Abstract
Context:
Irreversible airway obstruction is important sequelae of pulmonary tuberculosis (TB) that might contribute to a significant proportion of chronic obstructive pulmonary disease (COPD). India has the highest TB burden in the world. However, there are limited data on the prevalence and presentation of TB-associated COPD from this region.
Aims:
This study aims to evaluate the prevalence of TB-associated COPD among COPD patients presenting to a tertiary care hospital.
Settings and Design:
It was a case–control study conducted in a tertiary care hospital.
Subjects and Methods:
Stable COPD patients presenting to chest OPD and an equal number of healthy controls were enrolled. COPD patients were subjected to detailed clinical evaluation and lung function test. History of pulmonary TB was evaluated from both groups through self-reporting and/or checking previous records. TB-associated COPD patients were identified and their prevalence and distinguishing features evaluated.
Results:
Of 74 COPD patients, 24 (32.4%) had previous history of pulmonary TB. The odds of having a previous TB in COPD patients was 3.96 (95% confidence interval: 1.64–9.55; P = 0.002) as compared to controls. Patients with TB-associated COPD were younger (P = 0.02), had lesser pack-years of smoking (P = 0.027) but had more number of hospitalizations (P = 0.01). The airflow limitation was similar in both groups.
Conclusions:
TB-associated COPD constitutes a significant proportion of COPD patients. It is a distinct clinical entity with preponderance in young. It may be associated with frequent hospitalizations as compared to other COPD patients.
KEY WORDS: Airflow limitation, chronic obstructive pulmonary disease, pulmonary tuberculosis
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is estimated to affect 65 million people worldwide and is currently the third leading cause of death. Of the total number of deaths, 90% are in low- and middle-income countries which also harbor maximum number of pulmonary tuberculosis (TB).[1]
Apart from tobacco smoke, COPD has been known to occur in patients with previous pulmonary TB.[2,3] This COPD phenotype has been variably termed as posttubercular obstructive airway disease or TB-associated COPD.[3] Most of the evidence on the causative association between TB and COPD has been derived from studies on lung function evaluation in treated TB patients or from population-based surveys on COPD.[4,5,6,7,8] The difference in the study designs, criteria used for COPD diagnosis and previous TB detection has led to variable results in these studies. Moreover, the presence of confounding factors such as tobacco smoking, exposure to biomass fuel, and childhood respiratory illnesses has limited the strength of their association.
India is the highest TB burden country in the world and hence is likely to harbor significant burden of TB-associated COPD. Furthermore, India has a growing population of COPD and is in second place for harboring the most number of morbidity and mortality cases from obstructive airway disease, after China.[9] Ironically, there is paucity of studies on TB-associated COPD from this geographical region.[10,11] Moreover, it is not known whether the clinical presentation of this COPD phenotype is different from smoking-related COPD that might necessitate a different approach to its management.[12]
Hence, we planned to conduct a retrospective case–control study to find out hospital-based prevalence of TB-associated COPD among COPD patients and to evaluate its characteristic features.
SUBJECTS AND METHODS
It was a case–control study conducted in a tertiary care hospital over a period of 6 months (April 2016 to September 2016). Seventy-four consecutive patients of stable COPD attending chest OPD were enrolled as cases. An equal number of healthy subjects with similar age and gender distribution were taken as controls. Informed consent was taken from all subjects.
COPD patients with other pulmonary comorbidities such as obstructive sleep apnea, interstitial lung disease, lung cancer, congestive heart failure, unstable angina, recent myocardial infarction, and acute exacerbation of COPD in the past 4 weeks were excluded from the study. The study was approved by the Institutional Ethics Committee.
Detailed clinical history and medical examination were done with an emphasis on type and duration of symptoms, number of previous exacerbations, previous hospitalizations, tobacco smoke exposure, and occupational exposures to dust and smoke. The history of previous TB was elucidated through self-reporting by patients and/or by checking their medical records, if available. Specific points such as number of anti-tubercular treatment (ATT) courses, total duration of treatment, time elapsed since completion of treatment and their final outcome were recorded. Routine spirometry was performed as per the recent ATS guidelines[13] using RMS Helios 401 PC-based Spirometer. Postbronchodilator forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC values were recorded based on which patients were categorized into four stages of airflow limitation as per recent GOLD guidelines.[14] Room air oxygen saturation level was noted, body mass index (BMI) and routine X-ray chest (CXR) posteroanterior view was done.
Patients were labeled as TB-associated COPD only if their symptom of COPD started after the episode of pulmonary TB. The prevalence of TB-associated COPD group was calculated, and its different parameters were compared with rest of COPD patients.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation, median with range or frequencies, wherever appropriate. Comparison of continuous variables between two groups was done using Student's t-test for independent variables. Correlation between different variables was carried out using Pearson correlation. P < 0.05 was considered statistically significant. All statistical calculations were done using computer program (IBM) SPSS Statistics version 21.0.
RESULTS
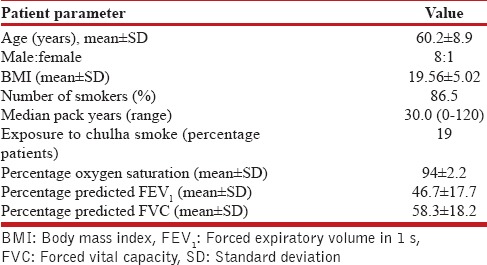
Elderly males comprised the majority of COPD patients in our study. Out of 74 patients, 64 (86.5%) had a history of >10 pack-years of tobacco smoking, out of which 19 were current smokers [Table 1].
Table 1.
Baseline features of chronic obstructive pulmonary disease patients (n=74)
The distribution of patients among different COPD stages is shown in Figure 1. Median duration of onset of symptoms was 4 years (range: 2 months–25 years). History of exacerbation in the preceding year was present in 31% (n = 23) patients. Thirty-five patients had a history of the previous hospitalization related to COPD. There was a history of 51 COPD-related hospitalizations in 35 patients that occurred at any point during their illness.
Figure 1.
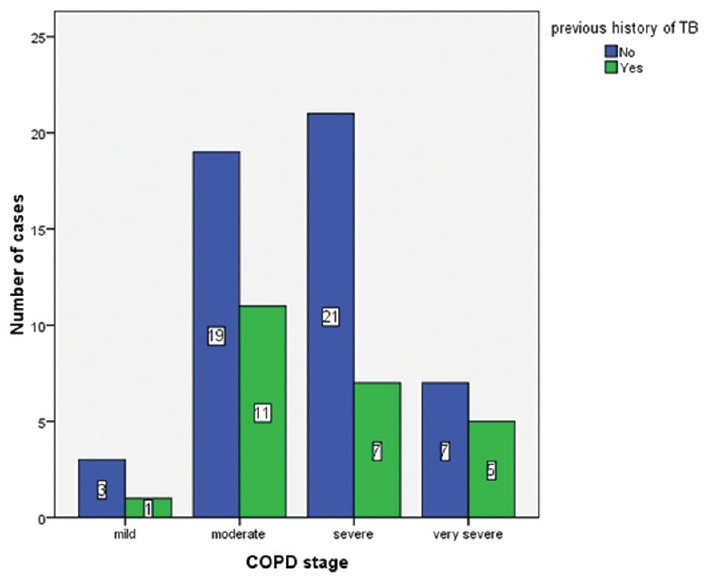
Distribution of patients among different stages of chronic obstructive pulmonary disease
Prevalence of tuberculosis-associated chronic obstructive pulmonary disease
Twenty-four patients (32.4%) in the COPD group and 8 patients in the control group had a history of TB. The unadjusted odds ratio for previous TB in the COPD group in comparison to controls was 3.96 (95% confidence interval: 1.64–9.55; P = 0.002). Median time elapsed since the completion of ATT was 4 years (range 1.5–35 years) in TB-associated COPD. Most of the patients (21/24) had taken a single course of ATT.
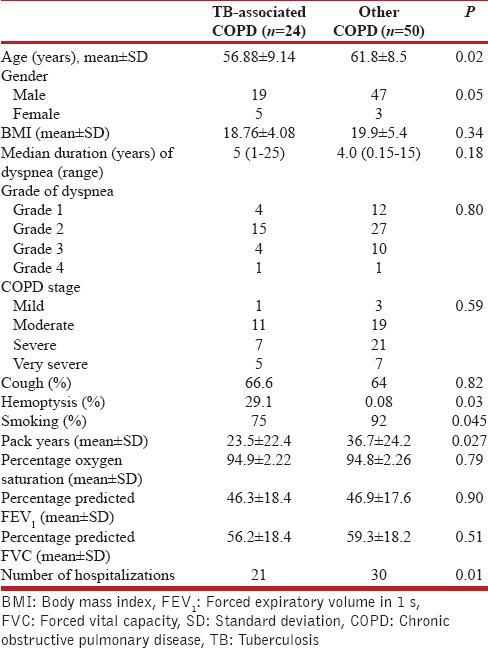
Comparison between tuberculosis-associated chronic obstructive pulmonary disease and other chronic obstructive pulmonary disease
TB-associated COPD patients were younger in age as compared to the rest of COPD patients (P = 0.02) with more proportion of females. They had similar symptoms except for high incidence of hemoptysis and frequent hospitalizations. Pack-years of smoking were less although airflow limitation was similar to other COPD patients [Table 2].
Table 2.
Comparison of features between tuberculosis-associated chronic obstructive pulmonary disease with other chronic obstructive pulmonary disease patients
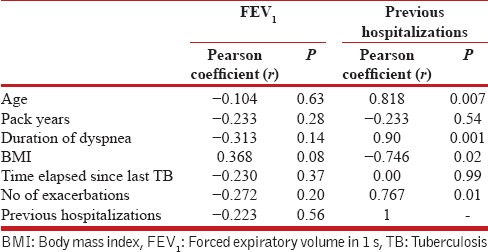
In TB-associated COPD patients, on univariate analysis, number of previous hospitalizations showed a positive correlation with age, duration of dyspnea, number of exacerbations in the last year and BMI [Table 3]. However, on multivariate analysis, duration of dyspnea retained positive correlation with hospitalizations (r = 0.82; P = 0.022) after adjusting for pack-years, age, and BMI. On the contrary, FEV1 did not have shown correlation with any of the parameters [Table 3].
Table 3.
Correlation of forced expiratory volume in 1 s and number of hospitalizations with different parameters in tuberculosis-associated chronic obstructive pulmonary disease
DISCUSSION
The present study was conducted to find out hospital-based prevalence of TB-associated COPD and to evaluate its characteristic features. The results showed that almost one-third of COPD patients (32.4%) had associated TB in the past. It was seen that patients with TB-associated COPD, despite being younger in age and having lesser pack-years of smoking, had similar grades of airway obstruction as compared to smoking-related COPD. This gives strong evidence in favor of casual association between TB and development of COPD.
In contrast to our study design, most of the published data on the association between TB and COPD has been derived from studies on previously treated TB patients. In a series of cohort studies, airflow obstruction was found in the range of 28%–70% in previously treated TB patients[4,15] whereas it was in the range of 11%–58%.[16] in hospital-based cross-sectional studies. In one of the largest population-based PLATINO studies, airflow obstruction was seen in 30.7% patients with previous TB as compared to 13.9% among those without a history.[7] In contrast, we evaluated “TB-associated COPD” among COPD patients at a tertiary care level in a case–control study design. We could not find other Indian study with a similar design in published literature.
In a landmark, population-based study (BOLD study)[6] on COPD in never smokers, authors did not found any association of TB in the development of COPD. In their study, 5.3% COPD patients had a history of the previous TB which is significantly lower than our results. This is likely due to the fact that the study was done in never smokers which excluded the causative effect of smoking in both COPD and TB.[17,18] Moreover, it was a community-based study from low TB prevalence countries which might have underscored the association between the two diseases. Our study enrolled COPD patients reporting to hospital with some symptoms and hence might have a higher chance of the previous TB. Another study from India enrolling patients with heavy pack years of smoking showed 57% prevalence of the previous TB in COPD patients.[19]
Different mechanisms have been proposed for the development of COPD in TB patients. It includes endobronchial involvement causing airway obstruction, bronchiolar narrowing, and bronchiolitis obliterans resulting from peribronchial fibrosis and accelerated emphysematous changes caused by residual chronic or recurrent inflammation affecting lung compliance.[16,20] A common link to the pathogenesis of both the conditions may lie in the destruction of pulmonary extracellular matrix due to increased activity of matrix metalloproteinases enzymes precipitated by TB.[21]
Risk of COPD has been found to increase with increase in radiological extent of TB,[4] increase in number of previous TB episodes[22] as well as with delay in initiating anti-TB therapy.[23] This could not be validated in our study as around 90% of patients with TB-associated COPD had a history of a single episode of TB as well as had minimal CXR changes (unilateral involvement and apical linear fibrotic strands). The results are similar to a Korean study which also showed lower FEV1 in TB patients with minimal CXR changes.[24] Moreover, our study did not include patients with mixed obstruction and restriction which are more likely to present with extensive CXR changes. The inclusion of these patients might has increased the prevalence of TB-associated COPD in our study.
Clinical behavior of TB-associated COPD in comparison to smoking-related COPD is not well defined. In our study, TB-associated COPD patients were younger in age as compared to other COPD which is likely due to the fact that TB is a disease of young adults as compared to smoking-related COPD which is uncommon in this age group.[12] Furthermore, TB-associated COPD patients had similar symptoms in our study except for high frequency of hemoptysis. The results were in accordance with another Korean study that showed no difference in the presentation of COPD patients with and without previous TB.[25] However, in contrast to our study, the authors showed a higher airway resistance in the TB-COPD group. In our study, there was no correlation between FEV1 and duration of symptoms in TB-COPD patients which hints toward nonprogressive nature of airflow limitation in TB-associated COPD. However, the fact can only be validated in longitudinal studies on TB-COPD patients with large sample size. Another interesting finding in our study was the higher number of hospitalizations seen in these patients that showed a positive correlation with age, BMI, and duration of symptoms. This was in contrast to a Korean study which did not show any difference in hospitalizations in the two groups.[25] Structural changes such as fibrosis, bronchiectasis, cavity occurring as sequelae of TB may act a nidus for recurrent infections in TB-associated COPD patients that warrant hospitalization.[19]
Our study gave a real life picture of TB-associated COPD giving an insight into its prevalence encountered in a routine OPD. It also suggests that adequate TB control might also aid in reducing the prevalence of TB-associated COPD. However, being a case–control study with an inherent recall bias associated with it, the causal association between TB and COPD could not be confirmed. TB-associated COPD can occur after a variable period of TB occurrence, ranging from the point of TB diagnosis[2] to several years after the treatment has ended. Moreover, airflow limitation seen in it may resolve with ATT and bronchodilators.[15] Hence, following TB patients till any point of time in a cohort study design may not give a true picture of their association. Evaluating COPD patients for the previous TB in a retrospective study design, similar to our study, seem to be a better platform to evaluate this COPD phenotype. Effect of confounding factors notably tobacco smoking could not be excluded in our study. Nevertheless, it did not seem to affect the outcome as the number of pack-years was significantly less in patients with TB-associated COPD as compared to other COPD patients.
CONCLUSION
TB-associated COPD constitutes a substantial proportion of COPD in a hospital setting. It is a distinct clinical entity different from a larger smoking-related COPD and hence may require different management. Being a high TB burden country, there is a need to conduct large-scale studies and population surveys on COPD patients in India to find out the burden and course of TB-associated COPD. Further, longitudinal studies on TB-associated COPD in never smokers might help to formulate guidelines for its management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birath G, Caro J, Malmberg R, Simonsson BG. Airways obstruction in pulmonary tuberculosis. Scand J Respir Dis. 1966;47:27–36. [PubMed] [Google Scholar]
- 3.Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis. 1971;103:625–40. doi: 10.1164/arrd.1971.103.5.625. [DOI] [PubMed] [Google Scholar]
- 4.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med. 1989;83:195–8. doi: 10.1016/s0954-6111(89)80031-9. [DOI] [PubMed] [Google Scholar]
- 5.Zakaria M, Moussa H. Chronic obstructive pulmonary disease in treated pulmonary tuberculous patients. Egypt J Bronchology. 2015;9:10–3. [Google Scholar]
- 6.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: Results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menezes AM, Hallal PC, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, et al. Tuberculosis and airflow obstruction: Evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30:1180–5. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 8.Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study) Chest. 2008;133:343–9. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- 9.Kalkana T, Moitra S, Jindal SK, Moitra S. Increasing burden of COPD in rural India: An example why India warrants primary healthcare reforms. ERJ Open Res. 2016;2:pii: 00032-2016. doi: 10.1183/23120541.00032-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma SK, Kumar S, Narayan K, Sodhi R. Post tubercular obstructive airway impairment. Indian J Allergy Asthma Immunol. 2009;23:95–9. [Google Scholar]
- 11.Gothi D, Shah DV, Joshi JM. Clinical profile of diseases causing chronic airflow limitation in a tertiary care centre in India. J Assoc Physicians India. 2007;55:551–5. [PubMed] [Google Scholar]
- 12.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. [Last accessed on 2016 Dec 01]. Available from: http://www.goldcopd.org/
- 15.Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998;12:351–6. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]
- 16.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86:76–85. doi: 10.1159/000350917. [DOI] [PubMed] [Google Scholar]
- 17.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med. 2007;4:e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies PD, Yew WW, Ganguly D, Davidow AL, Reichman LB, Dheda K, et al. Smoking and tuberculosis: The epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg. 2006;100:291–8. doi: 10.1016/j.trstmh.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishna R, Kumar P. Tuberculosis airway disease and bronchiectasis – A debilitating trio. J Evid Based Med Health. 2016;3:818–22. [Google Scholar]
- 20.Chakrabarti B, Calverley PM, Davies PD. Tuberculosis and its incidence, special nature, and relationship with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2:263–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–66. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–8. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CH, Lee MC, Lin HH, Shu CC, Wang JY, Lee LN, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One. 2012;7:e37978. doi: 10.1371/journal.pone.0037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SW, Kim YS, Kim DS, Oh YM, Lee SD. The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci. 2011;26:268–73. doi: 10.3346/jkms.2011.26.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003;97:1237–42. doi: 10.1016/s0954-6111(03)00255-5. [DOI] [PubMed] [Google Scholar]


