Abstract
Background:
This study compared the outcomes between patients with proximal humerus fractures (PHF) who underwent acute reverse total shoulder arthroplasty (RSA) to those who underwent an alternative initial treatment before requiring (secondary) RSA.
Methods:
Patients who underwent RSA after suffering a PHF were identified. Two year clinical follow-up was required for inclusion. Patients were divided into an acute group (RSA <4 weeks of fracture) and a secondary group. The secondary RSA group was subdivided by initial treatment (non-operative, hemiarthroplasty, open reduction internal fixation (ORIF)). Clinical and radiographic outcomes were compared.
Results:
Forty-seven patients met inclusion criteria with 15 in the acute RSA group and 32 in the secondary RSA group. The acute RSA group demonstrated better external rotation (28°) than the secondary RSA group (18°, P=0.0495). The acute RSA group showed a trend towards better Single Assessment Numeric Evaluation (SANE) scores. Tuberosity healing rate was higher in the acute RSA group.
Conclusion:
While acute and secondary RSA can yield successful outcomes, acute RSA results in a higher tuberosity healing rate and improved external rotation.
Keywords: Hemiarthroplasty, Non-operative treatment, Open reduction internal fixation, Proximal humerus fracture, Proximal humerus fracture sequelae, Reverse shoulder arthroplasty, Tuberosity healing
Introduction
ommonly selected surgical options for treatment of displaced proximal humerus fractures include open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty (RSA). Some studies have reported high rates of fixation failure with ORIF and high rates of tuberosity nonunion with hemiarthroplasty (1-4). Neer first described hemiarthroplasty as an alternative to ORIF and non-operative treatment in the elderly for early pain relief and improved function (5, 6). Follow-up studies using hemiarthroplasty for this indication have reported inconsistent results for function, power, and range of motion (1, 7). Functional outcome after hemiarthroplasty for proximal humerus fracture largely depends on the anatomic healing of the tuberosities to the humeral shaft and implant (7, 8). Unfortunately, the patients who sustain these injuries often have poor bone quality and/or comminuted tuberosities, which can compromise tuberosity healing (8).
There is recent support in the literature for the use of reverse shoulder arthroplasty (RSA) for acute displaced proximal humerus fractures in elderly patients (9-12). There is also literature supporting the use of RSA for proximal humerus fracture sequelae, recognizing that the expected outcomes are not as reliable as RSA performed for other etiologies, such as cuff tear arthropathy (13-16). A recent study shows that the incidence of use of RSA for fracture has increased significantly (from 2% to 38%) over the past 7 years (17). Several studies have shown improved early and mid-term outcomes with regards to forward elevation and validated outcome scores when RSA is compared to hemiarthroplasty for fracture (18-23). This must be balanced by an almost 20% rate of complications with RSA (24). The argument for RSA over other treatment modalities is predictable early pain relief and the decreased dependence on tuberosity healing for functional forward elevation. Despite the recent enthusiasm for RSA in the treatment of proximal humerus fractures, there is currently no study comparing outcomes when RSA is performed early for acute fracture to those when RSA is performed secondarily for failed alternative treatment of proximal humerus fractures. While a recent randomized controlled trial has reported equivalent outcomes between non operative and operative treatment of proximal humerus fractures, it remains unknown whether poor results after selection of initial non operative treatment, operative fixation, or hemiarthroplasty can be improved upon with delayed reverse arthroplasty (25). The purpose of this study is to compare the clinical outcomes, radiographic findings and rate of complications between patients who underwent acute RSA for displaced proximal humerus fracture to those patients who underwent an alternative initial treatment for their fracture and subsequently required RSA. We hypothesized that patients who underwent acute RSA would have better functional results than patients who underwent RSA as a secondary surgery for proximal humerus fracture.
Materials and Methods
After Institutional Review Board approval, all patients who had undergone RSA at one of two hospitals affiliated with our institution between 2008 and 2013 were identified. RSA cases were performed by 5 fellowship-trained shoulder surgeons. Operative indications and pre-operative diagnoses were reviewed. Patients who underwent RSA for the following diagnoses were identified: proximal humerus fracture, post-traumatic arthrosis, fracture malunion/nonunion, failed hemiarthroplasty performed for fracture, hardware failure after ORIF, and avascular necrosis after fracture with or without ORIF. Using these methods, a total of 73 patients were deemed eligible for this study.
Charts were retrospectively reviewed to collect data regarding demographics, estimated blood loss (EBL), postoperative range of motion, and complications (intraoperative and postoperative). Post-operative active forward elevation (AFE) and active external rotation (AER) were determined by review of the attending surgeon’s postoperative notes. Range of motion data was only considered if it was obtained at a minimum of 6 months after surgery. The three month and six month postoperative radiographs were reviewed to confirm tuberosity healing and baseplate tilt. The most recent post-operative radiographs were reviewed to assess the presence of scapular notching at a minimum of 1 year follow-up. Four views were obtained in standard fashion: anterior-posterior view, true anterior-posterior view, scapular-Y view, and axillary view. Notching was recorded and graded using the grading system described by Sirveaux et al (26). Measurement of baseplate tilt was recorded using the method described by Bries et al with positive values representing cephalad tilt and negative values caudal tilt relative to a line drawn from the super-o-medial scapular border to the superior border of the glenoid (27). Patients were contacted to obtain minimum 2-year functional outcomes using the American Shoulder and Elbow Surgeons (ASES), Single Assessment Numeric Evaluation (SANE), and Simple Shoulder Test scores (SST) (28-30). Of the 73 eligible patients, minimum 2-year outcomes scores could be obtained in 47 (64.4%); this included a subset of 5 patients treated with RSA for acute fracture on whom we have previously reported (22). Twenty-six of the eligible patients were deceased, incapable of completing or unwilling to complete the outcome survey, or lost to follow-up (1, 5, 19). The 47 patients with adequate follow-up were placed into groups based on the timing of RSA relative to the date of their fracture. The “acute RSA” group was defined as having undergone RSA within 4 weeks of fracture and as the initial treatment strategy. The “secondary RSA” group underwent RSA later than 4 weeks after the fracture and was divided into subgroups based on index treatment of the fracture prior to RSA (non-operative, hemiarthroplasty, and ORIF).
Statistical Analysis
Mann-Whitney test was performed to compare outcome variables between the acute RSA group and secondary RSA group. Fisher exact test was used to compare rates of tuberosity healing, scapular notching and complications. Dunnett’s test was performed to compare outcome variables between the acute RSA group and each secondary RSA subgroup (non-operative, hemiarthroplasty, ORIF). Spearman’s rho analysis was performed to evaluate a possible correlation between treatment groups and the aforementioned outcomes measures.
Results
Demographics
There were 15 patients in the acute RSA group and 32 patients in the secondary RSA group. Average age for the acute RSA group was 77.3 years (Range 64–87 years) compared to 70.6 (Range 50–85 years) for the secondary RSA group [Table 1]. The average body mass index (BMI) for the acute RSA group was 30.1 (Range 20–49) and the average BMI for the secondary RSA group was 33.1 (Range 21–53, P=0.264)) [Table 1]. The average clinical follow-up for all patients was 3.8 years (range 2–9 years).
Table 1.
Comparison between early and late RSA groups
| Early | Late | P-Value | |
|---|---|---|---|
| Patients | 15 | 32 | |
| Gender (Male : Female) | 2 : 13 | 6 : 26 | 1.0 |
| Age (Range) | 77.3 (64-87) | 70.6 (50-85) | 0.021 |
| BMI (Range) | 30.1 (20.5-49.4) | 33.1 (21.1-53.5) | 0.264 |
| Approach (Deltopectoral : Superior) | 13 : 2 | 30 : 2 | |
| Tuberosity Healed | 100% | 40% | |
| AFE (Range) | 129° (95°-160°) | 118° (70°-160°) | 0.127 |
| AER (Range) | 29° (10°-45°) | 13° (-10°-40°) | 0.002 |
| ASES (Range) | 77.0 (36.7-93.3) | 72.4 (41.7-98.3) | 0.173 |
| SANE (Range) | 80.9 (50-100) | 69.9 (20-100) | 0.070 |
| SST (Range) | 7.3 (2-11) | 7.2 (3-11) | 0.919 |
| EBL (Range) | 330.5 (75-800) | 435.4 (150-1200) | 0.352 |
| Scapular Notching | 0% | 28% (9/32, 7 grade 1 & 2 grade 2) | 0.041 |
| Baseplate Tilt | +3.7 (-22.2 – 22.4) | +15.7 (-4.2 – 38.5) | 0.004 |
| Complications | 0% | 22% (7/32) | 0.079 |
Body Mass Index (BMI); Active Forward Elevation (AFE); Active External Rotation (AER); American Shoulder and Elbow Surgeons Shoulder Score (ASES); Single Numeric Assessment Evaluation (SANE); Simple Shoulder Test (SST); Estimated Blood Loss (EBL)
Blood Loss
Average intra-operative blood loss was 330.5 ml (Range 75–800 ml) in the acute RSA group and 435.4 (Range 150–1200 ml, P=0.352) in the secondary RSA group. Comparing the subgroups within the secondary RSA group, the average blood loss for those previously treated with hemiarthroplasty, nonoperatively, or with ORIF was 464.3 ml (300–1000 ml, P=0.678), 387.5 ml (150–1200 ml, P=0.941), and 510.0 ml (150–1200 ml, P=0.547) [Table 2].
Table 2.
Comparison of early RSA and subgroups of late RSA groups
| Acute | Hemi | Non- operative | ORIF | ||||
|---|---|---|---|---|---|---|---|
| Patients | 15 | 10 | 15 | 7 | |||
| Gender (Male:Female) | 2 : 13 | 2: 8 | 0.948 | 3 : 12 | 0.933 | 1 : 6 | 1 |
| Age (Range) | 77.3 (64-87) | 66.8 (50-84) | 0.012 | 73.49 (55-85) | 0.489 | 69.6 (54-76) | 0.141 |
| BMI (Range) | 30.1 (20.5-49.4) | 35.7 (26.3-53.5) | 0.289 | 33.5 (22.0-47.1) | 0.589 | 28.6 (21.1-43.8) | 0.968 |
| Approach (Deltopectoral: Superior) | 13 : 2 | 10 : 0 | 13 : 2 | 7 : 0 | |||
| Tuberosity Healed (Percentage) | 15 (100%) | 3 (30%) | 6 (40%) | 4 (57%) | |||
| AFE (Range) | 129° (95°-160°) | 107° (70°-140°) | 0.046 | 127° (90°-160°) | 0.970 | 114° (80°-160°) | 0.311 |
| AER (Range) | 29° (10°-45°) | 13° (0°-35°) | 0.027 | 17° (-10°-40°) | 0.043 | 8° (-10° -20°) | 0.004 |
| ASES (Range) | 77.0 (36.7-93.3) | 69.2 (50-90) | 0.511 | 72.7 (46.7-98.3) | 0.813 | 76.4 (41.7-96.7) | 1 |
| SANE (Range) | 80.9 (50-100) | 68.0 (20-85) | 0.331 | 72.3 (25-100) | 0.568 | 63.7 (25-100) | 0.204 |
| SST (Range) | 7.3 (2-11) | 7 (3-11) | 0.984 | 7.1 (3-10) | 0.996 | 7.7 (4-11) | 0.974 |
| EBL (Range) | 330.5 (75-800) | 464.3 (300-1000) | 0.678 | 387.5 (150-1200) | 0.941 | 510 (150-1200) | 0.547 |
| Scapular Notching | 0% | 20% (2/10, 1 grade 1, 1 grade 2) | 20% | 57% (4/7, 3 grade 1, 1 grade 2) | |||
| Complications (Percentage) | 0 (0%) | 3 (30%) | 2 (14%) | 2 same patient (14%) |
Body Mass Index (BMI); Active Forward Elevation (AFE); Active External Rotation (AER); American Shoulder and Elbow Surgeons Shoulder Score (ASES); Single Numeric Assessment Evaluation (SANE); Simple Shoulder Test (SST); Estimated Blood Loss (EBL)
Range of Motion
Active Forward Elevation (AFE)
Average AFE for the acute RSA group was 128° (Range 95°–150°) compared to 118° degrees (Range 70°–160°, P=0.451) for the secondary RSA group. Compared to the acute RSA group, those previously treated with hemiarthroplasty, nonoperatively, or with ORIF had average forward elevation of 111° degrees (Range 70°–145°, P=0.216), 128° (Range 90°–160°, P=0.987) and 126° (Range 80°–160°, P=0.982), respectively.[Table 2].
Active External Rotation (AER)
When comparing post-operative AER, there was a significant difference found favoring the acute RSA group when compared to the secondary RSA group. Average AER for the acute RSA group was 28° (Range 10°–45°), while average external rotation for the secondary RSA group was 18° (Range -10°–45°, P=0.049) [Figure 1]. The average external rotation values for the secondary RSA group previously treated with hemiarthroplasty, nonoperatively, or with ORIF were 15° (Range 0°–35°, P=0.113), 22° (Range 0-45°, P=0.302), and 13° (Range -10°–45°, P=0.113), respectively [Table 2].
Figure 1.
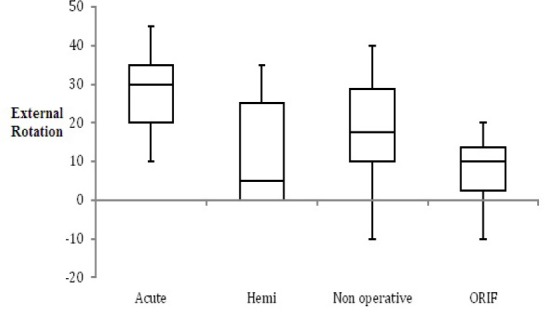
Box plot demonstrating post-operative active external rotation (AER) for each treatment group.
Outcome Scores
ASES
When comparing post-operative outcome scores, the acute RSA group had an average ASES score of 77.0 (Range 36.6–93.3), while the secondary RSA group had an average ASES score of 72.4 (Range 41.7–98.3, P=0.17). When comparing by subgroups within the secondary RSA group, the average ASES for those previously treated with hemiarthroplasty, nonoperatively, or with ORIF were 69.2 (Range 50.0–90.0, P=0.25), 72.6 (Range 46.7–98.3, P=0.52), and 76.4 (Range 41.7–96.7, P=0.79), respectively [Table 2].
SST
When comparing SST scores, the acute RSA group had an average SST of 7.3 (Range 2–11), while the secondary RSA group had an average SST of 7.2 (Range 4–11, P=0.91). Looking at individual subgroups with the secondary RSA group, the average SST for those previously treated with hemiarthroplasty, nonoperatively, or with ORIF were 7.0 (Range 3–11, P=0.98), 7.1 (Range 3–10, P=0.99), and 7.7 (Range 4–11, P=0.97), respectively [Table 2].
SANE
When comparing SANE scores, the acute RSA group had an average SANE of 80.9 (Range 50–100), while the secondary RSA group had an average SANE of 69.1 (Range 20–100) (P=0.07). The average SANE scores for those previously treated with hemiarthroplasty, nonoperatively, or with ORIF were 68 (Range 20–85, P=0.36), 72.3 (Range 25–100, P=0.25), and 63.7 (Range 25–100, P=0.20), respectively [Table 2].
Outcomes Measures
Analysis of the relationship between the acute and secondary groups and outcomes measures did not demonstrate any strong correlations. Of the outcome variables analyzed, the strongest relationship found was for external rotation with a spearman’s rank correlation coefficient of -0.24. The other outcomes measures had spearman’s rho values as follows: FE -0.08, ASES -0.13, SANE -0.18, and SST 0.02.
Radiographic Findings
The rate of tuberosity healing in the acute RSA group was significantly higher than in the secondary RSA group (100% vs. 42%, P<0.001). The average baseplate tilt in the acute RSA group was significantly lower (less cephalad tilt) than the secondary RSA group (+4.7 vs +15.8, P=0.019). The rate of scapular notching in the acute RSA group was significantly lower than the secondary RSA group (0% vs 42%, P=0.034). Of the 11 patients in the secondary RSA group with scapular notching, seven were grade 1 and four were grade 2.
Complications
There were fewer complications in the acute RSA group than the secondary RSA group (0 vs. 7, P=0.078). No intra-operative or post-operative complications had occurred in the acute RSA group at most recent follow-up. The seven complications (in six patients) in the secondary RSA group ranged from minor to major, with 2 patients requiring revision surgery [Table 3]. Three of the complications were directly related to removal of a well-fixed hemiarthroplasty stem.
Table 3.
Complications in the Late RSA Group
| Age, Gender | Prior Treatment | Complication | Required revision (Y/N) |
|---|---|---|---|
| 84, F | Hemiarthroplasty | Retained cement removal instrument |
N |
| 70, F | Hemiarthroplasty | Cortical perforation | N |
| 67, F | Hemiarthroplasty | Cortical perforation, cement extrusion |
N |
| 76, M | Non-operative | Extensive Heterotopic Ossification |
N |
| 69, F | Non-operative | Nickel Allergy | Y |
| 75, F | ORIF | 1. Dislocation 2. Infection |
Y |
Discussion
The most important finding of this study is that, while successful outcomes can be achieved with both acute and secondary RSA, there would be value in improving our ability to select patients who would benefit from acute RSA. Recent literature supports the use of RSA for acute, displaced proximal humerus fractures in elderly patients but little has been reported on the effect of timing on the outcomes of RSA done for proximal humerus fracture (10, 19, 22, 31, 32). Cicak et al. recently compared patients with and without previous surgery treated with RSA for proximal humerus fracture. They found that those who had surgery for the fracture prior to RSA had inferior results when compared to those without prior surgical treatment. This study focused on the influence of prior surgery on outcome of RSA for fracture rather than the influence of timing to RSA (33).
In this study, acute RSA was shown to improve post-operative AER when compared to secondary RSA. These findings can be explained by the improved ability to reduce and repair the greater tuberosity when surgery is performed in a time-sensitive manner.
Additionally, the biologic environment for bony healing may be more optimal in the acute setting. In the setting of secondary RSA for failed hemiarthroplasty, non-operative treatment, or ORIF, the greater tuberosity is often malunited, nonunited and migrated, or has undergone resorption making reduction and repair difficult or impossible. It has previously been shown in hemiarthroplasty and anatomic shoulder arthroplasty that management of a malunited greater tuberosity with an osteotomy to aid reduction leads to poor results (8, 34).
In this study, despite the difference in post-operative AER, we found that there was no clear difference in most patient perceived outcome scores, with both groups demonstrating successful outcomes. The failure to show a difference in subjective outcome scores may be explained by the fact that none of the three outcome instruments utilized in this study required or included tasks involving external rotation with the arm at the side. This limitation in commonly used outcomes measures has been previously reported (35). Conversely, it may be that the 15 degree difference in external rotation that was found between the acute and secondary RSA group does not have true clinical implications this patient population.
The rate of scapular notching was found to be significant lower in the acute RSA group (0%) compared to the secondary RSA group (42%). This can likely be attributed to technically easier deep dissection and glenoid exposure in the setting of acute fracture. Excellent glenoid exposure is important for appropriate placement of the baseplate at the inferior glenoid with neutral or inferior tilt and is often more difficult in the revision setting or in situations with excessive scar tissue and/or altered anatomy (malunion/nonunion). This is supported with our finding of increased cephalad tilt in the secondary RSA group. Though the long-term clinical implications of scapular notching are still debated, several studies have shown a relationship with poorer clinical outcomes in the mid-term (26, 36). Additionally, numerous studies have implicated scapular notching as a cause of glenoid component loosening (37-39). Other factors that can influence scapular notching include implant design as well as surgeon variability; however, this variability was consistent across the acute and secondary groups.
We recognize that this study has several limitations. First, this is a retrospective analysis with all the inherent limitations. Another limitation is the relatively small number of patients included. Only 64% of patients eligible for this study could be reached and evaluated at a minimum of 2 years after RSA. This increases the risk for bias as well as inadequate statistical power for some of the outcomes variables considered. The inability to include numerous eligible patients is at least partially a function of the injury studied and its predilection for occurring in elderly patients, many with diminishing cognitive abilities and some with limited life expectancy. Several studies have supported this concept, showing mortality rates within 2 years of surgery to be as high as 30% (10, 22). Though all patients completed outcome scores at a minimum of 2 years, the range of most recent physical exam data and radiographic data was at earlier time points. Other limitations of this study include: the lack of preoperative or standardized range of motion analysis performed by an independent investigator and the inclusion of multiple surgeons (five) with differing surgical techniques utilizing different implant designs and rehabilitation protocols. Though different implant manufactures were utilized, all were Grammont style implants with non-retentive liners. A final limitation of this study is the heterogeneity within the secondary RSA group, including patients previously treated by one of three modalities: non-operative, hemiarthroplasty, and ORIF. Recognition of this heterogeneity led to our decision to also compare the acute RSA group to each subgroup (based on previous treatment) within the secondary RSA group, which yielded some significant findings. Non-significant findings in the subgroup analysis should be viewed with caution, given the lack of statistical power to report negative results.
While both acute and secondary RSA for the treatment of proximal humerus fractures can yield successful clinical outcomes, acute RSA results in improved external rotation motion and decreased rate of complications scapular notching. The improved external rotation is likely related to the ease of reducing and repairing the greater tuberosity when RSA is performed in the acute setting. This study can be used to help counsel patients when discussing treatment options and expectations for acute proximal humerus fractures and for proximal humerus fracture that have failed alternative operative or non-operative treatment.
References
- 1.Antuna SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17(2):202–9. doi: 10.1016/j.jse.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Cai M, Tao K, Yang C, Li S. Internal fixation versus shoulder hemiarthroplasty for displaced 4-part proximal humeral fractures in elderly patients. Orthopedics. 2012;35(9):e1340–6. doi: 10.3928/01477447-20120822-19. [DOI] [PubMed] [Google Scholar]
- 3.Gerber C, Werner CM, Vienne P. Internal fixation of complex fractures of the proximal humerus. J Bone Joint Surg Br. 2004;86(6):848–55. doi: 10.1302/0301-620x.86b6.14577. [DOI] [PubMed] [Google Scholar]
- 4.Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569–77. doi: 10.1016/s1058-2746(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 5.Neer CS., 2nd Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077–89. [PubMed] [Google Scholar]
- 6.Neer CS., 2nd Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52(6):1090–103. [PubMed] [Google Scholar]
- 7.Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85(7):1215–23. doi: 10.2106/00004623-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401–12. doi: 10.1067/mse.2002.124527. [DOI] [PubMed] [Google Scholar]
- 9.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award. J Shoulder Elbow Surg. 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty;2006;15(5):527–40. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516–20. doi: 10.1302/0301-620X.89B4.18435. [DOI] [PubMed] [Google Scholar]
- 11.Kaisidis A, PAntOS PG, Heger H, Bochlos D, Selimas S, Oikonomoulas V. Reverse shoulder arthroplasty for the treatment of three and four part fractures of the proximal humerus in patients older than 75 years old. Acta Orthop Belg. 2014;80(1):99–105. [PubMed] [Google Scholar]
- 12.Klein M, Juschka M, Hinkenjann B, Scherger B, Ostermann PA. Treatment of comminuted fractures of the proximal humerus in elderly patients with the Delta III reverse shoulder prosthesis. J Orthop Trauma. 2008;22(10):698–704. doi: 10.1097/BOT.0b013e31818afe40. [DOI] [PubMed] [Google Scholar]
- 13.Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae) J Bone Joint Surg Am. 2014;96(24):2070–6. doi: 10.2106/JBJS.N.00405. [DOI] [PubMed] [Google Scholar]
- 14.Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367–72. doi: 10.3109/17453674.2010.487242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–85. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 16.Wellmann M, Struck M, Pastor MF, Gettmann A, Windhagen H, Smith T. Short and midterm results of reverse shoulder arthroplasty according to the preoperative etiology. Arch Orthop Trauma Surg. 2013;133(4):463–71. doi: 10.1007/s00402-013-1688-7. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo DC, Mann T, Abboud JA, Getz C, Baumhauer JF, Voloshin I. Reverse totalshoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg. 2014;23(9):1363–7. doi: 10.1016/j.jse.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Baudi P, Campochiaro G, Serafini F, Gazzotti G, Matino G, Rovesta C, et al. Hemiarthroplasty versus reverse shoulder arthroplasty: comparative study of functional and radiological outcomes in the treatment of acute proximal humerus fracture. Musculoskelet Surg. 2014;98(1):19–25. doi: 10.1007/s12306-014-0322-3. [DOI] [PubMed] [Google Scholar]
- 19.Boyle MJ, Youn SM, Frampton CM, Ball CM. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg. 2013;22(1):32–7. doi: 10.1016/j.jse.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050–5. doi: 10.2106/JBJS.L.01637. [DOI] [PubMed] [Google Scholar]
- 21.Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res. 2009;95(1):48–55. doi: 10.1016/j.otsr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703–8. doi: 10.3928/01477447-20120426-25. [DOI] [PubMed] [Google Scholar]
- 23.Sebastia-Forcada E, Cebrian-Gomez R, Lizaur-Utrilla A, Gil-Guillen V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg. 2014;23(10):1419–26. doi: 10.1016/j.jse.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Namdari S, Horneff JG, Baldwin K. Comparison of hemiarthroplasty and reverse arthroplasty for treatment of proximal humeral fractures: a systematic review. J Bone Joint Surg Am. 2013;95(18):1701–8. doi: 10.2106/JBJS.L.01115. [DOI] [PubMed] [Google Scholar]
- 25.Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Martin BC, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. JAMA. 2015;313(10):1037–47. doi: 10.1001/jama.2015.1629. [DOI] [PubMed] [Google Scholar]
- 26.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–95. doi: 10.1302/0301-620x.86b3.14024. [DOI] [PubMed] [Google Scholar]
- 27.Bries AD, Pill SG, Wade Krause FR, Kissenberth MJ, Hawkins RJ. Accuracy of obtaining optimal base plate declination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(12):1770–5. doi: 10.1016/j.jse.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Lippitt SB, Harryman DT, Matsen FA, Fu FH, Hawkins RJ. The shoulder: a balance of mobility and stability. Rosemont, IL: JAAOS; 1993. A practical tool for evaluating function: the simple shoulder test; pp. 501–18. [Google Scholar]
- 29.Richards RR, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347–52. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 30.Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the single assessment numeric evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214–21. doi: 10.1177/03635465990270021701. [DOI] [PubMed] [Google Scholar]
- 31.Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535–9. doi: 10.1302/0301-620X.92B4.22450. [DOI] [PubMed] [Google Scholar]
- 32.Ross M, Hope B, Stokes A, Peters SE, McLeod I, Duke PF. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg. 2015;24(2):215–22. doi: 10.1016/j.jse.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Nikola C, Hrvoje K, Nenad M. Reverse shoulder arthroplasty in acute fractures provides better results than in revision procedures for fracture sequelae. Int Orthop. 2015;39(2):343–8. doi: 10.1007/s00264-014-2649-7. [DOI] [PubMed] [Google Scholar]
- 34.Boileau P, Trojani C, Walch G, Krishnan SG, Romeo A, Sinnerton R. Shoulder arthroplasty for the treatment of the sequelae of fractures of the proximal humerus. J Shoulder Elbow Surg. 2001;10(4):299–308. doi: 10.1067/mse.2001.115985. [DOI] [PubMed] [Google Scholar]
- 35.Castagna A, Delcogliano M, de Caro F, Ziveri G, Borroni M, Gumina S, et al. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37(7):1297–305. doi: 10.1007/s00264-013-1907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 37.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189–95. doi: 10.1302/0301-620X.89B2.18161. [DOI] [PubMed] [Google Scholar]
- 38.Namdari S, Yagnik G, Ebaugh DD, Nagda S, Ramsey ML, Williams GR, et al. Defining functional shoulder range of motion for activities of daily living. J Shoulder Elbow Surg. 2012;21(9):1177–83. doi: 10.1016/j.jse.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]


