Abstract
BACKGROUND
Diagnoses of type 1 and type 2 diabetes in youths present a substantial clinical and public health burden. The prevalence of these diseases increased in the 2001–2009 period, but data on recent incidence trends are lacking.
METHODS
We ascertained cases of type 1 and type 2 diabetes mellitus at five study centers in the United States. Denominators (4.9 million youths annually) were obtained from the U.S. Census or health-plan member counts. After the calculation of annual incidence rates for the 2002–2012 period, we analyzed trends using generalized autoregressive moving-average models with 2-year moving averages.
RESULTS
A total of 11,245 youths with type 1 diabetes (0 to 19 years of age) and 2846 with type 2 diabetes (10 to 19 years of age) were identified. Overall unadjusted estimated incidence rates of type 1 diabetes increased by 1.4% annually (from 19.5 cases per 100,000 youths per year in 2002–2003 to 21.7 cases per 100,000 youths per year in 2011–2012, P = 0.03). In adjusted pairwise comparisons, the annual rate of increase was greater among Hispanics than among non-Hispanic whites (4.2% vs. 1.2%, P<0.001). Overall unadjusted incidence rates of type 2 diabetes increased by 7.1% annually (from 9.0 cases per 100,000 youths per year in 2002–2003 to 12.5 cases per 100,000 youths per year in 2011–2012, P<0.001 for trend across race or ethnic group, sex, and age subgroups). Adjusted pairwise comparisons showed that the relative annual increase in the incidence of type 2 diabetes among non-Hispanic whites (0.6%) was lower than that among non-Hispanic blacks, Asians or Pacific Islanders, and Native Americans (P<0.05 for all comparisons) and that the annual rate of increase among Hispanics differed significantly from that among Native Americans (3.1% vs. 8.9%, P = 0.01). After adjustment for age, sex, and race or ethnic group, the relative annual increase in the incidence of type 1 diabetes was 1.8% (P<0.001) and that of type 2 diabetes was 4.8% (P<0.001).
CONCLUSIONS
The incidences of both type 1 and type 2 diabetes among youths increased significantly in the 2002–2012 period, particularly among youths of minority racial and ethnic groups. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Centers for Disease Control and Prevention.)
Diagnoses of type 1 and type 2 diabetes in youths present a substantial clinical and public health burden owing to the challenges of disease management and the risks of acute and chronic complications.1 The SEARCH for Diabetes in Youth study (hereafter, the SEARCH study) previously showed increases in the prevalences of both diseases in the 2001–2009 period.2 However, data on the trends in incidence are needed to understand the current and potential burden of diabetes more fully.
Previous reports have shown that the incidence of type 1 diabetes has increased worldwide over the past three decades.3–8 Data from Australia showed a 5-year sinusoidal cyclical pattern from 2000 through 2011 in the incidence of type 1 diabetes among youths.9 However, a report from Finland suggested a stabilization of the incidence of type 1 diabetes in the 2005–2011 period,10 which was similar to trends in Norway.11 Although several U.S. registries have shown increases in the incidence of type 1 diabetes,12–15 such studies have been limited geographically or did not encompass diverse racial and ethnic groups.16
The SEARCH study previously showed the incidence of type 2 diabetes among children,17 and we are aware of one longitudinal study of incidence trends of type 2 diabetes among youths.18 Here, we report estimated trends in the incidences of type 1 and type 2 diabetes among youths from the five major racial and ethnic groups in the United States.
METHODS
STUDY DESIGN AND DATA COLLECTION
We analyzed data from the SEARCH study, a multicenter observational study that since 2002 has conducted population-based case ascertainment among youths who have received a diagnosis of nongestational diabetes before the age of 20 years.1,19 Youths were identified at five clinical centers — in California (all youths who were Kaiser Permanente Southern California health-plan enrollees in 7 counties), in Colorado (youths from all 64 counties, plus selected Native American reservations in Arizona and New Mexico), in Ohio (youths from 8 counties), in South Carolina (youths from all 46 counties), and in Washington (youths from 5 counties). All the surveillance networks included participating endocrinologists. Additional cases were identified by other health care providers, hospitals, community health centers, clinical and administrative data systems, and diabetes registries.
Case reports were validated on the basis of a physician’s diagnosis of diabetes in the medical record. Eligibility was based on age (<20 years), nonmilitary status, noninstitutionalized status, and county or area of residence for the centers in Colorado, Ohio, South Carolina, and Washington or health-plan membership (Kaiser Permanente Southern California enrollees or, for the Native American reservations coordinated by the Colorado center, Indian Health Service beneficiaries) at the time of diagnosis. After case validation and the deletion of duplicate cases, case patients were registered centrally. Diabetes type was noted as the physician-assigned diabetes type within 6 months after diagnosis. The case-ascertainment window was defined as 30 months after December 31 of each year in which the diagnosis was made (the incident year).
All registered case patients were invited to complete a survey that included questions about race and ethnic group that aligned with the U.S. Census questions. For the incident years of 2002 through 2006 and 2008 and 2012, all youths with diabetes other than diabetes that was due to a secondary cause were invited to a research visit. Written informed consent and assent, when appropriate, were obtained from all the participants or from parents or legal guardians for participants who were too young to provide written consent.19 Blood samples were analyzed for three diabetes autoantibodies — glutamic acid decarboxylase 65 (GAD65)20; insulinoma-associated 2 molecule (IA-2), with the use of a standardized protocol20; and zinc transporter 8 (ZnT8), with the use of a radioassay.21
The study steering committee led and approved the study design, and data were collected under standardized protocols that were approved by the institutional review board at each center, including case ascertainment and registration performed under a Health Insurance Portability and Accountability Act (HIPAA) waiver of written informed consent. The coordinating center was responsible for data quality control and analysis. All the investigators vouch for the completeness and accuracy of the data. Drafts of the manuscript were written by the first author, with all the authors providing review and input. The study publications committee and steering committee approved the manuscript before it was submitted for publication, as did the funding agencies, the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
STATISTICAL ANALYSIS
Patients with type 1 diabetes (including physiciandefined types 1, 1a, and 1b) who were younger than 20 years of age on December 31 of the incident year were included. For type 2 diabetes, we report the incidence rates among youths who were 10 to 19 years of age at diagnosis, because there were too few case patients who were younger than 10 years of age at diagnosis to produce stable rates (137 cases in the 2002–2012 period). Persons with all other types of diabetes, including secondary forms (e.g., diabetes due to cystic fibrosis or glucocorticoid-induced diabetes) were excluded (681 persons in the 2002–2012 period). Race and ethnic group were based on self-report when available from the participant survey (11,480 participants [81%]), from medical records (2217 [16%]), or from geocoding (i.e., assignment of a 2010 Census data–derived racial and ethnic-group proportion) for youths with missing data (394 [3%]).
The annual denominators included youths who were younger than 20 years of age on December 31 of the incident year and who were civilian residents of the geographic study areas, members of Kaiser Permanente Southern California for the included seven counties in California, or Indian Health Service beneficiaries at participating Native American reservations. For the geographically based centers, denominators used the bridged-race intercensal population estimates.22 For Kaiser Permanente Southern California, addresses were geocoded to the Census block level, and race and ethnic-group–specific proportions were applied to estimate the racial and ethnic-group composition of youths according to age and sex. For Native American reservations, the Indian Health Service user population for the previous 3 years was used in accordance with Indian Health Service definitions. Denominator estimates were then summed across all five centers. The distribution of demographic characteristics of the persons included in the denominators used in the current trial has been shown to be very similar to that of the general population in the United States over time.2
The annual incidence rates according to physician-assigned diabetes type were calculated as the number of the valid, registered patients (with duplicate cases deleted), regardless of subsequent participation in study surveys or visits, divided by the number of persons in the surveillance networks over the same period across the five centers. These rates are presented as 2-year moving averages and expressed per 100,000 youths, overall, and according to age group, sex, race or ethnic group, and study center. The 95% confidence intervals for the annual unadjusted rates were calculated with the use of the skew-corrected inverted-score test, assuming a binomial distribution.23 Adjustments for age, sex, race or ethnic group, and estimation of the annual rate of change were performed in a modeling framework.
Trends in incidence were tested with the use of a generalized autoregressive moving average (GARMA) to account for serial correlation.24 Likelihood-ratio tests were performed to compare three possible formulations: a first-order autoregressive and first-order moving-average model (GARMA [1, 1]), a first-order autoregressive model (GARMA [1, 0]), and a first-order moving-average model (GARMA [0, 1]). Model selection suggested that the first-order moving-average model (GARMA [0, 1]) provided the best fit for the majority of models. Trends that were adjusted for age, sex, and race or ethnic group and unadjusted trends in incidence were estimated with the use of a negative binomial distribution with logarithm link.
The model treated the observed number of diagnosed cases in each year as the outcome and the corresponding denominator as an offset. The stratification variable was removed from the list of covariates in each case to avoid multicollinearity. We performed homogeneity-of-effect tests to compare the observed trends in incidence across strata. The GARMA model did not reach convergence in a few cases in which the cell counts were particularly low. Negative binomial regressions were fitted in these cases. Likelihood-ratio tests for quadratic and cubic trends were also considered.
We assessed the completeness of case ascertainment for the four geographically based centers using the capture–recapture method25 in a two-mode ascertainment model. A total of 3068 of the 9782 cases (31%) were from hospital sources only, 270 (3%) were from other sources, and 6444 (66%) were reported by both hospital and other sources. The membership-based center did not have the independent data sources required for this method.
To ensure that trend analyses would not be affected by secular trends in the assignment of diabetes type by physicians, we compared the percentage of youth who had received a diagnosis from a provider of type 1 diabetes or type 2 diabetes with the percentage with type 1 or type 2 diabetes according to our assessment of etiologic type, using the chi-square test and Cochran–Armitage test for trend. Our assessment of etiologic type was based on diabetes autoantibody positivity and insulin resistance,26 as measured in a subgroup of cases that were diagnosed in 2004, 2008, and 2012 for which the participant had a research visit (including 917, 1101, and 1077 participants, respectively, with type 1 diabetes, and 202, 256, and 316, respectively, with type 2 diabetes). To estimate the number of youth in the United States with type 1 or type 2 diabetes, the incidence rates from the SEARCH study were applied to the total U.S. population for the five racial and ethnic groups for the years of interest.
RESULTS
STUDY POPULATION
For the incident years in the 2002–2012 period, a total of 11,245 youths with type 1 diabetes (0 to 19 years of age) were identified from a denominator of 54,239,600 person-years (an average of approximately 4.9 million youths per year in the surveillance networks), and 2846 youths with type 2 diabetes (10 to 19 years of age) were identified from a denominator of 28,029,000 person-years (approximately 2.5 million youths per year in the surveillance networks). Numerators that were based on 2-year moving averages for type 1 diabetes and type 2 diabetes are shown in Table 1. Case numbers according to age, sex, race or ethnic group, and study site are provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. Denominator data according to age, sex, and race or ethnic group are provided in Tables S2 and S3 in the Supplementary Appendix.
Table 1.
Number of Cases of Type 1 Diabetes and Type 2 Diabetes, According to Incident Year.*
| Diabetes Type | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |
| number of cases | ||||||||||
| Type 1 | 938.5 | 916.0 | 957.0 | 1009.5 | 1051.5 | 1091.0 | 1101.5 | 1027.5 | 1035.0 | 1097.0 |
|
| ||||||||||
| Type 2 | 225.5 | 209.5 | 205.0 | 207.0 | 231.0 | 256.0 | 282.0 | 312.0 | 321.5 | 321.5 |
The incident year was calculated as a 2-year moving average. Counts presented are the moving average, which was calculated as the average of the number of cases that were observed in the given incident year and the preceding year.
Capture–recapture analyses revealed consistent estimated completeness of case ascertainment over three time periods (2002–2005, 2006–2008, and 2009–2012) for type 1 diabetes (range, 98.5 to 98.8% complete) and for type 2 diabetes (range, 91.6 to 94.0% complete). The percentage of patients whose physician-diagnosed type 1 diabetes met our etiologic criteria for type 1 diabetes did not differ significantly over time (range, 95.8 to 96.9%; P = 0.60). Similarly, the percentage of patients with physician-diagnosed type 2 diabetes who met our etiologic criteria for type 2 diabetes did not differ significantly over time (range, 84.4 to 89.7%; P = 0.30).
INCIDENCE TRENDS OF TYPE 1 DIABETES
From unadjusted models, a significant upward trend in the incidence of type 1 diabetes was observed overall (from 19.5 cases per 100,000 youths per year in 2002–2003 to 21.7 cases per 100,000 youths per year in 2011–2012; annual increase, 1.4%; P = 0.03), with considerable variation across demographic subgroups of age, sex, and race or ethnic group (Table 2). The incidence decreased in the subgroup of participants who were 0 to 4 years of age (P = 0.03) and increased in the subgroups of participants who were 5 to 9 years of age (P = 0.048) and those who were 15 to 19 years of age (P = 0.03). There was no significant change in the subgroup of participants who were 10 to 14 years of age (P = 0.17). The incidence increased among boys (P = 0.003) but not among girls (P = 0.40). The incidence of type 1 diabetes increased among Hispanic youths (P = 0.009), but the trends were not significant among youths of other racial or ethnic groups. No significant trends were identified within any of the study centers.
Table 2.
Type 1 Diabetes Incidence Rates, with the Use of 2-Year Moving Averages.*
| Subgroup | Year | Unadjusted Model | Adjusted Model† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Annual Increase (95% CI) |
P Value | Annual Increase (95% CI) |
P Value | |
| no. of cases/100,000 youths/yr | % | % | ||||||||||||
| All participants | 19.5 | 19.1 | 19.9 | 20.8 | 21.4 | 22.0 | 22.0 | 20.4 | 20.5 | 21.7 | 1.4 (0.1 to 2.8) | 0.03 | 1.8 (1.0 to 2.6) | <0.001 |
|
| ||||||||||||||
| Age at diagnosis | ||||||||||||||
|
| ||||||||||||||
| 0–4 yr | 16.5 | 16.1 | 14.6 | 14.6 | 14.3 | 14.2 | 14.6 | 13.8 | 14.0 | 14.3 | −1.5 (−2.9 to −0.1) | 0.03‡ | −1.2 (−2.6 to 0.2) | 0.10‡ |
|
| ||||||||||||||
| 5–9 yr | 24.0 | 23.8 | 26.2 | 27.3 | 29.0 | 30.2 | 29.8 | 27.5 | 26.9 | 27.7 | 1.7 (0.0 to 3.4) | 0.048 | 2.5 (1.1 to 3.9) | <0.001 |
|
| ||||||||||||||
| 10–14 yr | 26.4 | 26.6 | 28.4 | 30.0 | 30.4 | 31.8 | 31.9 | 28.8 | 29.1 | 31.8 | 1.2 (−0.5 to 2.9) | 0.17 | 2.1 (0.5 to 3.7) | 0.009 |
|
| ||||||||||||||
| 15–19 yr | 11.0 | 9.7 | 10.4 | 11.4 | 12.3 | 12.5 | 12.5 | 12.0 | 12.1 | 12.9 | 1.8 (0.2 to 3.4) | 0.03‡ | 2.1 (0.5 to 3.6) | 0.009‡ |
|
| ||||||||||||||
| Sex | ||||||||||||||
|
| ||||||||||||||
| Girls | 19.2 | 19.1 | 19.3 | 19.7 | 20.7 | 21.6 | 21.6 | 19.7 | 19.5 | 19.9 | 0.7 (−0.9 to 2.3) | 0.40 | 1.4 (0.3 to 2.5) | 0.01 |
|
| ||||||||||||||
| Boys | 19.8 | 19.0 | 20.4 | 21.7 | 22.0 | 22.4 | 22.4 | 21.0 | 21.4 | 23.4 | 2.1 (0.7 to 3.5) | 0.003 | 2.2 (1.3 to 3.1) | <0.001 |
|
| ||||||||||||||
| Race or ethnic group | ||||||||||||||
|
| ||||||||||||||
| Non-Hispanic white | 23.9 | 23.5 | 24.2 | 25.2 | 26.3 | 27.4 | 27.5 | 25.4 | 25.3 | 27.0 | 0.8 (−0.5 to 2.2) | 0.22 | 1.2 (0.2 to 2.2) | 0.02 |
|
| ||||||||||||||
| Non-Hispanic black | 14.7 | 15.9 | 17.2 | 16.1 | 16.3 | 17.8 | 16.5 | 15.5 | 17.5 | 19.0 | 1.4 (−1.0 to 3.8) | 0.27 | 2.2 (0.4 to 4.1) | 0.02 |
|
| ||||||||||||||
| Hispanic | 13.7 | 12.2 | 13.9 | 16.1 | 16.4 | 16.1 | 16.9 | 16.2 | 15.2 | 14.8 | 3.7 (0.9 to 6.6) | 0.009 | 4.2 (2.5 to 5.9) | <0.001 |
|
| ||||||||||||||
| Asian or Pacific Islander | 7.9 | 7.3 | 7.0 | 8.5 | 8.4 | 6.6 | 6.9 | 5.5 | 7.4 | 9.7 | 4.1 (−2.1 to 10.6) | 0.20 | 3.7 (−0.5 to 8.1) | 0.08 |
|
| ||||||||||||||
| Native American | 6.6 | 5.7 | 3.9 | 4.7 | 5.0 | 4.4 | 5.0 | 5.4 | 5.7 | 6.5 | 1.4 (−5.6 to 8.9) | 0.71‡ | 0.4 (−6.5 to 7.7) | 0.92 |
|
| ||||||||||||||
| Study site | ||||||||||||||
|
| ||||||||||||||
| South Carolina | 16.8 | 16.6 | 17.7 | 17.6 | 18.8 | 20.4 | 19.9 | 19.1 | 19.0 | 18.6 | 1.4 (−0.4 to 3.3) | 0.13 | 2.2 (0.6 to 3.8) | 0.008 |
|
| ||||||||||||||
| Ohio | 24.7 | 27.1 | 25.0 | 23.3 | 23.8 | 24.4 | 24.2 | 25.1 | 25.8 | 26.1 | 0.7 (−0.9 to 2.3) | 0.41‡ | 0.9 (−0.7 to 2.5) | 0.27‡ |
|
| ||||||||||||||
| Colorado | 21.0 | 19.5 | 20.3 | 21.9 | 23.4 | 24.2 | 24.7 | 22.0 | 21.1 | 23.5 | 2.2 (−0.3 to 4.6) | 0.08 | 1.6 (0.4 to 2.9) | 0.01‡ |
|
| ||||||||||||||
| California | 14.5 | 13.9 | 16.5 | 18.0 | 17.4 | 16.4 | 16.7 | 16.8 | 16.1 | 16.4 | 0.9 (−1.4 to 3.2) | 0.45 | 2.0 (0.2 to 3.8) | 0.03 |
|
| ||||||||||||||
| Washington | 21.8 | 21.1 | 21.8 | 23.8 | 23.6 | 24.0 | 23.7 | 20.0 | 22.0 | 24.7 | 1.1 (−1.2 to 3.5) | 0.36 | 1.5 (0.2 to 2.8) | 0.02‡ |
Rates were based on the number of cases and the number at risk in the given year and the preceding year (2-year moving average). P values are from a generalized autoregressive moving average (GARMA) linear model unless otherwise noted. The 95% confidence intervals for the yearly data are provided in Table S4 in the Supplementary Appendix. CI denotes confidence interval.
The analysis was adjusted for age, sex, and race or ethnic group (age was adjusted for sex and race or ethnic group; sex was adjusted for age and race or ethnic group; race or ethnic group was adjusted for age and sex; and study site was adjusted for age, sex, and race or ethnic group).
P values are from linear negative binomial model, because the GARMA model was unable to estimate them.
After adjustment for age, sex, and race or ethnic group, significant (P<0.05) increases in trends were identified in all age groups except the group of participants who were 0 to 4 years of age, among both boys and girls, in each racial and ethnic group except Asian or Pacific Islanders and Native Americans, and within each study center except Ohio (Table 2). However, significant differences in the trends were not observed within demographic subgroups except within the subgroups of race or ethnic group (overall P<0.05).
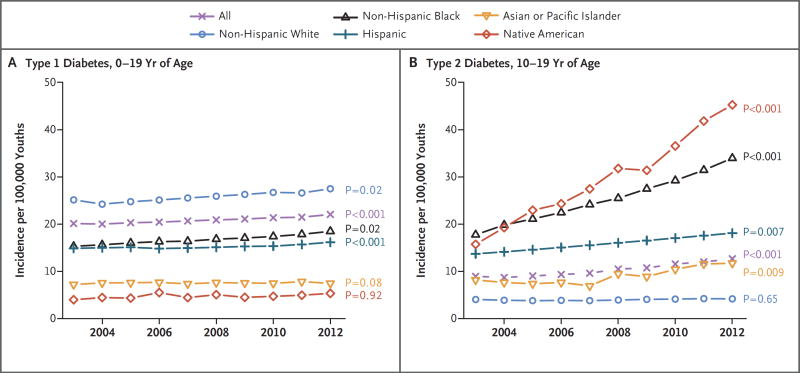
The adjusted incidence of type 1 diabetes increased significantly more among Hispanic youths (annual increase, 4.2%; 95% confidence interval [CI], 2.5 to 5.9) than among non-Hispanic white youths (annual increase, 1.2%; 95% CI, 0.2 to 2.2; P<0.001 for pairwise comparison) (Fig. 1). The test for a quadratic trend was not significant (t = −1.8, P = 0.08), so linear models were retained. We estimated that approximately 15,900 cases of type 1 diabetes were diagnosed annually in the United States in the 2002–2003 period,17 and this number increased to 17,900 cases annually in the 2011–2012 period. Overall, the adjusted annual relative increase in the incidence of type 1 diabetes was 1.8% (95% CI, 1.0 to 2.6; P<0.001).
Figure 1. Model-Adjusted Incidence Estimates.
Shown are model-adjusted incidence estimates per 100,000 youths. The incidence of type 1 diabetes was assessed among participants who were 0 to 19 years of age, and the incidence of type 2 diabetes among participants who were 10 to 19 years of age. P values are for the linear trend tests in each racial or ethnic group, according to type of diabetes. Significant results suggest a positive annual rate of increase during the study period.
INCIDENCE TRENDS OF TYPE 2 DIABETES
Among youths who were 10 to 19 years of age, unadjusted models revealed significant increases in the incidence of type 2 diabetes (from 9.0 cases per 100,000 youths per year in 2002–2003 to 12.5 cases per 100,000 youths per year in 2011–2012; annual increase, 7.1%; P<0.001), with increases observed across all age, sex, race or ethnic-group, and study-site subgroups (P<0.01 for all comparisons) except among non-Hispanic whites and among youths at the Ohio site (Table 3). In adjusted analyses, significant differences within demographic subgroups were observed with respect to race or ethnic group (overall P<0.05) (Table 3 and Fig. 1). Specifically, the pairwise comparisons of the adjusted percent annual increase in incidence showed that the trend among non-Hispanic whites (0.6%; 95% CI, −2.0 to 3.4) was lower than the trends among non-Hispanic blacks, Asians or Pacific Islanders, and Native Americans (P<0.05 for all pairwise comparisons). The trend of the increase in incidence among Hispanics (3.1%; 95% CI, 0.8 to 5.4) differed significantly from that among Native Americans (8.9%; 95% CI, 5.0 to 13.1; P = 0.01).
Table 3.
Type 2 Diabetes Incidence Rates, with the Use of 2-Year Moving Averages.*
| Subgroup | Year | Unadjusted Model | Adjusted Model† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Annual Increase (95% CI) |
P Value | Annual Increase (95% CI) |
P Value | |
| no. of cases/100,000 youths/yr | % | % | ||||||||||||
| All participants | 9.0 | 8.3 | 8.1 | 8.2 | 9.0 | 10.0 | 11.0 | 12.1 | 12.5 | 12.5 | 7.1 (5.2 to 9.0) | <0.001 | 4.8 (3.2 to 6.4) | <0.001 |
|
| ||||||||||||||
| Age at diagnosis | ||||||||||||||
|
| ||||||||||||||
| 10–14 yr | 8.0 | 7.3 | 8.0 | 8.0 | 8.6 | 9.7 | 11.0 | 11.9 | 12.2 | 12.1 | 6.8 (4.3 to 9.4) | <0.001 | 5.1 (3.1 to 7.2) | <0.001 |
|
| ||||||||||||||
| 15–19 yr | 10.0 | 9.4 | 8.3 | 8.3 | 9.5 | 10.3 | 11.0 | 12.3 | 12.7 | 12.9 | 4.3 (2.5 to 6.2) | <0.001‡ | 5.2 (3.2 to 7.2) | <0.001 |
|
| ||||||||||||||
| Sex | ||||||||||||||
|
| ||||||||||||||
| Girls | 11.1 | 9.7 | 9.2 | 9.7 | 11.4 | 12.4 | 13.6 | 15.7 | 15.9 | 16.2 | 9.0 (6.6 to 11.5) | <0.001 | 6.2 (4.2 to 8.2) | <0.001 |
|
| ||||||||||||||
| Boys | 7.0 | 7.0 | 7.1 | 6.6 | 6.8 | 7.7 | 8.5 | 8.7 | 9.2 | 9.0 | 4.2 (1.5 to 6.9) | 0.002 | 3.7 (1.6 to 5.8) | <0.001 |
|
| ||||||||||||||
| Race or ethnic group | ||||||||||||||
|
| ||||||||||||||
| Non-Hispanic white | 4.4 | 4.0 | 3.4 | 3.1 | 3.1 | 3.9 | 4.5 | 4.8 | 4.5 | 3.9 | 3.3 (−0.4 to 7.1) | 0.08 | 0.6 (−2.0 to 3.4) | 0.65 |
|
| ||||||||||||||
| Non-Hispanic black | 20.0 | 19.4 | 19.4 | 19.0 | 22.3 | 24.3 | 26.4 | 30.7 | 31.6 | 32.6 | 6.6 (4.5 to 8.7) | <0.001‡ | 6.3 (4.0 to 8.8) | <0.001 |
|
| ||||||||||||||
| Hispanic | 13.3 | 12.6 | 13.6 | 14.5 | 16.5 | 16.3 | 16.5 | 16.6 | 17.7 | 18.2 | 6.6 (3.6 to 9.7) | <0.001 | 3.1 (0.8 to 5.4) | 0.007‡ |
|
| ||||||||||||||
| Asian or Pacific Islander | 11.0 | 8.1 | 5.7 | 4.1 | 5.4 | 7.2 | 8.9 | 12.9 | 13.2 | 12.2 | 16.0 (7.0 to 25.7) | <0.001 | 8.5 (2.0 to 15.4) | 0.009 |
|
| ||||||||||||||
| Native American | 22.6 | 17.6 | 19.6 | 24.9 | 23.2 | 25.3 | 31.8 | 33.1 | 39.0 | 46.5 | 9.5 (4.8 to 14.4) | <0.001‡ | 8.9 (5.0 to 13.1) | <0.001 |
|
| ||||||||||||||
| Study site | ||||||||||||||
|
| ||||||||||||||
| South Carolina | 10.0 | 10.6 | 9.7 | 8.7 | 10.5 | 13.0 | 15.0 | 16.6 | 16.6 | 16.6 | 9.2 (5.9 to 12.6) | <0.001 | 7.5 (4.9 to 10.1) | <0.001 |
|
| ||||||||||||||
| Ohio | 11.8 | 9.3 | 9.3 | 9.0 | 9.2 | 9.4 | 9.4 | 8.6 | 7.9 | 9.7 | −2.3 (−5.8 to 1.3) | 0.20‡ | −2.6 (−6.3 to 1.2) | 0.18‡ |
|
| ||||||||||||||
| Colorado | 5.8 | 5.2 | 5.8 | 6.7 | 5.9 | 5.8 | 6.9 | 7.7 | 8.0 | 8.3 | 4.6 (1.8 to 7.5) | 0.001‡ | 5.1 (2.2 to 8.1) | <0.001 |
|
| ||||||||||||||
| California | 13.9 | 13.0 | 14.4 | 15.2 | 17.7 | 16.9 | 17.1 | 18.9 | 18.5 | 18.9 | 4.3 (1.3 to 7.4) | 0.004 | 2.8 (0.6 to 5.1) | 0.01‡ |
|
| ||||||||||||||
| Washington | 6.3 | 5.3 | 3.3 | 2.8 | 3.9 | 6.6 | 7.2 | 8.7 | 10.7 | 8.9 | 15.1 (6.5 to 24.4) | <0.001 | 7.9 (3.5 to 12.4) | <0.001‡ |
Rates were based on the number of cases and the number at risk in the given year and the preceding year (2-year moving average). P values are from the GARMA linear model unless otherwise noted. The 95% confidence intervals for the yearly data are provided in Table S5 in the Supplementary Appendix.
The analysis was adjusted for age, sex, and race or ethnic group (age was adjusted for sex and race or ethnic group; sex was adjusted for age and race or ethnic group; race or ethnic group was adjusted for age and sex; and study site was adjusted for age, sex, and race or ethnic group).
P values are from linear negative binomial model, because the GARMA model was unable to estimate them.
Some significant differences according to study center were observed. The incidence of type 2 diabetes increased at all study sites except Ohio (P<0.05 for all adjusted center-specific pairwise contrasts) and increased to a lesser extent in California than in South Carolina (P = 0.04) or Washington (P = 0.004). The test for a quadratic trend was not significant (t = −1.1, P = 0.27). We estimated that approximately 3800 cases of type 2 diabetes were diagnosed annually in the 2002–2003 period,17 and the number increased to 5300 annually in the 2011–2012 period. Overall, after adjustment for age, sex, and race or ethnic group, the annual relative increase in the incidence of type 2 diabetes was 4.8% (95% CI, 3.2 to 6.4; P<0.001).
DISCUSSION
The annual incidence of both type 1 diabetes and type 2 diabetes among youths in the United States showed significant linear increases in the 2002–2012 period. We previously found an increase in the prevalence of type 1 diabetes in the 2001–2009 period2 and an increase in the incidence of type 1 diabetes among non-Hispanic white youths in the 2002–2009 period.16 In the current analyses, the incidence of type 1 diabetes increased among Hispanic youths significantly more than among non-Hispanic white youths. Using data from the Colorado Insulin-Dependent Diabetes Mellitus Study Registry (1978–1988 period) and the SEARCH registry (2002–2004 period), Vehik et al.14 found an annual increase in the incidence of type 1 diabetes among both non-Hispanic white youths and Hispanic youths. From the same population,27 the frequency of the highest-risk type 1 diabetes genotype was higher among children who received a diagnosis between 1978 and 1988 than among those who received a diagnosis between 2002 and 2004. These data suggest an increased contribution of as-yet-unidentified environmental or behavioral factors, such as dietary, infectious, or psychosocial factors, to the incidence of type 1 diabetes.28
The increase in the incidence of type 1 diabetes suggests a growing disease burden that will not be shared equally. Studies have shown substantial differences among racial and ethnic groups in the methods of treatment29,30 and in clinical outcomes,31–34 as well as barriers associated with processes and quality of care.35 These findings highlight the critical need to identify approaches to reduce disparities among racial and ethnic groups.
Previously, we found that the prevalence of type 2 diabetes increased in the 2001–2009 period, with significant increases among non-Hispanic white youths, non-Hispanic black youths, and Hispanic youths. The increase in prevalence was not seen among Asian or Pacific Islander youths or among Native American youths.2 Here, we report a significant annual increase in the incidence of type 2 diabetes in all racial and ethnic groups except non-Hispanic whites. The numbers of cases in the Asian-Pacific Islander and Native American subgroups are markedly lower than in any other subgroup. Thus, the sample size accrued over a period of 11 years may have provided sufficient power to detect significant incidence trends that were not observable in the comparison of prevalence from only two time points.
Although there was no significant increase in the prevalence of obesity among U.S. youths from the 2003–2004 period to the 2011–2012 period overall,36 increases in the prevalence of obesity were observed among Hispanic girls and among non-Hispanic black boys.37 Variations in the underlying prevalence of obesity over time may contribute to variations in insulin resistance and to the increasing incidence of type 2 diabetes. Factors that contribute to compromised insulin secretion are not well known and may include epigenetic dysregulation, which is yet to be elucidated.38
This study has certain limitations. Despite a representative sample,2 a large number of youths in the surveillance networks, and the high estimated proportion of total cases that were ascertained, statistical power was limited in subgroup-specific analyses in demographic subgroups that had a low incidence of type 1 diabetes (e.g., Native Americans) or type 2 diabetes (e.g., non-Hispanic whites). Longer follow-up will be required in order to establish long-term trends.
We found significant increases in the annual incidence of both type 1 diabetes and type 2 diabetes among youths in the United States. We found variation across racial and ethnic groups, including high relative increases in the incidence of type 1 diabetes among Hispanic youths and in the incidence of type 2 diabetes in racial and ethnic groups other than non-Hispanic whites. Variation across demographic subgroups may reflect varying combinations of genetic, environmental, and behavioral factors that contribute to diabetes. As is consistent with the trends as modeled by Imperatore et al.,39 a linear increase in the incidences of type 1 diabetes and type 2 diabetes will substantially increase the number of youths with diabetes in the United States, particularly youths from minority racial and ethnic groups that are a growing proportion of the U.S. population.
Supplementary Material
Acknowledgments
Supported by a grant (1UC4DK108173-01) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and by the CDC. The Population Based Registry of Diabetes in Youth Study (RFP DP15-002) is funded by the CDC and supported by grants at the National Institute of Diabetes and Digestive and Kidney Diseases sites at Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U18DP006139, U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati’s Children’s Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200-2010-35171).
We thank the many youths and their families and the health care providers, whose participation made this study possible.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the official positions of the National Institute of Diabetes and Digestive and Kidney Diseases or the Centers for Disease Control and Prevention (CDC).
Dr. Marcovina reports receiving consulting fees from Denka-Saiken and MedTest DX. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336–44. doi: 10.2337/dc14-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–86. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes — the analysis of the data on published incidence trends. Diabetologia. 1999;42:1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 5.Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60:577–81. doi: 10.2337/db10-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142–7. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 7.Dahlquist G, Mustonen L. Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Acta Paediatr. 2000;89:1231–7. doi: 10.1080/080352500750027628. [DOI] [PubMed] [Google Scholar]
- 8.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide: Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–26. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 9.Haynes A, Bulsara MK, Bower C, Jones TW, Davis EA. Regular peaks and troughs in the Australian incidence of childhood type 1 diabetes mellitus (2000–2011) Diabetologia. 2015;58:2513–6. doi: 10.1007/s00125-015-3709-2. [DOI] [PubMed] [Google Scholar]
- 10.Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA. 2013;310:427–8. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- 11.Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G. Incidence of type 1 diabetes in Norway among children aged 0–14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia. 2014;57:57–62. doi: 10.1007/s00125-013-3090-y. [DOI] [PubMed] [Google Scholar]
- 12.Smith TL, Drum ML, Lipton RB. Incidence of childhood type I and non-type 1 diabetes mellitus in a diverse population: the Chicago Childhood Diabetes Registry, 1994 to 2003. J Pediatr Endocrinol Metab. 2007;20:1093–107. doi: 10.1515/jpem.2007.20.10.1093. [DOI] [PubMed] [Google Scholar]
- 13.Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care. 2013;36:1597–603. doi: 10.2337/dc12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–9. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 15.Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatr. 2012;161(4):652–7. e1. doi: 10.1016/j.jpeds.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence JM, Imperatore G, Dabelea D, et al. Trends in incidence of type 1 diabetes among non-Hispanic white youth in the U.S., 2002–2009. Diabetes. 2014;63:3938–45. doi: 10.2337/db13-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Kim YM, Kwak MJ, et al. Incidence trends and associated factors of diabetes mellitus in Korean children and adolescents: a retrospective cohort study in Busan and Gyeongnam. Ann Pediatr Endocrinol Metab. 2015;20:206–12. doi: 10.6065/apem.2015.20.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SEARCH Study Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–71. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–7. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;135:1–55. [PubMed] [Google Scholar]
- 23.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–26. [Google Scholar]
- 24.Benjamin MA, Rigby RA, Stasinopoulos DM. Generalized autoregressive moving average models. J Am Stat Assoc. 2003;98:214–23. [Google Scholar]
- 25.Verlato G, Muggeo M. Capture-recapture method in the epidemiology of type 2 diabetes: a contribution from the Verona Diabetes Study. Diabetes Care. 2000;23:759–64. doi: 10.2337/diacare.23.6.759. [DOI] [PubMed] [Google Scholar]
- 26.Dabelea D, D’Agostino RB, Jr, Mason CC, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54:78–86. doi: 10.1007/s00125-010-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vehik K, Hamman RF, Lezotte D, et al. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care. 2008;31:1392–6. doi: 10.2337/dc07-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H, Hagopian W. Environmental factors in the development of type 1 diabetes. Rev Endocr Metab Disord. 2006;7:149–62. doi: 10.1007/s11154-006-9024-y. [DOI] [PubMed] [Google Scholar]
- 29.Pihoker C, Badaru A, Anderson A, et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care. 2013;36:27–33. doi: 10.2337/dc12-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424–34. doi: 10.1542/peds.2014-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155(5):668–72. e1–3. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016;17:327–36. doi: 10.1111/pedi.12295. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2006;29:1891–6. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez BL, Dabelea D, Liese AD, et al. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for Diabetes in Youth study. J Pediatr. 2010;157(2):245–251. e1. doi: 10.1016/j.jpeds.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164(6):1369–75. e1. doi: 10.1016/j.jpeds.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168:561–6. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 38.Dayeh T, Ling C. Does epigenetic dysregulation of pancreatic islets contribute to impaired insulin secretion and type 2 diabetes? Biochem Cell Biol. 2015;93:511–21. doi: 10.1139/bcb-2015-0057. [DOI] [PubMed] [Google Scholar]
- 39.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35:2515–20. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.