Abstract
Transtracheal oxygen therapy is a well-established modality for improving oxygenation in patients with chronic obstructive pulmonary disease, sleep apnea, pulmonary fibrosis, and other conditions causing hypoxic respiratory failure. In spite of its proven track record, the device remains underutilized. This article reviews benefits and complications related to the use of this modality with an illustrative case presentation.
KEY WORDS: Chronic hypoxic respiratory failure, granulation tumor and tracheal ulcer, transtracheal oxygen therapy
INTRODUCTION
Heimlich pioneered transtracheal oxygen therapy (TTOT) in 1982, using a 16-gauge intravenous catheter.[1] Three years later, he reported successful use of TTOT in 100 patients.[2] Subsequent efforts by Christopher et al.,[3] Kampelmacher,[4] and, Hoffman et al.,[5] showed improvement in compliance with oxygen therapy and demonstrated the safety and efficacy of this therapeutic modality, which was cosmetically more pleasing than nasal cannulas and allowed for lower flow rates.[6] Despite a demonstrated role for TTOT in severely hypoxemic patients, this therapeutic modality is not without complications, some of which are not broadly described in the available literature. We present a review of the literature regarding the complications of TTOT and an illustrative case that highlights some unexpected potential complications, as well. In addition, we provide a discussion about therapeutic approaches to minimize these complications.
ILLUSTRATIVE CASE
A 66-year-old man with rheumatoid arthritis-associated interstitial lung disease (ILD) and obstructive sleep apnea (OSA) was evaluated for daytime hypoxia refractory to continuous nasal oxygen supplementation at 5 L/min. He was also on nocturnal continuous positive airway pressure (CPAP) at 10 cm of water pressure. Based on the authors' prior experience[7] and data supporting the use of TTOT in patients with OSA,[8] this patient was deemed appropriate for TTOT. Using the Seldinger technique, he underwent a two-stage procedure for insertion of an 11 cm TTOT catheter without any procedure-related complication. On subsequent follow-up, he had adequate oxygen saturations and increased exercise tolerance. Nevertheless, 6 months later he experienced problems related to recurrent blockage of the catheter with mucus plugs and difficulty reinserting the catheter after cleaning it. On one occasion, he was unable to reinsert it and unfortunately presented to our pulmonary office 3 days later. By then, the physician was unsuccessful at replacing the catheter in the usual manner, and the catheter was later replaced under bronchoscopic guidance. Bronchoscopy revealed a granulation tumor (8 mm × 5 mm × 2.5 mm) on the anterior tracheal wall just superior to the site of insertion of the TTOT catheter [Figure 1]. In addition, there was an area of superficial ulceration on the middle third of the posterior tracheal wall. A new stage 1 catheter (for tract formation) was then placed, and the area of granulation tumor was treated with cryotherapy using a 2.4 mm cryoprobe (Erbe USA Inc., Marietta GA, USA). The patient returned 8 days later for placement of the 11 cm TTOT definite catheter and for airway reinspection. The granulation tumor responded well to cryotherapy demonstrating white necrotic material, which was debulked with further cryotherapy performed to its base. Biopsies of this area confirmed necrotic granulation material. The area of previous ulceration had healed, and the new 11.0 cm TTOT catheter was placed without any difficulty. The patient was also given a 9.0 cm catheter so that he could alternate between the 9.0 cm and 11.0 cm catheters to avoid potential ulceration of the posterior tracheal wall related to the catheter. He was also prescribed nebulized albuterol 4 times a day and oral guaifenesin.
Figure 1.
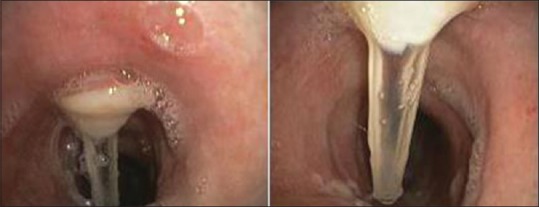
Bronchoscopic view of the anterior tracheal wall showing entry of the transtracheal catheter and the granulation tissue surrounding then entry point. Once catheter is removed for cleaning this potentially can occlude the lumen of catheter entrance
He returned 2 months later reporting hemoptysis. He had not alternated catheters, as instructed. Repeat bronchoscopy demonstrated a linear superficial ulcer on the posterior wall which was 1.0 cm × 0.2 cm in dimension and extended 1.0 cm beyond the visualized distal tip of the catheter, but noticing the catheter could extend into the distal portion of this ulcer with neck movements. The catheter was then replaced with a new one that was shortened to 7.5 cm to avoid further traumatizing the area of ulceration. Further bronchoscopic cryotherapy was done to treat an area of mild residual granulation tissue superior to the catheter insertion site. He was discharged with instructions not to use the TTOT catheter for the following 2 weeks. Repeat bronchoscopy 2 weeks later showed complete healing of the posterior tracheal wall ulcer [Figure 2], but there was still mild residual granulation tissue (5 mm × 3 mm) at the superior edge of the catheter entrance point, which was further treated with cryotherapy. He was instructed to resume TTOT after 2 weeks and use a 9 cm catheter initially. He was instructed to cap the catheter at night and use CPAP through full face mask with in-line oxygen instead to minimize mucosal desiccation. He was again instructed to continue to alternate from the 9 cm to 11 cm TTOT catheter every 2 weeks. Following this intervention, he progressed well with good tolerance and without any complication.
Figure 2.
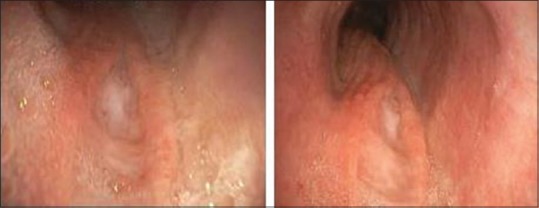
Bronchoscopic views of the healing ulcer on the posterior tracheal wall
DISCUSSION AND REVIEW OF THE LITERATURE
TTOT benefits include improved patients' compliance with oxygen therapy and have proved to be cosmetically more appealing. It is preferred over nasal oxygen by most patients as it allows the tubing to be hidden under clothing. There is a less nasal irritation which further improves patients' comfort with the therapy and thereby improving compliance. It seems well accepted by nearly all patients as Kampelmacher reported a 97% acceptance rate.[4]
As it improves oxygenation at lower flow rates, consequentially it increases exercise tolerance. Furthermore, by insufflating oxygen near the carina, the anatomic dead space is decreased, which increases alveolar ventilation and thereby decreases work of breathing.[9] Eventually patients are less homebound as they can be away from home longer with decreased oxygen flow rates. The later should impact quality of life.
Given these benefits, TTOT became a useful tool for rehabilitating primarily chronic obstructive pulmonary disease (COPD) patients in the early 80s.[10,11,12] A growing body of evidence supported its use in several other conditions such as ILD,[2,13] OSA,[7,14,15,16] and in patients with overlap syndrome.[7] While initially used to conserve oxygen (allowing increased oxygen delivery time with the same size reservoirs) in ambulatory COPD patients, more recently it was proven to be effective in treating severe refractory hypoxemia in patients with advanced lung diseases, including pulmonary fibrosis.
In spite of its proven record, the device remains underutilized, partly because of perceived misconceptions among physicians related to its complications[17] and partly related to complications specific to its use. Some complications associated with TTOT are linked to the type of technique used to place the transtracheal oxygen catheter. The modified Seldinger technique and the fast tract (FT) procedure are the most commonly employed methods. In the former, a “stent” catheter is initially placed using a guidewire which is then replaced with a functional TTOT catheter after 1 week.[18,19] Tract maturation usually requires 6 weeks, and the most common complications encountered are catheter dislodgments and formation of keloids.[4] These complications are presumably less frequent with the newer FT method though it is noted there appears to be less experience with this technique. This was developed by Alan Lipkin, and though it is a more aggressive initial surgical approach (therefore not always the best initial approach for all), it arguably offers some benefits including earlier initiation of TTOT, rapid tract maturation, and reduced incidence of lost tracts.[20] Nevertheless, the modality of initial catheter insertion does not prevent the development of other long-term complications. These include mucous ball formation, tract granulation and stenosis, tracheal ulcerations, and hemoptysis. Our case illustrates a patient with refractory hypoxemia where TTOT was beneficial, but in whom several complications developed, all of which were successfully managed.
The most common postoperative complication is formation of mucous balls within the catheter. In 1998, Orvidas et al. published their experience with TTOT and noted that this complication occurred in up to 38% of the patients receiving TTOT.[21] Harrow et al.[22] further reported cases where large mucus plugs resulted in partial tracheal obstruction and in one case report, the mucus plugging of the catheter even resulted in the patient's death due to complete tracheal obstruction.[23] Similarly, it is noted that patients can often develop mucus impaction leading to dyspnea, therefore indicating the need for frequent TTOT catheter changes. The latter can also on occasions lead to malplacement of the catheter with formation of granulation tumor at the insertion site.
Hoffman et al.[24] described a technique of prophylactic catheter “stripping” to prevent mucous ball formation. This involves a visit to doctor's office where, after topical lidocaine administration, the catheter is removed over a guidewire. The guidewire is then left in place (or can be “held” in place for safety) while the catheter is being cleaned with antimicrobial soap water and then reinserted over the same guidewire. If a mucus ball is identified, then the patient is seen more frequently for “stripping” particularly in the early postoperative period.[25] Importantly, the frequency of these visits, use of guidewire at home, and use of humidification can be tailored according to each patient's unique needs.
Erosions of the tracheal or bronchial mucosa due to TTOT have also been previously reported. Sciurba et al.[13] noted mucosal ulcerations in two patients of small stature with restrictive lung disease who presented with cough and hemoptysis. In both patients, the 11.0 cm catheter was replaced with a 9.0 cm catheter with a significant symptomatic resolution. Walsh and Govan[26] in their case review of 37 patients reported hemoptysis in 2 patients, associated with ulceration of the posterior tracheal wall. Another case report[27] documented ulcerative tracheitis while Menon et al.[28] reported a posterior tracheal wall perforation resulting in pneumothorax and pneumomediastinum prompting discontinuation of TTOT. While these complications are uncommon, they require immediate recognition and prompt correction.
Formation of granulation tumor at the insertion site of the TTOT catheter has been previously reported by Punzal et al., and in their report, tracheal obstruction occurred due to granulation tissue at the site of the catheter in a patient with COPD, who was successfully treated with neodymium-doped yttrium aluminum garnet laser therapy.[29] The physician should maintain a high index of suspicion for this complication. Patients who experience difficulty removing and replacing TTOT catheters should undergo early bronchoscopic evaluation to identify possible granulation tissue formation. This allows early detection and ablation of the granulation tissue, preventing potentially life-threatening complications. Cryotherapy should be considered as a potential first-line therapeutic modality for the treatment of granulation tissue formation associated with the TTOT catheter in patients requiring high oxygen flow rates because of the risk of airway fires with laser coagulation or use of electrocautery techniques.
Formation of ulcers on the posterior tracheal wall as a result of oxygen flow directed at the mucosa was also noted in our case. This is different from “friction-related” mucosal damage as previously reported. The “oxygen flow-related” ulcer formation can be deep and usually extends beyond the distal edge of the TTOT catheter. Ulcers can lead to fistula formation if left unattended. Prompt bronchoscopic evaluation if patients develop hemoptysis while being treated with TTOT is indicated. In certain cases, consideration should also be made to alter the length of the TTOT catheter to avoid both catheter-associated friction-related tracheal wall injury and/or flow-related mucosal ulceration. The latter is of particular concern in patients with severe hypoxemia where high requirements of oxygen flow will cause mucosal desiccation, which can be prevented by alternating catheter length (to avoid hitting always the same spot), but also by intermittently switching to alternate oxygen delivery modalities (either nasal cannula or positive airway pressure with in-line oxygen supplementation), as clinically indicated.
In summary and though there is a lack of studies supporting specific methods to reduce the incidence of complications associated with long-term TTOT, the concomitant use of humidified oxygen, scheduled nebulized albuterol, guaifenesin, and the avoidance of TTOT at night could potentially reduce the incidence of some of these complications. Finally, as noted above, the use of alternating catheter lengths may prevent the same area of mucosa from being continuously exposed to the desiccating effects of high oxygen flow rates, thereby minimizing the development of ulcerative lesions and associated hemoptysis. It must be understood that desiccation of the mucosa can lead to severe mucosal lesions, regardless of the underlying pathological process.
Despite the above complications, TTOT has therapeutic and important palliative benefits in patients with serious, end-stage lung disease. This unique case demonstrates “oxygen flow-related ulcers” and subsequent granulation tumors which complicate catheter insertion and maintenance. These complications can be proactively dealt with the use of bronchoscopic techniques such as cryotherapy and laser coagulation. High awareness of these potential complications leads to improve management. The early institution of techniques, such as alternating the length of the TTOT catheter, frequent surveillance bronchoscopies, and airway humidification, can help prevent serious complications. In turn, a good understanding of the indications and complications of TTOT can help us safely reincorporate this rather “forgotten” technique of oxygen therapy in clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Heimlich HJ. Respiratory rehabilitation with transtracheal oxygen system. Ann Otol Rhinol Laryngol. 1982;91(6 Pt 1):643–7. doi: 10.1177/000348948209100626. [DOI] [PubMed] [Google Scholar]
- 2.Heimlich HJ, Carr GC. Transtracheal catheter technique for pulmonary rehabilitation. Ann Otol Rhinol Laryngol. 1985;94(5 Pt 1):502–4. doi: 10.1177/000348948509400518. [DOI] [PubMed] [Google Scholar]
- 3.Christopher KL, Spofford BT, Brannin PK, Petty TL. Transtracheal oxygen therapy for refractory hypoxemia. JAMA. 1986;256:494–7. [PubMed] [Google Scholar]
- 4.Kampelmacher MJ, Deenstra M, van Kesteren RG, Melissant CF, Douze JM, Lammers JW. Transtracheal oxygen therapy: An effective and safe alternative to nasal oxygen administration. Eur Respir J. 1997;10:828–33. [PubMed] [Google Scholar]
- 5.Hoffman LA, Wesmiller SW, Sciurba FC, Johnson JT, Ferson PF, Zullo TG, et al. Nasal cannula and transtracheal oxygen delivery. A comparison of patient response after 6 months of each technique. Am Rev Respir Dis. 1992;145(4 Pt 1):827–31. doi: 10.1164/ajrccm/145.4_Pt_1.827. [DOI] [PubMed] [Google Scholar]
- 6.Christopher KL, Spofford BT, Petrun MD, McCarty DC, Goodman JR, Petty TL. A program for transtracheal oxygen delivery.Assessment of safety and efficacy. Ann Intern Med. 1987;107:802–8. doi: 10.7326/0003-4819-107-6-802. [DOI] [PubMed] [Google Scholar]
- 7.Biscardi FH, Rubio ER. Transtracheal oxygen and positive airway pressure: A salvage technique in overlap syndrome. Lung India. 2014;31:67–9. doi: 10.4103/0970-2113.125988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farney RJ, Walker JM, Elmer JC, Viscomi VA, Ord RJ. Transtracheal oxygen, nasal CPAP and nasal oxygen in five patients with obstructive sleep apnea. Chest. 1992;101:1228–35. doi: 10.1378/chest.101.5.1228. [DOI] [PubMed] [Google Scholar]
- 9.Nahum A. Equipment review: Tracheal gas insufflation. Crit Care. 1998;2:43–7. doi: 10.1186/cc124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimlich HJ, Carr GC. The micro-trach.A seven-year experience with transtracheal oxygen therapy. Chest. 1989;95:1008–12. doi: 10.1378/chest.95.5.1008. [DOI] [PubMed] [Google Scholar]
- 11.Heimlich HJ. Oxygen delivery for ambulatory patients.How the micro-trach increases mobility. Postgrad Med. 1988;84:68. doi: 10.1080/00325481.1988.11700463. [DOI] [PubMed] [Google Scholar]
- 12.Banner NR, Govan JR. Long term transtracheal oxygen delivery through microcatheter in patients with hypoxaemia due to chronic obstructive airways disease. Br Med J (Clin Res Ed) 1986;293:111–4. doi: 10.1136/bmj.293.6539.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sciurba FC, Hoffman LA, Wesmiller SW, Mazzocco MC, Dauber JH. The use of a short-length transtracheal oxygen catheter in patients of small stature with restrictive lung disease. Chest. 1992;101:1165–7. doi: 10.1378/chest.101.4.1165. [DOI] [PubMed] [Google Scholar]
- 14.Sériès F, Forge JL, Lampron N, Cormier Y. Transtracheal air in the treatment of obstructive sleep apnoea hypopnoea syndrome. Thorax. 2000;55:86–7. doi: 10.1136/thorax.55.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauncey JB, Aldrich MS. Preliminary findings in the treatment of obstructive sleep apnea with transtracheal oxygen. Sleep. 1990;13:167–74. [PubMed] [Google Scholar]
- 16.Breitenbücher A, Keller-Wossidlo H, Keller R. Transtracheal oxygen therapy in obstructive sleep apnea syndrome. Schweiz Med Wochenschr. 1989;119:1638–41. [PubMed] [Google Scholar]
- 17.Castillo D, Güell R, Casan P. Oxygen-conserving devices: A forgotten resource. Arch Bronconeumol. 2007;43:40–5. doi: 10.1016/s1579-2129(07)60019-6. [DOI] [PubMed] [Google Scholar]
- 18.Spofford B, Christopher KL, Goodman JR. Transtracheal oxygen therapy. In: Christopher KL, editor. Problems in Respiratory Care: The Current Status of Oxygen Therapy. Philadelphia, PA: JB Lippincott Company; 1990. pp. 600–21. [Google Scholar]
- 19.Christopher KL, Schwartz MD. Transtracheal oxygen therapy. Chest. 2011;139:435–40. doi: 10.1378/chest.10-1373. [DOI] [PubMed] [Google Scholar]
- 20.Lipkin AF, Christopher KL, Diehl S, Jorgenson S. Otolaryngologist's role in transtracheal oxygen therapy: The minitrach procedure. Otolaryngol Head Neck Surg. 1995;113:P158. doi: 10.1177/019459989611500516. [DOI] [PubMed] [Google Scholar]
- 21.Orvidas LJ, Kasperbauer JL, Staats BA, Olsen KD. Long-term clinical experience with transtracheal oxygen catheters. Mayo Clin Proc. 1998;73:739–44. doi: 10.4065/73.8.739. [DOI] [PubMed] [Google Scholar]
- 22.Harrow EM, Oldenburg FA, Lingenfelter MS, Leonard J. Respiratory failure and cor pulmonale associated with tracheal mucoid accumulation from a SCOOP transtracheal oxygen catheter. Chest. 1992;101:580–1. doi: 10.1378/chest.101.2.580. [DOI] [PubMed] [Google Scholar]
- 23.Burton GG, Wagshul FA, Henderson D, Kime SW. Fatal airway obstruction caused by a mucous ball from a transtracheal oxygen catheter. Chest. 1991;99:1520–3. doi: 10.1378/chest.99.6.1520. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman LA, Reinke LF, Wesmiller SW, Sciurba FC. Airway obstruction by a mucus ball from a transtracheal oxygen catheter. Chest. 1992;101:1740–1. doi: 10.1378/chest.101.6.1740. [DOI] [PubMed] [Google Scholar]
- 25.Insertion Technique for Physicians. Transtracheal systems. 14 Inverness Dr. E, Suite H, 100 Englewood CO 80112 USA. [Last accessed on 2017 July 21]. Available from: http://tto2-com.deskpal.net/Home/Clinicians/FAQ .
- 26.Walsh DA, Govan JR. Long term continuous domiciliary oxygen therapy by transtracheal catheter. Thorax. 1990;45:478–81. doi: 10.1136/thx.45.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borer H, Frey M, Keller R. Ulcerous tracheitis and mucus ball formation: A nearly fatal complication of a transtracheal oxygen catheter. Respiration. 1996;63:400–2. doi: 10.1159/000196586. [DOI] [PubMed] [Google Scholar]
- 28.Menon AS, Carlin BW, Kaplan PD. Tracheal perforation.A complication associated with transtracheal oxygen therapy. Chest. 1993;104:636–7. doi: 10.1378/chest.104.2.636. [DOI] [PubMed] [Google Scholar]
- 29.Punzal PA, Myers R, Ries AL, Harrell JH., 2nd Laser resection of granulation tissue secondary to transtracheal oxygen catheter. Chest. 1992;101:269–71. doi: 10.1378/chest.101.1.269. [DOI] [PubMed] [Google Scholar]


