Abstract
Eosinophilic lung diseases are a diverse group of pulmonary disorders with an extensive list of differential diagnoses. Multiple drugs particularly antibiotics can cause pulmonary eosinophilia with variable pulmonary manifestations. Cutaneous drug reactions are common. Diagnosis is usually made on clinical history and blood eosinophilia with an accumulation of eosinophils in alveolar spaces on histologic analysis. Imaging findings are nonspecific. Stopping the offending agent is often enough while a short course of corticosteroids can hasten recovery. We present a unique case of eosinophilic pneumonia due to meropenem that highlights the importance of keeping a low threshold of suspicion regarding the etiology of drug-induced lung diseases as the current list is not exhaustive, and new agents are being identified continuously. A 51-year-old African American woman presented with fever, dyspnea, and diffuse pustular rash. She had been treated with meropenem intravenously through a peripherally inserted central catheter for 6 weeks before presentation for Pseudomonas aeruginosa septic arthritis of the left knee. She had a temperature of 102.2 F and SpO2 of 86% on room air. Chest roentgenogram had scattered infiltrates and chest tomography showed bilateral ground-glass opacities. Laboratory workup showed peripheral eosinophilia. Bronchoalveolar lavage revealed a white blood cell of 2230 with 89% eosinophils. Skin lesions' biopsies showed pustular dermatosis, compatible with acute drug-induced eosinophilic lung disease with skin involvement. As meropenem was the only medication she had been exposed to, it was stopped and systemic steroids were initiated with improvement in respiratory and clinical status and complete recovery on follow-up.
KEY WORDS: Eosinophilia, meropenem, pulmonary
INTRODUCTION
Eosinophilic lung diseases are a diverse group of pulmonary disorders in which the lungs become infiltrated by eosinophils. Various classifications schemes have been proposed, but at present, there is no agreed upon classification of these disorders. The most commonly reported etiology of circulating and/or alveolar eosinophilia with pulmonary infiltrates are drug reactions and the sheer number of causative agents that have been identified can make establishing a diagnosis challenging.[1]
CASE REPORT
A 51-year-old African American woman presented to the emergency department with 1 week history of progressively worsening shortness of breath, fever, sore throat, and diffuse pruritic rash.
She had a previous medical history of untreated chronic hepatitis C and recurrent Pseudomonas aeruginosa septic arthritis of her left knee that started 8 months before presentation. She had been treated initially with 4 weeks of intravenous cefepime but 6 months later had a recurrence of symptoms requiring drainage and reinitiating antibiotic therapy with meropenem intravenously through a peripherally inserted central catheter (PICC). She had been on it for approximately 6 weeks before the onset of her symptoms. She is a current smoker with forty pack-year smoking history. No personal history of asthma. She denied any illicit drug use. She previously worked as a housekeeper but had been unemployed since the onset of her knee arthritis.
In the emergency department, her vitals were as follows: blood pressure of 119/79, temperature of 102.2 F, heart rate of 97 beats/min, respiratory rate of 22 breaths/min and oxygen saturation of 86% on room air, 91% on 4 L through nasal cannula. She was tachypneic and in moderate respiratory distress on examination. Her lungs were clear to auscultation. She had a diffuse pustular rash that was only sparing her face. Her left knee was neither erythematous nor warm to touch.
Chest X-ray was notable for scattered opacities bilaterally. A noncontrast enhanced chest tomography showed bilateral ground-glass opacities, predominantly in the upper lungs without consolidation, but with borderline mediastinal lymphadenopathy [Figures 1 and 2]. Pertinent laboratory workup findings included leukocytosis with white blood cell (WBC) of 13.6 K/μL and absolute eosinophil count of 2.6 K/μL (19%). Urine drug screen was negative. She underwent punch biopsy of her rash. She also underwent bronchoscopy with bronchoalveolar lavage (BAL). Meropenem was continued and she was started on broad spectrum antibiotics with vancomycin, azithromycin, and levofloxacin while awaiting results of the workup. Bronchoscopy was performed and BAL cell count revealed 2230 WBC including 89% eosinophils, 11% lymphocytes, and 0% neutrophils. Pathological analysis of BAL showed numerous eosinophils [Figures 3 and 4] but was negative for Pneumocystis jiroveci pneumonia, fungal organisms, and malignancy. Serum IgE level was 18,218 IU/ml. Infectious workup including Legionella and Streptococcus pneumoniae antigens, influenza, rubella, measles, mycoplasma, Epstein–Barr virus, parvovirus B19, coxsackie, echovirus, and HIV was negative. Antineutrophil cytoplasmic antibody panel was also negative. Pathological exam of skin biopsy showed pustular dermatosis with rare eosinophils. The clinical picture was compatible with acute drug-induced eosinophilic pneumonia (EP) and skin reaction. As meropenem was the only medication she had been exposed to before presentation, it was felt to be the culprit. Meropenem along with the empiric broad-spectrum antibiotic therapy was stopped and oral prednisone 20 mg twice daily was initiated. Within 24 hours, her fever subsided, oxygen requirements decreased, and she was discharged home on a steroid taper 5 days after admission without supplemental oxygen. On follow-up in clinic 1 month later, she was noted to have complete clinical recovery and resolution of previous imaging findings.
Figure 1.
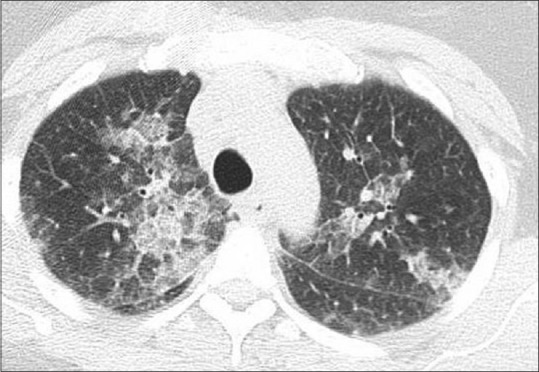
Computed tomography of chest without contrast on admission with bilateral ground glass opacities
Figure 2.
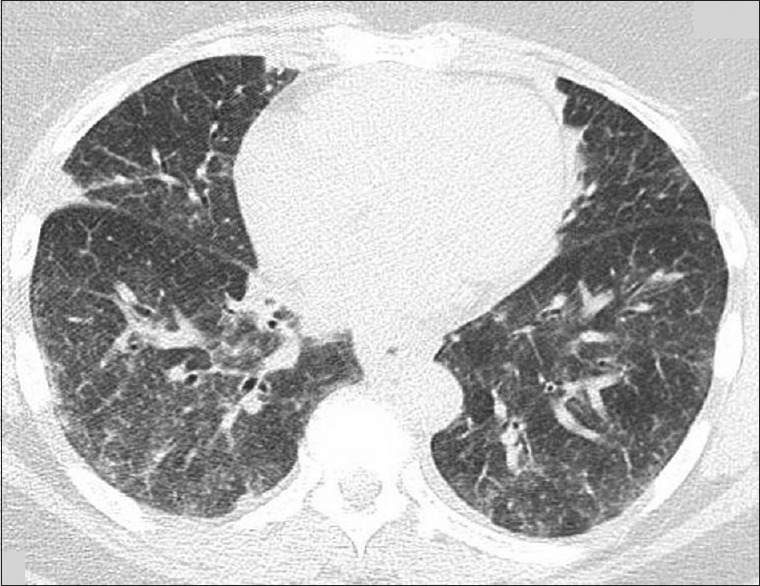
Computed tomography of chest without contrast on admission with bilateral ground glass opacities
Figure 3.
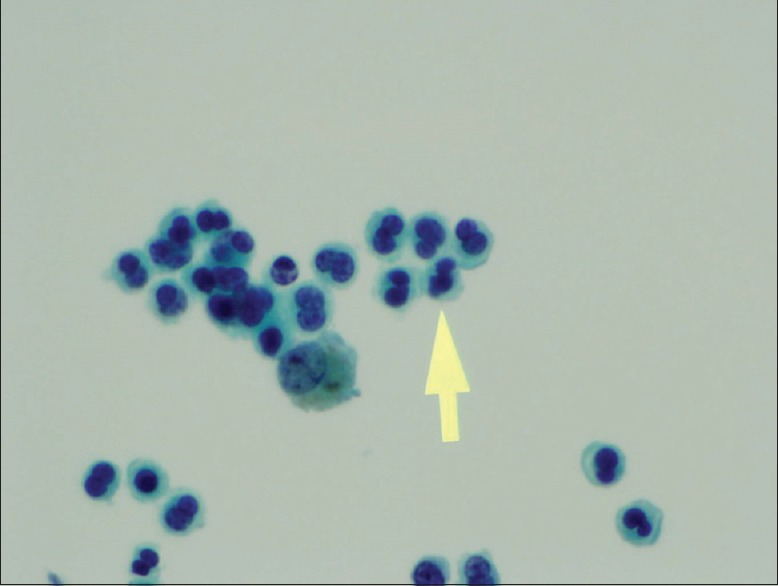
Bronchoalveolar lavage showing many eosinophils
Figure 4.
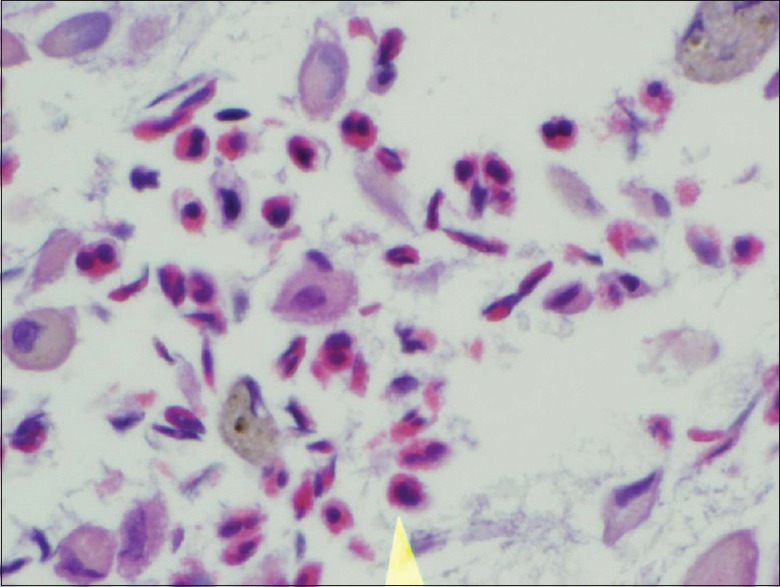
Cell block from bronchoalveolar lavage showing eosinophils and alveolar histiocytes (H&E)
DISCUSSION
As previously stated the most commonly reported etiology of circulating and/or alveolar eosinophilia with pulmonary infiltrates are drug reactions.[1] Nonsteroidal anti-inflammatory drugs constitute the most common causes of EP.[2] However, the list extends to include several other drugs ranging from acetaminophen to chemotherapeutic agents like bleomycin and antidepressants such as duloxetine.[3,4,5] In addition, several antibiotics have been described in the literature as causative agents for EPs. A complete and updated list of drugs suspected of causing lung disease can be found on a website maintained by the Department of Pulmonary and Intensive Care at the University Hospital in Dijon, France with the following electronic address http://www.pneumotox.com.[6,7]
Patients with drug-induced EP can vary in clinical presentation from those who are asymptomatic, to those with mild pulmonary symptoms, pulmonary infiltrates, and peripheral eosinophilia, to those with fulminant respiratory failure similar to acute EP. Symptoms usually develop within 2 months of starting the medication but can be delayed up to 5 years.[3,6]
The diagnosis is usually made by clinical history in addition to bronchoscopy with BAL demonstrating accumulation of eosinophils in the alveolar spaces on histologic analysis. Peripheral eosinophilia can either be present or absent.[8] Lung biopsy is often unnecessary to establish the diagnosis in drug-induced EP, it is however performed when the causative agent is uncertain or to rule out other disease entities that could explain the clinical picture.[2]
Imaging findings are nonspecific varying from diffuse ground-glass opacities, to consolidation, pleural effusion, reticulonodular densities and hilar adenopathy.[2,9] Cutaneous drug reactions are also common and can be life-threatening as is the case with drug rash with eosinophilia and systemic symptoms (DRESS).[9] The challenge is often determining the causal link between a particular drug and the pulmonary manifestations. This is crucial since stopping the offending agent is often enough for recovery. A short course of corticosteroids can hasten recovery and is warranted particularly in the setting of hemodynamic instability and respiratory failure.[1,9,10,11]
Solomon and Schwarzsuggested the following criteria to link an EP to an offending drug. They include, establishing the diagnosis of EP, determining a potential candidate drug that fits the time frame, ruling out any other causes of EP such as fungal and parasitic infections, noting improvement after cessation of the drug and relapse on reexposure. The latter is often unnecessary as reexposure can be dangerous.[2]
The exact pathophysiology for the development of drug-induced eosinophilic diseases remains unclear. Hypersensitivity-like reactions have been described with ingestion of the antigen by alveolar macrophages, recruitment of T-helper 2 lymphocytes, and release of interleukin 5 leading to recruitment and production of eosinophils at the alveolar level.[6,12] Another proposed mechanism for eosinophilic lung diseases like acute EP is dysregulation in the metabolism of pulmonary surfactant proteins with altered levels reported in blood and/or BAL.[13] Treatment options with exogenous administration of purified surfactant proteins in the setting of eosinophilic lung diseases are currently being investigated in allergic animal models.[13]
Meropenem is an intravenous B-lactam antibiotic that belongs to the subgroup of carbapenems. Its broad spectrum coverage makes it a very attractive option in treating resistant organisms particularly P. aeruginosa. Meropenem has been linked to DRESS syndrome in one patient[14] and hypersensitivity syndrome along with Imipenem in an immunocompromised patient.[15] Cutaneous manifestations dominated the picture in both cases; drug-induced EP was not described nor suspected, and neither patient underwent bronchoscopic evaluation. Foong et al. had described a case of acute EP induced by imipenem-cilastatin.[16] There were also case reports of EP secondary to cephalosporins.[12] An extensive review of the literature, however, did not demonstrate that meropenem had been directly implicated in eosinophilic lung disease. In our case, however, the patient's clinical presentation, radiographic and histologic findings were compatible with an acute drug reaction and suggestive of drug-induced eosinophilic lung disease. After ruling out infectious processes, the discontinuing of meropenem and the initiation of steroids was both diagnostic and therapeutic as it resulted as expected in rapid recovery with resolution of symptoms and radiological findings on follow-up. Since meropenem was the only drug our patient had been exposed to before the presentation, it was suspected to be the underlying etiology for her presentation. Using the World Health Organization Uppsala Monitoring Center (WHO-UMC) system for standardized case causality assessment and the Naranjo algorithm, the causality was determined to be probable.[17,18]
CONCLUSION
This case illustrates the importance of keeping a high index of suspicion regarding the etiology of drug-induced lung diseases as the current list is not exhaustive and new causative agents are continually being identified. This case is unique as meropenem has not been previously reported as a cause of EP and thus should be added to the drug-induced respiratory disease registries. In addition, the spectrum of EP is variable based on the culprit agent warranting the inclusion of drug-induced EP in the differential diagnosis of pulmonary manifestations of unclear etiology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1423–38. doi: 10.1164/ajrccm.150.5.7952571. [DOI] [PubMed] [Google Scholar]
- 2.Solomon J, Schwarz M. Drug-, toxin-, and radiation therapy-induced eosinophilic pneumonia. Semin Respir Crit Care Med. 2006;27:192–7. doi: 10.1055/s-2006-939522. [DOI] [PubMed] [Google Scholar]
- 3.Espeleta VJ, Moore WH, Kane PB, Baram D. Eosinophilic pneumonia due to duloxetine. Chest. 2007;131:901–3. doi: 10.1378/chest.06-1659. [DOI] [PubMed] [Google Scholar]
- 4.Hapani S, Chu D, Wu S. Eosinophilic pneumonia associated with bleomycin in a patient with mediastinal seminoma: A case report. J Med Case Rep. 2010;4:126. doi: 10.1186/1752-1947-4-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saint-Pierre MD, Moran-Mendoza O. Acetaminophen use: An unusual cause of drug-induced pulmonary eosinophilia. Can Respir J. 2016;2016:4287270. doi: 10.1155/2016/4287270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalogeropoulos AS, Tsiodras S, Loverdos D, Fanourgiakis P, Skoutelis A. Eosinophilic pneumonia associated with daptomycin: A case report and a review of the literature. J Med Case Rep. 2011;5:13. doi: 10.1186/1752-1947-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu SY, Faust AC, Dand HM. Daptomycin-induced eosinophilic pneumonia treated with intravenous corticosteroids. J Pharm Pract. 2015;28:275–9. doi: 10.1177/0897190014568678. [DOI] [PubMed] [Google Scholar]
- 8.Buelow BJ, Kelly BT, Zafra HT, Kelly KJ. Absence of peripheral eosinophilia on initial clinical presentation does not rule out the diagnosis of acute eosinophilic pneumonia. J Allergy Clin Immunol Pract. 2015;3:597–8. doi: 10.1016/j.jaip.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Jeong YJ, Kim KI, Seo IJ, Lee CH, Lee KN, Kim KN, et al. Eosinophilic lung diseases: A clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617–37. doi: 10.1148/rg.273065051. [DOI] [PubMed] [Google Scholar]
- 10.Cottin V, Cordier JF. Eosinophilic lung diseases. Immunol Allergy Clin North Am. 2012;32:557–86. doi: 10.1016/j.iac.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: A study of 22 patients. Am J Respir Crit Care Med. 2002;166:1235–9. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths CL, Gutierrez KC, Pitt RD, Lovell RD. Eosinophilic pneumonia induced by ceftaroline. Am J Health Syst Pharm. 2014;71:403–6. doi: 10.2146/ajhp130441. [DOI] [PubMed] [Google Scholar]
- 13.Ledford JG, Addison KJ, Foster MW, Que LG. Eosinophil-associated lung diseases. A cry for surfactant proteins A and D help? Am J Respir Cell Mol Biol. 2014;51:604–14. doi: 10.1165/rcmb.2014-0095TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prados-Castaño M, Piñero-Saavedra M, Leguísamo-Milla S, Ortega-Camarero M, Vega-Rioja A. DRESS syndrome induced by meropenem. Allergol Immunopathol (Madr) 2015;43:233–5. doi: 10.1016/j.aller.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Shimizu F, Takeo N, Okamoto O, Katagiri K, Ikewaki J, et al. Drug-induced hypersensitivity syndrome due to carbapenem antibiotics. J Dermatol. 2010;37:374–7. doi: 10.1111/j.1346-8138.2010.00820.x. [DOI] [PubMed] [Google Scholar]
- 16.Foong KS, Lee A, Pekez M, Bin W. Imipenem/cilastatin-induced acute eosinophilic pneumonia. BMJ Case Rep 2016. 2016:pii: Bcr2016214804. doi: 10.1136/bcr-2016-214804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Use of the WHO-UMC System for Standardized Case Causality Assessment. [Last accessed on 2017 Mar 31]. Available from: https://www.who-umc.org/media/2768/standardised-case-causality-assessment.pdf .
- 18.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


