Abstract
Neisseria gonorrhoea is a common sexually transmitted infection worldwide. Disseminated gonococcal infection is an infrequent presentation and rarely can be associated with septic arthritis. Incidence of this infection is rising, both internationally and in older age groups. We present the first documented case of N. gonorrhoea prosthetic joint infection which was successfully treated with laparoscopic debridement and antimicrobial therapy.
Keywords: Gonorrhoea, prosthetic joint infection, gonococcal arthritis, DGI.
Introduction
Neisseria gonorrhoea is an aerobic facultative intracellular gram negative bacteria typically appearing as diplococci 1. It is sexually acquired and a common cause of urethritis, tenosynovitis and migratory polyarthralgia 2. In approximately 0.5 - 3% of infections disseminated gonococcal infection (DGI) may occur through bacteraemia 3, 4. Localised septic arthritis occurs in approximately 40% of DGI infections 1. We present the unique case of DGI with associated prosthetic joint infection of the knee.
Case
Mr AJ is a sixty-year-old Caucasian man originally from Australia, currently residing in Papua New Guinea who presented to our facility with an acutely inflamed right knee associated with a febrile illness, malaise, and sweats. Two weeks prior to presentation he described self-limiting dysuria without urethral discharge, followed 1 week later by pain and swelling of his right first metacarpophalangeal joint. He denied any history of recent illness, trauma, and denied any history of sexual contact since separation from his wife 15 years earlier. His past medical history was notable for a right total knee replacement four years prior and poorly diet controlled type 2 diabetes mellitus, HbA1C: 8.3% (4.3-6.0).
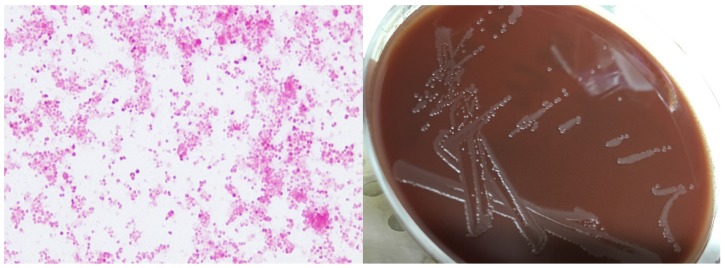
Clinical examination was remarkable for a warm, tender, oedematous and erythematous right knee, with associated decreased range of movement and limited mobility. No dermatological lesions were evident and genital examination revealed no urethral discharge, testicular tenderness nor inguinal lymphadenopathy. Blood investigations demonstrated a neutrophilia of 13.6 cells/mm3 (2.0 -8.0) and elevated C-reactive protein of 86 mg/L (<5.0). The white cell count from the initial joint fluid aspiration in the emergency department was 57,500 x106/L, of which 83% were polymorphonuclear cells; gram stain was negative. The patient underwent laparoscopic washout of the knee the following day. The knee fluid aspiration was centrifuged and the spun deposit was cultured onto blood agar (aerobically and anaerobically), chocolate agar, and inoculated into Brewer thioglycollate Broth. These plates and broth were incubated in a CO2 enriched environment at 35oC except for the anaerobic blood agar plate. Blood cultures were also collected and performed using BacT/Alert (BioMerieux Marcy l'Etoile, France). On day two of admission the patient's blood and aspirate culture results returned positive for growth of Neisseria gonorrhoea, Figure 1. Bacterial Identification was made based on MALDI-TOF technology using the Vitek MS (BioMerieux Marcy l'Etoile, France) and sugar fermentation. Further confirmation was obtained from the aspirate and surgical washout fluid samples using in house Real Time Polymerase Chain Reaction (RT-PCR). Antibiotic susceptibility testing using NNN agar dilution and Minimum Inhibitory Concentration (MIC) revealed that the organism was sensitive to ceftriaxone (MIC: 0.008mg/L), ciprofloxacin (MIC: 0.03mg/L), azithromycin (MIC: 0.12mg/L), and spectinomycin (MIC: 64mg/L). The organism was resistant to penicillin (MIC: 4mg/L) and tetracycline (MIC: 16mg/L). Notably urine, throat and rectal RT-PCR testing was negative for gonorrhoea and chlamydia. However, a urine RT-PCR was positive for Trichomonas vaginalis. Additional investigations including human immunodeficiency virus (HIV), syphilis, and hepatitis viruses were all negative. Complement levels were within normal range. Surgical management involved one arthroscopic surgical washout with synovectomy and retention of prosthesis. Antibiotic therapy included a two week course of intravenous ceftriaxone 2g daily. Given concerns over prosthetic joint infection a twelve week course of oral ciprofloxacin 500mg twice daily was completed. The patient tolerated therapy without incident and had no signs of relapse 60 days after completion.
Figure 1.
A. Gram stain revealing gram negative diplococci; B. N. gonorrhoea growth on chocolate agar.
Discussion
N. gonorrhoea is a common sexually transmitted infection 5, 6. While DGI and bacteraemia are uncommon, it is often associated with septic arthritis, occurring in 60% as a triad of migratory polyarticular disease, dermatological manifestations usually macules or papules, and tenosynovitis 2. The most commonly affected joints include knees and wrists 2, 7. Localized septic arthritis occurs in up to 40% of DGI cases 1, 2. There are multiple reported cases of rare or unusual presentations of gonococcal infection 8-10. However, on review of the literature including PubMed, Medline, and Google Scholar, we believe this to be the first description of N. gonorrhoea prosthetic joint infection.
Our patient's initial presentation with urethritis, accompanied by fever, malaise and polyarthritis is classic 1, 11. On multiple occasions the patient denied recent female sexual contact, no male sexual contact ever, nor any other risk factors for either sexually transmitted infections or blood borne viruses. Given both the DGI and T. vaginalis infection, it is likely that the true aetiology of the infection was sexual contact. Considering the risk of acquisition from a female partner is approximately 20% per sexual encounter, and the time for infection to symptoms is 1-9 days, he is likely to have had recent intercourse 1, 12. Aside from initial infection, our patient's risk profile for septic arthritis included age and diabetes mellitus 13, 14. Interestingly, from a gonococcal arthritis risk perspective our patient is not female, did not have an asymptomatic mucosal infection, no low socio-economic status, not an intravenous drug user and HIV negative 15. Complement deficiency has been associated with increased susceptibility to Neisseria infections associated with up to 13% of DGI cases 1, 15. Although our patient had multiple positive blood and synovial fluid cultures, he did not have complement deficiency.
In terms of microbiological samples, our patient's culture results are uncommon. Gram stains are negative in over 75% of cases of gonococcal septic arthritis 15, 16. Culture results are positive in up to approximately 50% of synovial fluid samples and less than 30% of blood cultures 7, 16. Although PCR has markedly improved the diagnosis with a specificity of 96-98% and sensitivity of 78-80%, it was only confirmatory in this case 15.
Sexually transmitted infections are rising both in Australia and abroad 5, 6. The incidence of gonorrhoea in Australia has more than doubled over the last decade, and notably, the incidence in the over 40 age category has almost tripled in the same timeframe with a male predominance of 2.9:1 5. Similarly, there has been an increase in both joint replacement procedures and prosthetic joint infections worldwide 17. Given an aging population, increased prevalence of joint prostheses and a rising rate of sexually transmitted infections in an older population, the case described herein may portend an emerging pathology 5, 6, 17.
Acknowledgments
The authors would like to thank the patient for their consent in documenting this case.
References
- 1.Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (Gonorrhea). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (Eighth edition) Philadelphia, PA: Elsevier/Saunders; 2015. pp. 2446–62. [Google Scholar]
- 2.O'Brien JP, Goldenberg DL, Rice PA. Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. Medicine (Baltimore) 1983;62:395–406. [PubMed] [Google Scholar]
- 3.Holmes KK, Counts GW, Beaty HN. Disseminated gonococcal infection. Ann. Intern. Med. 1971;74:979–93. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- 4.Barr J, Danielsson D. Septic gonococcal dermatitis. Br Med J. 1971;1:482–5. doi: 10.1136/bmj.1.5747.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: Annual Surveillance Report 2016. UNSW Australia, Sydney NSW 2052: The Kirby Institute; 2016. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2015. Atlanta: U.S. Department of Health and Human Services; 2016. [Google Scholar]
- 7.Belkacem A, Caumes E, Ouanich J, Jarlier V, Dellion S, Cazenave B. et al. Changing patterns of disseminated gonococcal infection in France: cross-sectional data 2009-2011. Sex Transm Infect. 2013;89:613–5. doi: 10.1136/sextrans-2013-051119. [DOI] [PubMed] [Google Scholar]
- 8.El Mezouar I, Tahiri L, Lazrak F, Berrada K, Harzy T. Gonococcal polyarthritis with sternoclavicular joint involvement in pregnant woman: a case report. Pan Afr Med J. 2014;17:242. doi: 10.11604/pamj.2014.17.242.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jitmuang A, Boonyasiri A, Keurueangkul N, Leelaporn A, Leelarasamee A. Gonococcal subcutaneous abscess and pyomyositis: a case report. Case Rep Infect Dis. 2012;2012:790478. doi: 10.1155/2012/790478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas SB, Unglaub F, Dragu A, Gessner A, Horch RE. An unusual case of gonococcal arthritis of the finger. Arch Orthop Trauma Surg. 2009;129:1335–8. doi: 10.1007/s00402-008-0727-2. [DOI] [PubMed] [Google Scholar]
- 11.Geelhoed-Duyvestijn PH, van der Meer JW, Lichtendahl-Bernards AT, Mulder CJ, Meyers KA, Poolman JT. Disseminated Gonococcal Infection in Elderly Patients. Arch Intern Med. 1986;146:1739–40. [PubMed] [Google Scholar]
- 12.Hooper RR, Reynolds GH, Jones OG, Zaidi A, Wiesner PJ, Latimer KP. et al. Cohort study of venereal disease. I: the risk of gonorrhea transmission from infected women to men. Am J Epidemiol. 1978;108:136–44. doi: 10.1093/oxfordjournals.aje.a112597. [DOI] [PubMed] [Google Scholar]
- 13.Kaandorp CJ, Van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 1995;38:1819–25. doi: 10.1002/art.1780381215. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerli W, Sendi P. Orthopedic Implant-Associated Infections. Mandell, Douglas, and Bennett's principles and practice of infectious diseases (Eighth edition) Philadelphia, PA: Elsevier/Saunders; 2015. pp. 1328–40. [Google Scholar]
- 15.Bardin T. Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003;17:201–8. doi: 10.1016/s1521-6942(02)00125-0. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-De La Torre I, Nava-Zavala A. Gonococcal and nongonococcal arthritis. Rheum Dis Clin North Am. 2009;35:63–73. doi: 10.1016/j.rdc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–45. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]