Abstract
Bacterial toxins introduce protein modifications such as ADP-ribosylation to manipulate host cell signaling and physiology. Several general mechanisms for toxin function have been established, but the extent to which previously uncharacterized toxins utilize these mechanisms is unknown. A study of an Escherichia coli pertussis-like toxin demonstrates that this protein acts on a known toxin substrate but displays distinct and dual chemoselectivity, suggesting this E. coli pertussis-like toxin may serve as a unique tool to study G-protein signaling in eukaryotic cells.
Introduction
Bacterial pathogens utilize a variety of strategies to attack their host. One strategy is the release of protein toxins that catalyze post-translational modifications (PTMs)2 of host proteins; these PTMs then interfere with a central biological function to elicit a pathological phenotype. For example, protein toxins targeting components of the actin cytoskeleton can modulate motility, toxins targeting components of the ribosome and accessory factors can disrupt protein synthesis, and toxins targeting G-proteins, including monomeric G-proteins and heterotrimeric G proteins, can influence cell growth and metabolism (1). This functional diversity can come even in the context of conserved structural elements, providing motivation to characterize novel toxins with the goal of gaining insights into bacterial function, identifying possible novel mechanisms of pathogenesis and discovering unique tools to dissect eukaryotic signaling pathways. A new study from Littler et al. (2) provides a compelling example in these respects, describing the structural and functional characterization of an Escherichia coli toxin that acts via an unusual mechanism to cause an unexpected cellular outcome.
The most common PTMs catalyzed by protein toxins are glucosylation, deadenylation, proteolysis, and ADP-ribosylation, in which toxins catalyze the transfer of ADP-ribose from NAD to host proteins (3). Two archetypical examples of ADP-ribosylating toxins act on components of heterotrimeric G-proteins: Cholera toxin ADP-ribosylates Gαs, enforcing an activated conformation that alters ion transport and water flow leading to diarrhea (4), whereas pertussis toxin ADP-ribosylates Gαi, blocking the ability of Gαi to interact with its associated receptor, resulting in whooping cough and alterations in cell migration behavior (5). In cultured cells, cholera toxin stimulates cell elongation, whereas pertussis toxin stimulates cell clustering, phenotypes that are specific for each toxin and thus can be used to identify toxin action. ADP-ribosylating toxins, including cholera and pertussis toxin, use a conserved “AB” architecture, where A is the modifying enzyme and B binds cell surface receptors and mediates internalization of A. The specific details of the A and B structures, however, can vary. For example, diphtheria toxin is a single chain AB protein that is proteolytically cleaved to create a disulfide-linked N-terminal catalytic domain and a C-terminal translocation receptor-binding domain. Cholera toxin is an AB5 protein where the catalytic A1 domain is linked to an A2 domain that inserts noncovalently into the channel of a B pentamer. Although the catalytic A domains of the ADP-ribosylating toxins share limited primary amino acid homology, they share overall three-dimensional structure and contain several conserved amino acids, including an active site glutamic acid (6). As a result, scanning genomes for A and B sequences can point not only to uncharacterized toxins, but what the likely structure, and potential function, of the toxin might be.
Extra-intestinal E. coli, including the uropathogenic E. coli and neonatal meningitis E. coli, normally reside in the gut but can damage the host when they invade other systems, such as the urinary tract or nervous system, respectively. Similar to Vibrio cholera, Bordetella pertussis, and pathogenic E. coli such as enterotogenic E. coli and enterohemorrhagic E. coli, extra-intestinal E. coli are known to encode AB5 toxins, but whether these toxins are functional and whether their functions follow established mechanisms are unknown.
To study this question, Littler et al. (2) queried whole and partial E. coli genomes in the NCBI database using known E. coli A and B gene sequences and found a group of genes encoding AB5 toxins related to pertussis toxin that the authors termed E. coli-pertussis-like toxins (EcPlt), which were subjected to biological and biochemical characterization. Purified EcPlt elicited a pertussis toxin-like clustering of cultured cells, and the isolated A domain of EcPlt (EcPltA) ADP-ribosylated a 41-kDa host protein with the same molecular weight as pertussis toxin-treated Gαi proteins, suggesting some functional conservation. Introduction of a point mutation to the presumed active site glutamic acid (E118D) in EcPltA also reduced activity by 1000-fold, consistent with Glu-118 being the conserved active site residue within this family of toxins.
EcPlt also diverged from pertussis toxin in several ways: A systemic assessment of substrate specificity showed that EcPltA ADP-ribosylated each of the three Gαi isoenzymes, but with slightly different preferences to that of pertussis toxin. Unexpectedly, mass spectrometry analysis showed that EcPltA ADP-ribosylated one of these isoenzymes, Gαi3, at Lys-345 and Asn-347, rather than at Cys-351 ribosylated by pertussis toxin or indeed any of the Cys residues present in native Gαi3. Although there are precedents from other protein toxins and the endogenous host transferase to ADP-ribosylate multiple sites within a protein (7), EcPlt is unique in modifying both Lys and Asn amino acids. Mutagenesis studies suggested the two sites could both be necessary for a concerted mechanism of substrate recognition, the details of which will be fascinating to learn. The authors then tested the impact of EcPlt in a cellular assay of forskolin-mediated G protein recruitment to GPCRs, in which mutation of the pertussis toxin modification site Cys-351 prevents pertussis toxin from blocking recruitment. In the case of EcPlt, however, the authors observed only a modest rescue provided by the N347A mutation and no obvious rescue provided by the K345A mutation. These limited effects may reflect the intrinsic differences between agonist activation and forskolin activation or may suggest Lys-345 and Asn-347 modifications affect Gαi action by a different mechanism than Cys-351 modification, possibly uncoupling G-protein signaling through different contacts with the G-protein-coupled receptor.
Finally, Littler et al. (2) solved the crystal structure of EcPlt at 2.4 Å (Fig. 1), which demonstrates structural homology, and possibly functional homology, with typhoid toxin (8). Structural comparisons of EcPlt in the inactive oxidized state and the activated reduced state, containing a Cys-41–Cys-192 disulfide bond, further showed how EcPlt is activated via movement of the activation loop to expose the NAD-binding domain. This activation mechanism is different than that of cholera toxin (9) and may provide insight into the less studied activation mechanisms of typhoid toxin and other pertussis-like toxins.
Figure 1.
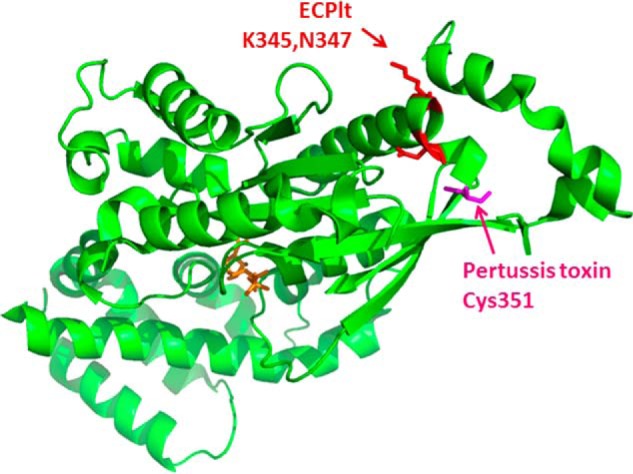
ADP-ribosylation of Gαi by EcPlt and pertussis toxin. EcPlt and pertussis toxin ADP-ribosylate Gαi (Protein Data Bank code 3FFB) on its C-terminal tail, EcPlt at Lys-345 and Asn-347 (red), and pertussis toxin at Cys-351 (pink), which mediates the interaction with a GPCR. Binding of agonist to the extracellular domain of the GPCR is detected by Gαi initiating a conformational change that exchanges GDP (orange) with GTP, leading to intracellular cell signaling.
The study from Littler et al. (2) provides exciting new information on the molecular and biophysical properties of an understudied subset of AB5 toxins. In addition to raising questions about the basis of chemoselectivity and revealing new conformational pathways, EcPlt may offer another benefit: Pertussis toxin has proven a useful reagent to dissect not only the molecular basis for the pathogenesis of Bordetella pertussis, but also to dissect the basis for GPCR signaling (10). Because EcPlt may have a different basis for uncoupling G-protein signaling, EcPlt may provide a new tool to continue dissection of this important eukaryotic signaling pathway.
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
- PTM
- posttranslational modification
- EcPlt
- E. coli-pertussis-like toxin
- NAD
- nicotinamide adenine dinucleotide
- GPCR
- G-protein-coupled receptor.
References
- 1. Aktories K., and Barbieri J. T. (2005) Bacterial cytotoxins: targeting eukaryotic switches. Nat. Rev. Microbiol. 3, 397–410 [DOI] [PubMed] [Google Scholar]
- 2. Littler D. R., Ang S. Y., Moriel D. G., Kocan M., Kleifeld O., Johnson M. D., Tran M. T., Paton A. W., Paton J. C., Summers R., Schrembri M., Rossjohn J., and Beddoe T. T. (2017) Structure–function analyses of a pertussis-like toxin from pathogenic Escherichia coli reveal a distinct mechanism of inhibition of trimeric G-proteins. J. Biol. Chem. 292, 15143–15158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon N. C., Aktories K., and Barbieri J. T. (2014) Novel bacterial ADP-ribosylating toxins: structure and function. Nat. Rev. Microbiol. 12, 599–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vanden Broeck D., Horvath C., and De Wolf M. J. (2007) Vibrio cholerae: cholera toxin. Int. J. Biochem. Cell Biol. 39, 1771–1775 [DOI] [PubMed] [Google Scholar]
- 5. West R. E. Jr., Moss J., Vaughan M., Liu T., and Liu T. Y. (1985) Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J. Biol. Chem. 260, 14428–14430 [PubMed] [Google Scholar]
- 6. Carroll S. F., McCloskey J. A., Crain P. F., Oppenheimer N. J., Marschner T. M., and Collier R. J. (1985) Photoaffinity labeling of diphtheria toxin fragment A with NAD: structure of the photoproduct at position 148. Proc. Natl. Acad. Sci. U.S.A. 82, 7237–7241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ganesan A. K., Mende-Mueller L., Selzer J., and Barbieri J. T. (1999) Pseudomonas aeruginosa exoenzyme S, a double ADP-ribosyltransferase, resembles vertebrate mono-ADP-ribosyltransferases. J. Biol. Chem. 274, 9503–9508 [DOI] [PubMed] [Google Scholar]
- 8. Song J., Gao X., and Galán J. E. (2013) Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 499, 350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mekalanos J. J., Collier R. J., and Romig W. R. (1979) Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J. Biol. Chem. 254, 5855–5861 [PubMed] [Google Scholar]
- 10. Moss J., Bruni P., Hsia J. A., Tsai S. C., Watkins P. A., Halpern J. L., Burns D. L., Kanaho Y., Chang P. P., and Hewlett E. L. (1984) Pertussis toxin-catalyzed ADP-ribosylation: effects on the coupling of inhibitory receptors to the adenylate cyclase system. J. Recept. Res. 4, 459–474 [DOI] [PubMed] [Google Scholar]


