Abstract
In daily practice, clinicians are often confronted with obese type 2 diabetes mellitus (T2DM) patients for whom the treatment plan fails and who show an inadequate glycemic control and/or no sustainable weight loss. Untreated hypogonadism can be the reason for such treatment failure. This case describes the profound impact testosterone therapy can have on a male hypogonadal patient with metabolic syndrome, resulting in a substantial and sustained loss of body weight, pronounced improvement of all critical laboratory values and finally complete remission of diabetes.
Learning points:
Hypogonadism occurs frequently in men with T2DM.
In case of pronounced abdominal fat deposition and T2DM, the male patient should be evaluated for testosterone deficiency.
Untreated hypogonadism can complicate the successful treatment of patients with T2DM.
Under testosterone therapy, critical laboratory values are facilitated to return back to normal ranges and even complete remission of diabetes can be achieved.
Background
Diabetes, dyslipidemia, obesity and hypertension are cardiovascular risk factors often encountered in clinical routine. For obese patients, weight loss represents the most important target; however, it is well known that the majority of patients are not successful to slim down in a sustainable manner. Bariatric surgery can provide substantial effects on weight loss and significant improvements in obesity-attributable comorbidities including improvement or resolution of diabetes (1, 2) but bears the risk of complication, reoperation and death. In non-bariatric patients, prolonged remission of diabetes is extremely rare (3).
Dieting leads to depletion of both, fat and muscle tissue; for the necessary increase of lean body mass, a reduced calorie diet must therefore be combined with simultaneous building of muscle mass. Testosterone is one essential factor for the protein synthesis in the mechanism of muscle mass regulation, and thus, correction of subnormal testosterone levels is important for hypogonadal, obese men. Several studies have shown that the weight loss of hypogonadal, obese men on diet under testosterone therapy was almost exclusively due to loss of body fat and diet-associated loss of lean mass was prevented (4, 5). Untreated hypogonadism will be rather a severe hindrance to improve body composition.
Complex interlinks exist between obesity, testosterone and T2DM in men. From a number of studies, subnormal testosterone levels were reported to be present in 25–40 percent of men with T2DM (6). Consequently, the Endocrine Society Clinical Practice Guideline recommends to measure the total testosterone level in T2DM patients on a routine basis (7).
As testosterone plays a key role in human physiology, the importance of testosterone deficiency and its treatment goes far beyond symptoms of sexual dysfunction and one must not underestimate the influence of hypogonadism on life-threatening complications of associated diseases such as diabetes, cardiovascular disease and metabolic syndrome.
Case presentation
A 63-year-old man suffering from recurrent prostatitis and lower urinary tract symptoms (LUTS), combined with loss of libido and erectile dysfunction was referred to a urologist. At the time of referral, the obese patient (BMI: 37.1 kg/m2) had T2DM for 4 years, was on metformin for the same period and, in addition, on insulin treatment for 2 years at 22 IU per day. Under insulin, the patient had gained 5 kg. Doctors’ instructions to lose weight by diet and exercise had been unsuccessful. Comorbidities comprised dyslipidemia, mild hypertension and coronary artery disease (CAD).
Investigation
At the time of presentation to our office, the patient neither smoked nor consumed alcohol. When questioned about family history regarding major cardiovascular events (MACE), the patient reported that his father had had a stroke and had died three years later of myocardial infarction (MI). Also, the father’s brother had suffered three MIs and died of the third. The patient’s history included a previous diagnosis of CAD. Upon enquiry, we learned that the CAD had been diagnosed 4 years prior to the referral. The patient had had mild hypertension since he had been in his thirties. He never suffered a MACE. The cardiologist recommended physical activity and the patient started riding a bicycle every day and walking one hour, three times a week, a regimen the patient has maintained until today. The cardiologist had also found an elevated HbA1c and referred the patient to a diabetologist.
At the time of referral to our urology practice, laboratory workup showed inadequate glycemic control (fasting glucose: 8.5 mmol/L, HbA1c: 9.4%) combined with dyslipidemia (LDL/HDL ratio: 3.3). HOMA-IR test revealed profound insulin resistance (11.7). C-reactive protein (CRP) was elevated (3.2 mg/dL). Blood pressure measurement showed mild hypertension (159/96 mmHg). Total testosterone was measured in early morning samples on two occasions and showed each time a value below the lower limit of the reference range of 12.1 nmol/L (8). In our urology office setting, neither SHBG nor gonadotropins were measured for cost reasons. Since the patient had class II obesity, it is safe to assume that he had functional hypogonadism (4, 9).
Treatment
In addition to the medication at the time of referral (anti-hypertensives, alpha-blockers, anti-diabetics and statins), testosterone therapy was started with testosterone undecanoate injections (Nebido® injected i.m. every three months following an initial 6-week interval).
Outcome and follow-up
The patient was routinely monitored prior to the next injection at 3-month intervals for 10 years.
Testosterone normalized at the first control and remained in the normal range henceforth (Fig. 1). Under testosterone therapy, the patient continuously lost weight; finally, 24 kg weight loss (20.2%) and 12 cm reduction of waist circumference were achieved (Fig. 2A). BMI dropped to 29.6 kg/m2 (Fig. 2B). In addition, the critical laboratory values related to diabetes and other comorbidities substantially improved and even returned to normal range. Right from the beginning of testosterone therapy, fasting glucose declined and remained below 6 mmol/L from the 3rd year onward. A progressive decrease of insulin resistance and HbA1c occurred allowing to gradually reduce insulin dose: from 22 IU/day to 18 IU/day at the end of year 1, to 14 IU/day at 30 months and to 12 IU/day in the 6th year. Finally, with an HbA1c value of 6.7% achieved in year 7, insulin use was discontinued. In the following time, without further administration of insulin, HbA1c further decreased to 6.1% (Fig. 3A) and HOMA-IR to 2.8 in year 8 (Fig. 3B). Metformin was discontinued in year 10. Dyslipidemia resolved completely and LDL/HDL ratio returned to normal (from 3.3 to 1.4) (Fig. 4). Blood pressure normalized from 159/96 mmHg to 125/75 mmHg. CRP normalized from 3.2 mg/dL to 0.1 mg/dL. LUTS improved in terms of International Prostate Symptom Scale (IPSS) from moderate to mild symptoms and residual bladder volume decreased from 80 to 10 mL.
Figure 1.
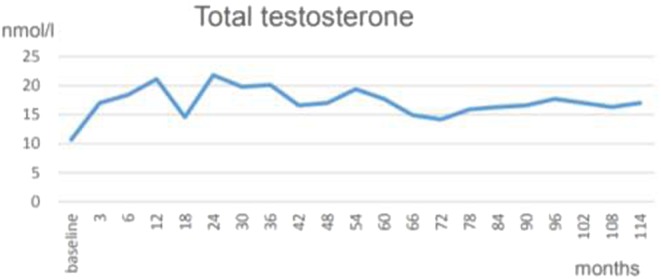
Total testosterone normalized under testosterone therapy. The graph shows trough levels, measured at the end of an interval and prior to the following injection.
Figure 2.
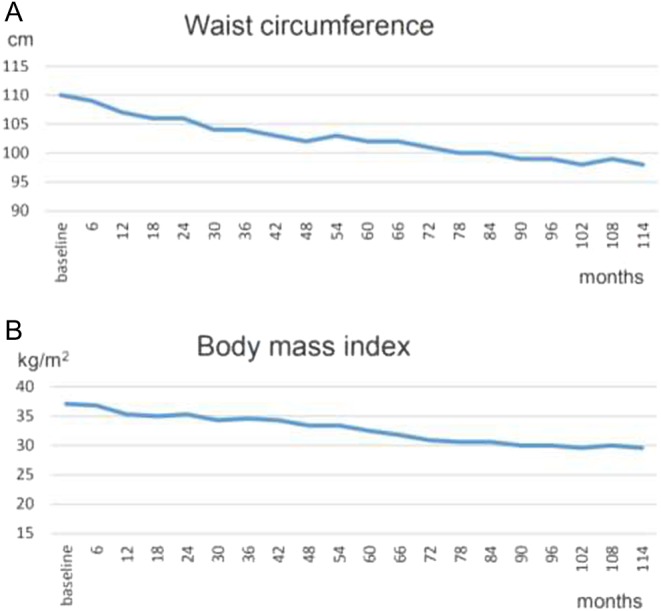
(A) Waist circumference of 110 cm at baseline decreased to 98 cm in month 114. (B) Body mass index of 37.1 kg/m2 at baseline decreased to 29.6 kg/m2 in month 114.
Figure 3.
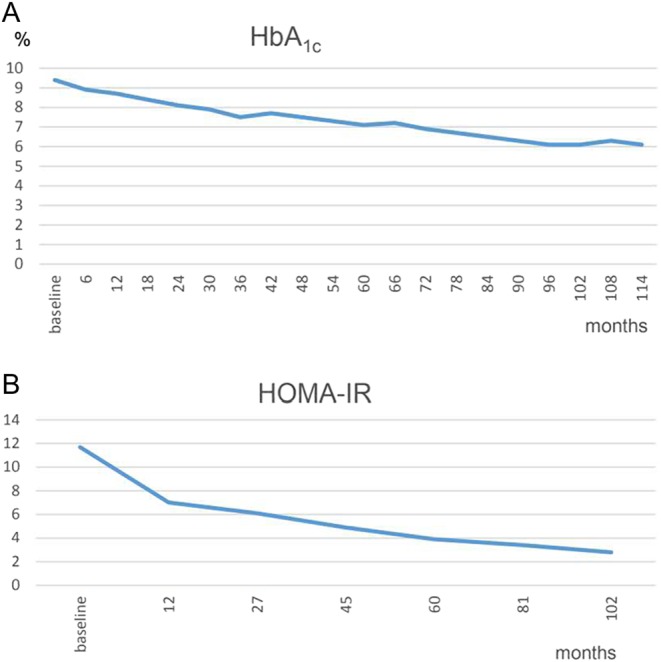
(A) HbA1c – baseline value of 9.4% decreased down to 6.1% in month 114. (B) HOMA-IR – baseline value of 11.7 declined to 2.8 in month 102.
Figure 4.
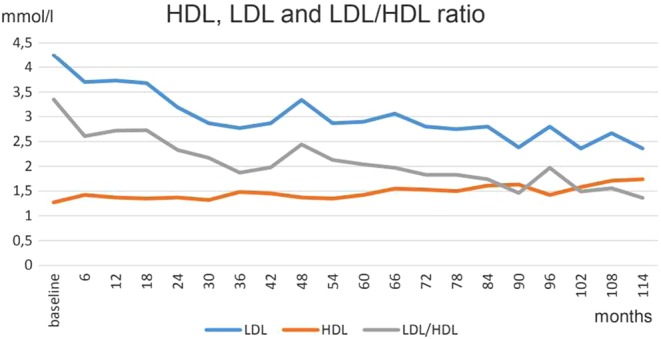
HDL increased and LDL decreased over time. LDL/HDL ratio of 3.3 at baseline normalized to 1.4 in month 114.
The patient’s libido was assed as part of the Aging Males’ Symptoms (AMS) scale, which improved both in general and in every single item. Erectile function was assessed by the International Index of Erectile Function – Erectile Function domain (IIEF-EF) with a maximal score of 30. At baseline, the patient had only mild erectile dysfunction (ED), which improved to ‘no ED’, a status that was maintained over the entire observation time despite the fact that the patient’s age increased by 10 years.
Discussion
For this hypogonadal, obese diabetic patient, all classical cardiovascular disease risk factors such as blood pressure, lipid pattern, glycemic control and obesity were improved under testosterone therapy and for the most part even resolved. Testosterone significantly contributes to metabolic activities, and thus, the correction of hypogonadism contributes to the recovery of a previously disturbed biochemical balance. The described clinical outcome can be seen as an indirect result from the regulation of the testosterone level. Moreover, the return from the hypogonadal to the eugonadal state stimulates and supports the biochemical activities to achieve fat loss, in particular abdominal fat, and to increase muscle mass, thus increasing basal metabolic rate. The strong relationship between testosterone therapy and weight loss in obese men is well established and has been demonstrated in a series of studies. In particular, the sustainability of weight loss under testosterone therapy is a remarkable aspect. This has been shown in long-term observational studies such as a 5-year controlled study (10) or the analysis from 8-year follow-up data from registry studies (11). Like in the present case report, these studies showed sustainable weight loss under testosterone therapy resulting in a significant reduction of blood glucose and insulin sensitivity, improvement of lipid profile and inflammatory markers as well as blood pressure (10, 11).
The American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) recommend in their Clinical Practice Guidelines for medical care of patients with obesity to measure testosterone in all men with increased waist circumference, obesity and/or T2DM, and that men with hypogonadism should be considered for testosterone therapy (12). We have shown previously that testosterone therapy is particularly beneficial in obese men with T2DM (13) but, when analyzing 6-year data, had not yet seen a case with complete remission. Moreover, recent study results showed that the beneficial effects of testosterone treatment were not sustained after cessation (14). This underlines the importance of long-term and potentially lifelong treatment with testosterone and may suggest a slow normalization of a metabolic disease state that had slowly developed over decades. It is sufficiently established that hypogonadal men, in particular those with metabolic syndrome, benefit from testosterone therapy. Prerequisites are a proper diagnosis according to guideline recommendations from The Endocrine Society (7) and other medical societies and continuous follow-up examinations. The present case is an example of clinically meaningful improvements of health outcomes a patient can receive, provided that testosterone therapy is performed adequately and with thorough and careful monitoring.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
A Haider has received partial funding for a registry study and travel grants from Bayer AG. F Saad is a full-time employee of Bayer AG.
Author contribution statement
A Haider is the physician responsible for the patient. A Haider and K Haider have collected the data for this case report. F Saad drafted the manuscript, which was reviewed by all authors.
Patient consent
Written informed consent was obtained from the patient for publication of this case.
References
- 1.Chang S, Stoll CRT, Song J, Varela JE, Eagon CJ, Colditz GA. 2014. The effectiveness and risks of bariatric surgery. An updated systematic review and meta-analysis, 2003–2012. JAMA Surgery 149 275–287. ( 10.1001/jamasurg.2013.3654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjöholm K, Pahunen P, Jacobson P, Karason K, Sjöström CD, Torgerson J, Carlsson LMS, Sjöström L, Peltonen M. 2015. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia 58 1448–1453. ( 10.1007/s00125-015-3591-y) [DOI] [PubMed] [Google Scholar]
- 3.Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. 2014. Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care 37 3188–3196. ( 10.2337/dc14-0874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossmann M, Matsumoto AM. 2017. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. Journal of Clinical Endocrinology and Metabolism 102 1067–1075. ( 10.1210/jc.2016-3580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, Grossmann M. 2016. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Medicine 14 153 ( 10.1186/s12916-016-0700-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandona P, Dhindsa S. 2011. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. Journal of Clinical Endocrinology and Metabolism 96 2643–2651. ( 10.1210/jc.2010-2724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. 2010. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology and Metabolism 95 2536–2559. ( 10.1210/jc.2009-2354) [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, et al. 2011. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. Journal of Clinical Endocrinology and Metabolism 96 2430–2439. ( 10.1210/jc.2010-3012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saboor Aftab SA, Kumar S, Barber TM. 2013. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clinical Endocrinology 78 330–337. ( 10.1111/cen.12092) [DOI] [PubMed] [Google Scholar]
- 10.Francomano D, Lenzi A, Aversa A. 2014. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. International Journal of Endocrinology article ID 527470. ( 10.1155/2014/527470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad F, Yassin A, Doros G, Haider A. 2016. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. International Journal of Obesity 40 162–170. ( 10.1038/ijo.2015.139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R, Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines 2016. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocrine Practice 22 (Supplement 3) 1–203. ( 10.4158/EP161365.GL) [DOI] [PubMed] [Google Scholar]
- 13.Haider A, Yassin A, Doros G, Saad F. 2014. Effects of long-term testosterone therapy on patients with ‘diabesity’: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. International Journal of Endocrinology 2014 article ID 683515. ( 10.1155/2014/683515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng Tang Fui M, Hoermann R, Zajac JD, Grossmann M. 2017. The effects of testosterone on body composition in obese men are not sustained after cessation of testosterone treatment. Clinical Endocrinology Epub. ( 10.1111/cen.13385) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a