Abstract
A 51 year old man presented with sepsis in the setting of thioamide-induced agranulocytosis. Empiric broad-spectrum antibiotics was followed by directed narrow-spectrum antibiotics, and his neutrophil count recovered with support from granulocyte-colony stimulating factor (G-CSF) analogue transfusions. After a brief period of multi-modal therapy for nine days including potassium iodide (Lugol’s iodine), cholestyramine, propanolol and lithium to temper his persisting hyperthyroidism, a total thyroidectomy was performed while thyroid hormone levels remained at thyrotoxic levels. Postoperative recovery was uncomplicated and he was discharged home on thyroxine. There is limited available evidence to guide treatment in this unique cohort of patients who require prompt management to avert impending clinical deterioration. This case report summarises the successful emergent control of thyrotoxicosis in the setting of thioamide-induced agranulocytosis complicated by sepsis, and demonstrates the safe use of multi-modal pharmacological therapies in preparation for total thyroidectomy.
Learning points:
Thioamide-induced agranulocytosis is an uncommon but potentially life-threatening complication of which all prescribers and patients need to be aware.
A multi-modal preoperative pharmacological approach can be successful, even when thioamides are contraindicated, when needing to prepare a thyrotoxic patient for semi-urgent total thyroidectomy.
There is not enough evidence to confidently predict the safe timing when considering total thyroidectomy in this patient cohort, and therefore it should be undertaken when attempts have first been made to safely reduce thyroid hormone levels.
Thyroid storm is frequently cited as a potentially severe complication of thyroid surgery undertaken in thyrotoxic patients, although the evidence does not demonstrate this as a common occurrence.
Background
Thioamide-induced agranulocytosis is an uncommon but potentially serious complication. In the absence of infection, antithyroid medication can normally be ceased and other management options considered, such as radioactive iodine therapy or total thyroidectomy. When the patient also presents with sepsis and remains thyrotoxic, the risks of performing surgery on an immunosuppressed patient in this setting are largely unknown, but potentially high. Although the safety and efficacy of the therapies used in this case have been previously reported, there are few data to support combined anti-thyroid medication therapy to prepare for safe total thyroidectomy. This case highlights the potential seriousness of agranulocytosis secondary to thioamide therapy, and importantly, it demonstrates the safety of performing total thyroidectomy in thyrotoxic patients when necessary.
Case presentation
A 51 year old man presented to a regional tertiary hospital Emergency Department with palpitations, shortness of breath, bilateral lower leg oedema and general malaise. His medical history included unexplained macrocytic anaemia eight years earlier, and nasopalatine cyst surgery in recent weeks. He had a family history significant for Hashimoto’s thyroiditis (uncle).
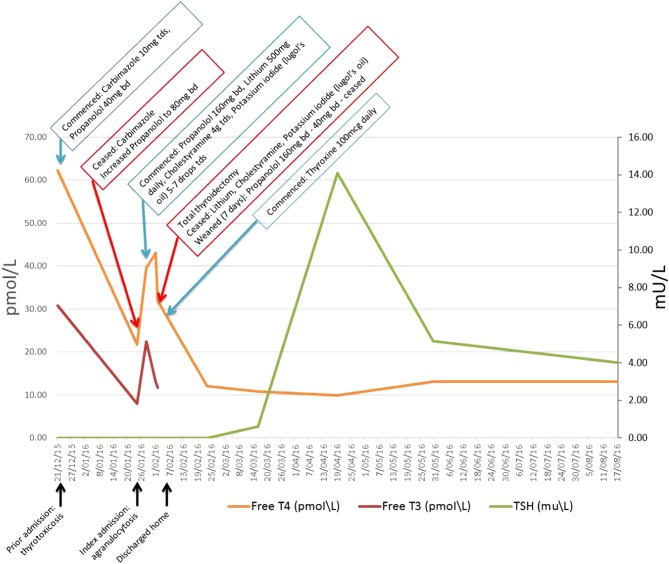
A computed tomography (CT) pulmonary angiogram excluded pulmonary embolus and, after he complained of abdominal symptoms, a CT abdomen and pelvis with contrast excluded abdominal pathology. Thyroid studies revealed thyrotoxicosis, with a suppressed thyroid-stimulating hormone (TSH) (reference range: 0.3–4.0 mU/L), a free triiodothyronine (T3) of 30.8 pmol/L (reference range: 3.5–6.5 pmol/L) and a free thyroxine (T4) of 62.3 pmol/L (reference range: 9.8–18.8 pmol/L) (Fig. 1). An electrocardiogram excluded atrial fibrillation, demonstrating sinus in origin. A chest xray was unremarkable.
Figure 1.
Multi-modal anti-thyroid therapy temporises thyrotoxicosis with reduction in thyroid hormone levels prior to semi-urgent total thyroidectomy.
He was commenced on carbimazole 10 mg three times daily orally and propanolol 40 mg twice daily orally (Fig. 1). One month later, he was transferred from a rural hospital to the same regional tertiary hospital with a temperature of 39.1 degrees celsius and systemic features of infection including diaphoresis, nausea and sinus tachycardia.
He did not exhibit clinical findings of a goitre, orbitopathy, ophthalmopathy, dermopathy, periorbital oedema or nail changes consistent with Graves’ disease.
Investigation
Full blood examination revealed a white cell count of 1.3 × 109/L (reference range: 4.0–11.0/L) and neutrophil count of 0.0 × 109/L (reference range: 2.0–8.0/L) (Table 1). His C-Reactive Protein (CRP) result peaked at 92.6 mg/L (reference range: <2.9 mg/L). He was hyperthyroid, with a suppressed TSH, a T3 of 8.0 pmol/L and a T4 of 21.8 pmol/L (Table 1). A CT sinus demonstrated widespread sinusitis, and microscopy and culture of a neck ulcer subsequently revealed a Methicillin Sensitive Staphylococcus Aureus (MSSA) site infection. Blood cultures remained negative.
Table 1.
Laboratory investigations on presentation demonstrated agranulocytosis one month after commencement of carbimazole for a new diagnosis of Graves’ disease.
| Laboratory test | Value | Reference range |
|---|---|---|
| Full blood examination | ||
| Lymphocyte total | 0.9 × 109/L | 1.0–4.0 × 109/L |
| WCC | 1.3 × 109/L | 4.0–11.0 × 109/L |
| Neutrophil | 0.0 × 109/L | 2.0–8.0 × 109/L |
| Haemoglobin | 122 g/L | 125–175 g/L |
| Red Cell Count | 4.3 × (10 × 12/L) | 4.5–5.9 × (10 × 12/L) |
| Haematocrit | 0.36 L/L | 0.41–0.53 L/L |
| Thyroid function tests (1 month post starting Carbimazole) | ||
| TSH | <0.005 mU/L | 0.300–4.000 mU/L |
| Free T3 | 8.0 pmol/L | 3.5–6.5 pmol/L |
| Free T4 | 21.8 pmol/L | 9.8–18.8 pmol/L |
A diagnosis of Graves’ disease was confirmed with an elevated TSH-receptor autoantibody titre of 1.9 U/L at first hospital admission, increasing to 3.7 U/L one month later on repeat testing (reference range: <1.8 U/L). Although a thyroid ultrasound was performed prior to thyroidectomy, a thyroid nuclear uptake scan was not performed in this case.
Treatment
Carbimazole was ceased immediately (Fig. 1). Piperacillin-tazobactam 4.5 g three times daily intravenously was commenced as empiric therapy for sepsis of unknown focus. This was changed to amoxicillin/clavulanic acid 875 mg/125 mg twice daily orally when the neutrophil count was >0.5 × 109/L and culture revealed MSSA, and continued for seven days. A G-CSF analogue, filgrastim, was commenced at 300 µg daily subcutaneously for three days, increased to 480 µg daily subcutaneously for three days, and ceased when the neutrophil plus bands count reached a total of 1 × 109/L.
The patient developed persistent hypertension and an increased pulse rate during his admission, raising concerns for emerging thyroid storm. His blood pressure peaked at 195/85 and pulse rate at 100 beats per minute despite doses of propanolol which commenced at 40 mg twice daily orally, and increased in increments to 80 mg four times daily prior to thyroidectomy.
Within five days of ceasing carbimazole, his T3 levels had increased sharply to 33.4 pmol/L and T4 to 39.7 pmol/L. He was commenced on lithium 500 mg daily orally, cholestyramine 4 g three times daily orally, and Lugol’s iodine five to seven drops three times daily orally in preparation for total thyroidectomy, which was performed by Ear Nose & Throat Surgeons on day nine of admission (Fig. 1). A lithium level of 0.2 mmol/L (therapeutic range: 0.5–1.0 mmol/L) was performed three days after commencement of lithium.
On the day of surgery, thyroid function results had improved but were still elevated, with a suppressed TSH, a T4 of 31.9 pmol/L and a T3 of 11.8 pmol/L (Fig. 1).
Thyroid gland histology further supported the diagnosis of Graves’ disease. The thyroid gland weighed 50.5 g. Microscopically, there were focal hyperplastic changes.
Post surgery, lithium, cholestyramine and Lugol’s iodine were ceased. Propanolol was gradually weaned over seven days to 40 mg twice daily orally before ceasing. Thyroxine 100 µg daily orally was commenced on discharge (Fig. 1).
Outcome and follow-up
The patient was discharged home following successful total thyroidectomy. There were no complications from surgery, specifically with regard to bleeding, recurrent laryngeal nerve injury or hypoparathyroidism. He has remained stable on thyroid hormone replacement therapy, with regular thyroid function monitoring with his General Practitioner. At follow up nine months post surgery he remained euthyroid on replacement thyroxine (Fig. 1) with a TSH of 4.0 mU/L and a T4 of 13.1 pmol/L.
Discussion
Agranulocytosis caused by thioamides is uncommon, with incidence considered to be around 0.2% and almost always developing in the first 90 days of commencement of therapy (1, 2). Although the mechanism is not entirely known, it is thought that a myelosuppressive effect on granulocyte production leads to suppression of neutrophil development, possibly due to anti-granulocyte auto-antibodies and/or direct toxicity at the haematopoietic stem cell level (2).
There are a number of pharmacological options used historically as monotherapy when thioamides are contraindicated in hyperthyroid patients. They each have a unique mechanism of action, which are likely to be synergistic.
Propanolol is a beta-blocker used to attenuate hyperadrenergic symptoms of palpitations, anxiety, heat intolerance, shortness of breath and tremor (1). At a starting dose of 40–160 mg daily, it has an advantage over other beta-blockers in helping decrease the peripheral conversion of T4–T3 by being more lipid soluble and through inhibition of the 5-monodeiodinase enzyme (1). A study published in NEJM in 1978 reported a series of 100 patients with thyrotoxicosis who underwent successful subtotal thyroidectomy following treatment with propanolol as monotherapy, without major perioperative or postoperative morbidity and importantly, with no cases of thyroid storm (3).
Lithium has a number of beneficial actions in the treatment of thyrotoxicosis. Firstly, it inhibits the coupling of iodotyrosine residues preventing synthesis of T4 and T3, likely through inhibiting the action of TSH on the cyclic adenosine monophosphate (cAMP) second messenger system (4). Secondly, it inhibits thyroid hormone release from the follicular cell (4). Finally, it acts as an adjunct to radioactive iodine treatment by promoting its retention in the thyroid gland (4).
Lugol’s iodine exerts its effect primarily via the autoregulatory Wollf-Chaikoff effect of inhibiting iodination of thyroglobulin due to large influx of exogenous iodide to suppress new thyroid hormone synthesis (1). It has the added benefit of reducing thyroid gland vascularity and risk of bleeding in thyroidectomy, supporting its role in mitigating the risk of postoperative haemorrhage (1, 5).
Cholestyramine is a bile acid sequestrant. Thyroid hormones are normally metabolised in the liver where they are conjugated with glucuronide and sulphate and excreted in the bile. Free thyroid hormones are then released in the intestine and are finally reabsorbed. Thyrotoxic states are characterised by significantly increased enterohepatic circulation of thyroid hormones. Cholestyramine reduces these increased hormone levels by binding to them and interfering with enterohepatic circulation and the recycling of thyroid hormone, resulting in enhanced faecal excretion (6).
Corticosteroids reduce the conversion of T4 to T3 and thyroid hormone release from the follicular cell, and although not administered to this patient, have previously been recommended for rapid preparation of emergent thyroid surgery (7). They are also used to treat and hinder the progression of established ophthalmopathy associated with Grave’s disease (8).
There are little available data to support combined pharmacological therapy regimes for hyperthyroidism or for urgent surgical intervention when a patient presents profoundly thyrotoxic with the added burden of neutropaenic sepsis. The 2016 American Thyroid Association guidelines for management of hyperthyroidism recommend that patients with Graves’ disease be rendered euthyroid with methimazole prior to thyroidectomy, and support combined use of beta-blockade and potassium iodide in the preoperative period (9). They also acknowledge that if the need for thyroidectomy is urgent or it is not possible to render a patient euthyroid, the patient should then be adequately treated preoperatively with beta-blockade and potassium iodide, and priority made for a surgeon and anaesthetist with suitable experience (9).
Radioactive iodine therapy has been used for hyperthyroid patients to successfully render them euthyroid in the setting of thioamide-induced agranulocytosis, and may represent a safe treatment alternative for patients with a more stable clinical presentation (10). Prior iodine exposure from two recent CT scans with intravenous iodine would require a clinically unacceptable delay for radioactive iodine therapy in our patient, who remained symptomatic in the setting of increasing thyroid hormone levels following cessation of carbimazole. Secondly, standard practice in Australia is to continue thioamide therapy to control hyperthyroidism following radioactive iodine administration until the patient is rendered euthyroid. The thioamide-induced agranulocytosis in this case represented a contraindication for ongoing thioamide therapy.
There are published case reports describing a potential role for plasmapheresis to control thyroid hormone levels and thyroid storm in the preoperative management of patients with thioamide-induced agranulocytosis (11, 12). However, the most recent American guidelines on the use of therapeutic apheresis do not support the use of plasmapheresis to manage thyroid hormone excess and was therefore not considered in this case (13).
A major concern has always been the potential for thyroid storm induced by the trauma of surgery in patients who are already hyperthyroid. However, there are many case reports of successful and safe total thyroidectomies in patients with thyrotoxicosis or with agranulocytosis in the setting of thyrotoxicosis (14). A recent retrospective study in the United States on 165 patients who underwent total thyroidectomy demonstrated that of the 42% of patients who were hyperthyroid on the day of surgery, although 37% developed some form of complication, none of them developed thyroid storm (14).
This case presents the unique challenges of managing thioamide-induced agranulocytosis complicated by thyrotoxicosis and sepsis. The concern for emerging thyroid storm during admission added complexity to the decision to proceed with early thyroidectomy. There were also logistical issues to consider relating to availability of a surgeon experienced in thyroidectomy in thyrotoxic patients, and availability of an operating theatre.
This case validates the role for multi-modal therapy to ameliorate thyrotoxicosis prior to semi-urgent total thyroidectomy. Although thioamide-induced agranulocytosis in the setting of Graves’ disease is rare, there is a risk of rapid deterioration for the patient who develops sepsis in this setting.
There remains a grey area when it comes to safe timing of surgery in a patient with marked Graves’ thyrotoxicosis, and a gap in the knowledge when in comes to recommendations on combined pharmacological anti-thyroid therapy duration in the immediate preparatory phase for surgery. This case demonstrates that semi-urgent thyroidectomy may be safe in the setting of thyrotoxicosis. Non-thioamide based therapies may be of benefit to temporise the clinical state and may reduce the risk of complications.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient for publication of the submitted article and the accompanying images.
Author contribution statement
C L K is the primary author of the manuscript. S D C, J K, M B and M K provided further intellectual input and supervision into the content of the manuscript.
Acknowledgement
The authors would like to acknowledge Mr Jean-Paul Berthelot for his contribution in preparing figure 1.
References
- 1.Burch HB, Cooper DS. 2015. Management of graves disease: a review. JAMA 314 2544–2554. ( 10.1001/jama.2015.16535) [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H, Miyauchi A, Miyawaki N, Imagawa J. 2013. Analysis of 754 cases of antithyroid drug-induced agranulocytosis over 30 years in Japan. Journal of Clinical Endocrinology Metabolism 98 4776–4783. ( 10.1210/jc.2013-2569) [DOI] [PubMed] [Google Scholar]
- 3.Toft AD, Irvine WJ, Sinclair I, McIntosh D, Seth J, Cameron EH. 1978. Thyroid function after surgical treatment of thyrotoxicosis. A report of 100 cases treated with propranolol before operation. New England Journal of Medicine 298 643–647. ( 10.1056/NEJM197803232981202) [DOI] [PubMed] [Google Scholar]
- 4.Prakash I, Nylen ES, Sen S. 2015. Lithium as an alternative option in Graves’ thyrotoxicosis. Case Report Endocrinology 2015 1–4. ( 10.1155/2015/869343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erbil Y, Ozluk Y, Giris M, Salmaslioglu A, Issever H, Barbaros U, Kapran Y, Ozarmagan S, Tezelman S. 2007. Effect of lugol solution on thyroid gland blood flow and microvessel density in the patients with Graves’ disease. Journal of Clinical Endocrinology Metabolism 92 2182–2189. ( 10.1210/jc.2007-0229) [DOI] [PubMed] [Google Scholar]
- 6.Kaykhaei MA, Shams M, Sadegholvad A, Dabbaghmanesh MH, Omrani GR. 2008. Low doses of cholestyramine in the treatment of hyperthyroidism. Endocrine 34 52–55. ( 10.1007/s12020-008-9107-5) [DOI] [PubMed] [Google Scholar]
- 7.Baeza A, Aguayo J, Barria M, Pineda G. 1991. Rapid preoperative preparationin hyperthyroidism. Clinical Endocrinology 35 439–442. ( 10.1111/j.1365-2265.1991.tb03562.x) [DOI] [PubMed] [Google Scholar]
- 8.Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, et al. 2008. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid 18 333–346. ( 10.1089/thy.2007.0315) [DOI] [PubMed] [Google Scholar]
- 9.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, et al. 2016. American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016 1343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Zhu YJ, Zhong JJ, Zhang J, Weng WW, Liu ZF, Xu Q, Dong MJ. 2016. Characteristics of Antithyroid Drug-Induced Agranulocytosis in patients with hyperthyroidism: a retrospective analysis of 114 cases in a single institution in China involving 9690 patients referred for radioiodine treatment over 15 years. Thyroid 26 627–633. ( 10.1089/thy.2015.0439) [DOI] [PubMed] [Google Scholar]
- 11.Lew WH, Chang CJ, Lin JD, Cheng CY, Chen YK, Lee TI. 2011. Successful preoperative treatment of a Graves’ disease patient with agranulocytosis and hemophagocytosis using double filtration plasmapheresis. Journal of Clinical Apheresis 26 159–161. ( 10.1002/jca.20282) [DOI] [PubMed] [Google Scholar]
- 12.Vyas AA, Vyas P, Fillipon NL, Vijayakrishnan R, Trivedi N. 2010. Successful treatment of thyroid storm with plasmapheresis in a patient with methimazole-induced agranulocytosis. Endocrine Practice 16 673–676. ( 10.4158/EP09265.CR) [DOI] [PubMed] [Google Scholar]
- 13.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH. 2016. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: the seventh special issue. Journal of Clinical Apheresis 31 149–162. ( 10.1002/jca.21470) [DOI] [PubMed] [Google Scholar]
- 14.Shinall MCJ, Broome JT, Nookala R, Shinall JB, Kiernan C, Parks Lr, Solorzano CC. 2013. Total thyroidectomy for Graves’ disease: compliance with American Thyroid Association guidelines may not always be necessary. Surgery 154 1009–1015. ( 10.1016/j.surg.2013.04.064) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a