016/j.visres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Rhodes MG, Castel AD, Jacoby LL. Associative recognition of face pairs by younger and older adults: The role of familiarity-based processing. Psychology and Aging. 2008;23:239–249. doi: 10.1037/0882-7974.23.2.239. [DOI] [PubMed] [Google Scholar]
- Shannon C, Weaver W. Mathematical theory of communication. Urbana, IL: University of Illinois Press; 1949. [Google Scholar]
- Shimamura AP, Ross JG, Bennett HD. Memory for facial expressions: The power of a smile. Psychonomic Bulletin & Review. 2006;13(2):217–222. doi: 10.3758/BF03193833. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging. 1995;10:527–539. doi: 10.1037/0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31(1):137–149. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. Journal of Neuroscience. 2010;30(29):9890–9897. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neuroscience & Biobehavioral Reviews. 2002;26(7):849–857. doi: 10.1016/S0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of Gerontology. 1993;48(4):157–171. doi: 10.1093/geronj/48.4.P157. [DOI] [PubMed] [Google Scholar]
- Watkins MJ, Ho E, Tulving E. Context effects in recognition memory for faces. Journal of Verbal Learning and Verbal Behavior. 1976;15(5):505–517. doi: 10.1016/0022-5371(76)90045-1. [DOI] [Google Scholar]
- Werheid K, Gruno M, Kathmann N, Fischer H, Almkvist O, Winblad B. Biased recognition of positive faces in aging and amnestic mild cognitive impairment. Psychology and Aging. 2010;25(1):1–15. doi: 10.1037/a0018358. [DOI] [PubMed] [Google Scholar]
- Winograd E, Rivers-Bulkeley NT. Effects of changing context on remembering faces. Journal of Experimental Psychology: Human Learning and Memory. 1977;3(4):397–405. doi: 10.1037/0278-7393.3.4.397. [DOI] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory & Language. 2002;46:441–517. doi: 10.1006/jmla.2002.2864. [DOI] [Google Scholar]