Abstract
Von Willebrand factor (VWF) mediates blood platelet adhesion and accumulation at sites of blood vessel injury, and also carries coagulation factor VIII (FVIII) that is important for generating procoagulant activity. Von Willebrand disease (VWD), the most common inherited bleeding disorder, affects males and females, and reflects deficiency or defects of VWF that may also cause decreased FVIII. It may also occur less commonly as an acquired disorder (acquired von Willebrand syndrome). This article briefly summarizes selected features of the March 2008 evidence-based clinical and laboratory diagnostic recommendations from the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel for assessment for VWD or other bleeding disorders or risks. Management of VWD is also addressed in the NHLBI guidelines, but is not summarized here. The VWD guidelines are available at the NHLBI Web site (http://www.nhlbi.nih.gov/guidelines/vwd).
Introduction
Bleeding symptoms or concerns about them are relatively frequent in adults as well as children. Common bleeding symptoms include nosebleeds, bruising, gingival bleeding, bleeding from small wounds, and menorrhagia or postpartum bleeding in women. Other bleeding symptoms include bleeding with surgery or other invasive procedures including dental extractions, gastrointestinal or urinary bleeding, hematomas or hemarthroses, hemoptysis, and central nervous system bleeding.
Appropriate clinical and laboratory evaluation of bleeding symptoms, disorders, and risks is challenging. Bleeding can occur in normal persons or those with anatomic or pathological conditions predisposing to bleeding. Bleeding also occurs in persons with a hereditary or acquired bleeding disorder. However, diagnosed bleeding disorders are less common than are bleeding symptoms or the presence of conditions associated with increased bleeding risk.
In March 2008, the National Heart, Lung, and Blood Institute (NHLBI) published evidence-based guidelines for evaluating and managing the most common hereditary bleeding disorder, von Willebrand disease (VWD). These guidelines—the first from NHLBI for any blood disorder—also provide recommendations for the initial clinical and laboratory evaluation of patients with bleeding symptoms, history, or medical conditions associated with increased bleeding risk with invasive procedures. These guidelines were developed for practicing primary care physicians—including family physicians, internists, obstetrician-gynecologists, pediatricians, and nurse-practitioners—as well as hematologists and laboratory medicine specialists.
The full guidelines document [1] is available online at the NHLBI web site (http://www.nhlbi.nih.gov/guidelines/vwd), as is a Pocket Guide [2] synopsis for practitioners, and a patient education brochure. An edited version of these guidelines was also published in the March 2008 issue of Hemophilia journal [3]. In March 2008, the British Committee for Standards in Haematology independently published guidelines for bleeding risk assessment before surgery or invasive procedures [4]. In December 2008, the American Society of Hematology published a Quick Reference synopsis of the NHLBI VWD guidelines [5].
Congenital (Hereditary) von Willebrand Disease
Von Willebrand disease (VWD) is the most common inherited bleeding condition, affecting males and females in approximately equal proportions, and occurring in up to 1% of the U.S. and world populations. VWD reflects deficiency or dysfunction of von Willebrand factor (VWF), a multimeric plasma glycoprotein that mediates platelet adhesion and aggregation at sites of vascular injury, and that also carries and stabilizes blood coagulation factor VIII (FVIII) in the circulation.
Persons with VWD may experience easy bruising, nosebleeds, or other mucosal bleeding such as gastrointestinal or heavy menstrual bleeding (in women), and may be at risk of bleeding following surgery or invasive procedures, traumatic injury, or childbirth. Symptoms can range from mild bleeding in Type 1 VWD to severe, life-threatening bleeding in Type 3 VWD.
The three main types of VWD are as follows: Type 1 (partial quantitative deficiency), Type 2 (qualitative deficiency) with four subtypes (2A, 2B, 2M, 2N), and Type 3 (virtually complete quantitative deficiency) [1,3,6]. Type 1 VWD accounts for ~75% of symptomatic persons, and nearly all the remaining affected persons have Type 2 variants, with Type 2A more common than types 2B, 2M, or 2N. Type 3 VWD is rare, affecting only ~1 person in 1,000,000.
Acquired von Willebrand Syndrome
Acquired von Willebrand syndrome (AVWS) is less common than congenital (hereditary) VWD and is typically etiologically associated with a number of different mechanisms and medical conditions (see Table I, and published guidelines [1,3]). Laboratory findings in AVWS are similar to those in VWD. AVWS, and disorders causing it, should be considered in persons found to have abnormal VWF test results and bleeding symptoms, without a personal and/or family history consistent with hereditary VWD. Conversely, when bleeding occurs in association with one of the causative conditions, AVWS should be considered and initial VWD testing performed if indicated.
TABLE I.
Disorders Pathophysiologically Associated with Acquired von Willebrand Syndrome (AVWS)
| Pathophysiologic category | Disease or association |
|---|---|
| Antibodies to VWF | Lymphoproliferative diseases, monoclonal gammopathies, or autoimmune diseases such as systemic lupus erythematosus |
| Shear-induced VWF conformational changes leading to increased proteolysis of VWF | Ventricular septal defect, aortic valvular stenosis, hypertrophic obstructive cardiomyopathy, left ventricular assist device, or primary pulmonary hypertension |
| Markedly elevated blood platelet count | Essential thrombocythemia, polycythemia vera, myeloid metaplasia with myelofibrosis, or other myeloproliferative disorders |
| Removal of VWF from circulation by aberrant binding to tumor cells | Wilm’s tumor and certain lymphoproliferative or plasma cell proliferative disorders |
| Decreased VWF synthesis | Hypothyroidism |
| Drugs associated with AVWS | Ciprofloxacin, valproic acid, hydroxyethyl starch, and griseofulvin |
AVWS, acquired von Willebrand syndrome; VWF, von Willebrand factor.
Diagnosis and Evaluation
Clinical evaluation
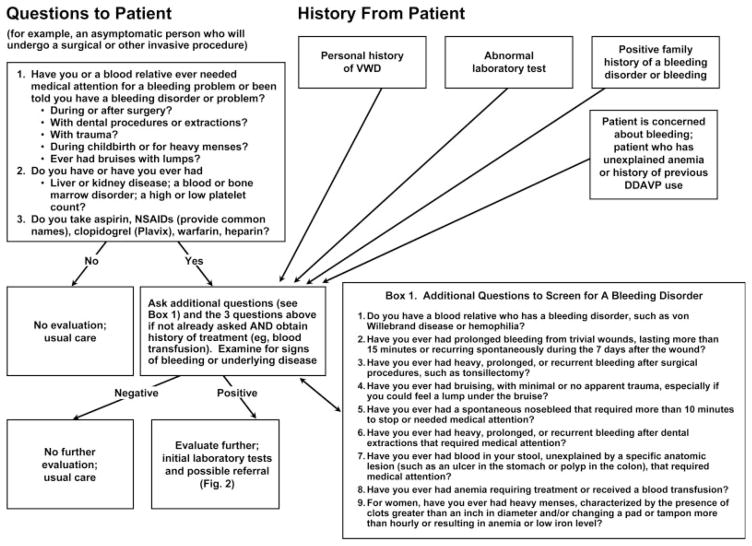
Figure 1 (from the NHLBI VWD Guidelines) provides an algorithm for initial clinical evaluation for VWD or other bleeding disorders. Two main scenarios are envisioned: (1) Asymptomatic persons who will undergo a surgical or invasive procedure and who should be assessed for bleeding risk; or (2) Persons presenting with a personal and/or family history of bleeding symptoms or bleeding disorder, abnormal hemostasis laboratory tests, or concerns about bleeding symptoms.
Figure 1.
Initial clinical evaluation strategy to determine which patients would benefit most from further diagnostic evaluation for von Willebrand disease (VWD) or other bleeding disorders. Individuals (for example, an asymptomatic person who will undergo a surgical or other invasive procedure) would be asked three questions (left upper box), which, if any responses are positive, would lead to a second set of nine questions selected for sensitivity and specificity for VWD (Box 1, lower right). Individuals presenting with specific information or a concern about bleeding would be asked the Box 1 questions and the initial three questions if not already asked, and would also undergo laboratory evaluation. DDAVP, desmopressin; NSAIDs, nonsteroidal anti-inflammatory drugs. Modified from Ref. 2, Fig. 1.
The left upper box in Fig. 1 provides three recommended questions for preoperative screening of asymptomatic persons for bleeding risks or disorders. “Box 1” (lower right box) provides nine questions recommended for further evaluation of persons answering positively to the initial three questions, or for evaluation of persons who have specific hemostasis issues. All questions are grade B recommendations that are based on limited published research, except for questions 2 and 3 in the left upper box that primarily reflect expert opinion (grade C recommendations). Persons answering positively to one or more questions should be considered for further evaluation such as hemostasis consultation and focused laboratory testing. “An increasing number of positive responses to the questions about bleeding (the initial three questions, and the nine additional questions), and abnormal findings on physical examination, increase the likelihood that an individual has a bleeding disorder including possible VWD” [1,3].
Physical examination, directed to assess evidence for a bleeding disorder, should also be performed, such as identifying ecchymoses, hematomas, petechiae, and other evidence of recent bleeding. The examination should also focus on findings that may suggest other causes of increased bleeding such as evidence of liver disease (e.g., jaundice), splenomegaly, joint and skin laxity (e.g., Ehlers-Danlos syndrome), telangiectasia (e.g., hereditary hemorrhagic telangiectasia), signs of anemia, or anatomic lesions on gynecologic examination.
Laboratory evaluation
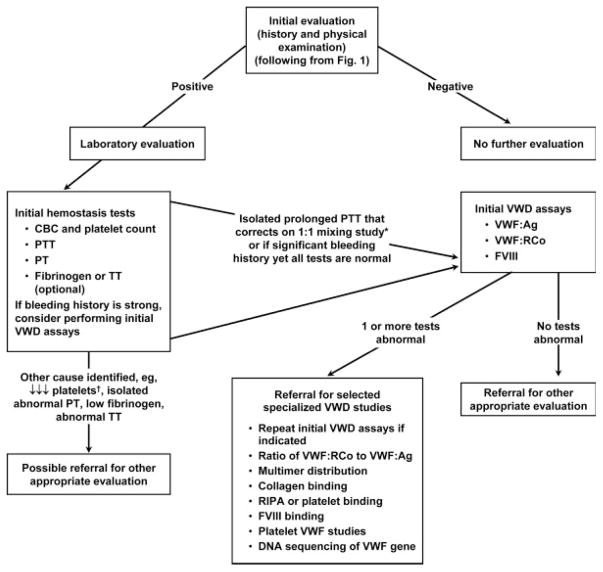
Figure 2 (from the NHLBI VWD Guidelines) provides an algorithm for initial laboratory evaluation for VWD or other bleeding disorders. If the initial clinical evaluation suggests a bleeding disorder, the “initial hemostasis tests” (box on the left) should be ordered. These tests include a complete blood count (CBC), prothrombin time (PT), and activated partial thromboplastin time (PTT), reflecting grade C recommendations. This testing does not evaluate for VWD, but it can suggest whether coagulation factor deficiency or thrombocytopenia (or thrombocytosis) might be the potential cause of clinical bleeding. If the mucocutaneous bleeding history is strong, consider ordering initial VWD assays (box on the right) with the initial visit.
Figure 2.
Laboratory assessment algorithm for VWD or other bleeding disorders. If the initial clinical hemostasis evaluation (see Fig. 1) suggests a bleeding disorder, the “initial hemostasis tests” should be ordered, followed by or along with the next tests (“initial VWD assays”) indicated in the laboratory algorithm. Referral to a hemostasis specialist is appropriate for help in interpretation, repeated testing, and specialized VWD studies. *Correction in the PTT mixing study immediately and after 2-hr incubation removes a factor VIII (FVIII) inhibitor from consideration. Investigation of other intrinsic factors and lupus anticoagulant may also be indicated. †Isolated decreased platelets may occur in VWD Type 2B. ↓Refers to a decrease in function or in the test result compared with the laboratory reference range. Modified from Ref. 2, Fig. 2.
The initial three VWD tests are grade B recommendations and include measurements of blood plasma:
VWF antigen (VWF:Ag);
VWF ristocetin cofactor activity (VWF:RCo); and
factor VIII coagulant activity (FVIII).
All three tests are recommended for initial evaluation, and the results may not only establish the diagnosis but also suggest the type and severity of VWD if it is present. If one or more test results are abnormally low and/or if the ratio of VWF:RCo to VWF:Ag is abnormally low (below 0.5–0.7), selected specialized VWD assays should be considered for reflexive testing or with evaluation of another plasma sample (lower box in Fig. 2).
Multimer analysis visualizes the distribution of plasma VWF multimers, is technically complex, is qualitatively interpreted in conjunction with results of the initial three tests and available clinical information, and is used to help determine the VWD subtype. Therefore, VWF multimer analysis is not recommended for initial VWD screening, [1,3] and should only be performed if initial VWD testing identifies an abnormal result (e.g., abnormally low VWF:RCo or ratio of VWF:RCo to VWF:Ag) or clinical information suggests a high likelihood of abnormal VWF multimer analysis.
Although VWF:RCo values are typically decreased (with variably decreased VWF:Ag and/or FVIII) in AVWS, sometimes only VWF multimer analysis is abnormal, with mild reduction or loss of the highest molecular weight multimers. Evolving information [7–11] suggests this latter situation is more likely for AVWS associated with enhanced VWF proteolysis reflecting shear-induced VWF conformational changes leading to increased proteolysis of VWF, such as with aortic valvular stenosis and other conditions causing abnormally high shear blood flow (Table I).
Some centers add a bleeding time or a platelet function analyzer (PFA-100) assay to their initial laboratory tests, but there are conflicting data with regard to sensitivity and specificity for VWD, and current evidence does not support their routine use as screening tests for VWD.
This laboratory evaluation algorithm does not address additional considerations such as evaluation for platelet hypofunction or for certain other bleeding disorders, acquired or hereditary. Also, the laboratory evaluation of a person for possible VWD or AVWS is relatively complex, particularly because of potential variabilities of laboratory testing results contributed by conditions of the patient, the blood sample, and the laboratory methodology (Table II). In interpreting test results, it is important to be aware of these variabilities. Following initial assessment, consultation or discussion with a hemostasis specialist may be useful for further evaluation of a suspected or diagnosed bleeding disorder.
TABLE II.
Conditions of the Patient, Blood Sample, and Laboratory Testing Affecting Laboratory Evaluation for von Willebrand Disease (VWD)
| Phlebotomy conditions: An atraumatic blood draw limits the exposure of tissue factor from the site and the activation of clotting factors, minimizing falsely high or low values. Lipemia should be avoided, as it may interfere with photo-optical testing methods, especially some used for VWF:RCo assay. |
| Patient stress level: Undue stress, such as struggling or crying in children or anxiety in adults, may falsely elevate VWF and FVIII levels. Very recent exercise can also elevate VWF levels. |
| Additional conditions in the person: The presence of an acute or chronic inflammatory illness may elevate VWF and FVIII levels, as may pregnancy or administration of estrogen/oral contraceptives. Individuals with blood group O have VWF levels 325% lower than those of other ABO blood groups. African-Americans have higher VWF levels than Caucasians. |
| Sample processing: To prevent cryoprecipitation of VWF and other proteins, blood samples for VWF assays should be transported to the laboratory at room temperature. Plasma should be separated from blood cells promptly at room temperature, and the plasma should be centrifuged thoroughly to remove platelets. If plasma samples will be assayed within 2 hr, they should be kept at room temperature. Frozen plasma samples should be carefully thawed at 37°C and kept at room temperature for <2 hr before assay. |
| Sample storage: Plasma samples that will be stored or transported to a reference laboratory must be frozen promptly at or below −40°C and remain frozen until assayed. A control sample that is drawn, processed, stored, and transported under the same conditions as the tested person’s sample may be helpful in indicating problems in the handling of important test samples. Activity of FVIII typically is 10–20% lower in frozen-thawed plasma than in fresh (nonfrozen) plasma, and can be even lower if blood processing or storage conditions are suboptimal. |
| Laboratory testing: Calibrators for assays of VWF:Ag, VWF:RCo, and FVIII should be referenced to the World Health Organization (WHO) plasma standard. These three tests have relatively high coefficients of variation (CVs of 10–30%), especially the VWF:RCo assay. The quality of laboratory testing also varies considerably among laboratories (high interlaboratory CV). Test results can be reported as international units per deciliter (IU/dL), rather than as a percentage (%) of mean normal, if WHO- linked calibrators are used. Referencing VWF testing results to the population reference range, rather than to ABO-stratified reference ranges, may be clinically useful. |
Synthesis of clinical and laboratory evaluation for VWD
Table III outlines classification of VWD [6] and provides prototypical laboratory values [1,2,3,5]. Diagnosis, especially for individuals with mildly decreased VWF (30–50% or IU/dL), requires correlation of clinical assessment (personal and family history of bleeding) and results of laboratory testing, the latter preferably performed in the absence of conditions associated with elevation of baseline VWF and with careful attention to blood specimen collection, processing, transportation, and storage (see Table II and full documents for details [1,3]).
TABLE III.
VWD Classification and Laboratory Valuesa
| Condition | Description | VWF:Rco (IU/dL) | VWF:Ag (IU/dL) | FVIII | VWF:RCo/VWF:Ag Ratiob |
|---|---|---|---|---|---|
| Type 1 | Partial quantitative VWF deficiency | <30c | <30c | ↓ or Normal | >0.5–0.7 |
| Type 2A | ↓ VWF-dependent platelet adhesion with selective deficiency of high-molecular-weight VWF multimers | <30 | <30–200 | ↓ or Normal | <0.5–0.7 |
| Type 2B | Increased VWF affinity for platelet GP Ib; ± ↓ platelet numbers | <30 | <30–200 | ↓ or Normal | Usually <0.5–0.7 |
| Type 2M | ↓ VWF-dependent platelet adhesion without selective deficiency of high-molecular-weight VWF multimers | <30 | <30–200 | ↓ or Normal | <0.5–0.7 |
| Type 2N | Markedly decreased VWF binding affinity for FVIII | 30–200 | 30–200 | ↓↓ | >0.5–0.7 |
| Type 3 | Virtually complete deficiency of VWF | <3 | <3 | ↓↓↓(<10 IU/dL) | Not applicable |
| “Low VWF”c | 30–50 | 30–50 | Normal | >0.5–0.7 | |
| Normal | 50–200 | 50–200 | Normal | >0.5–0.7 |
Modified from Ref. 2 (“A Pocket Guide to The Diagnosis, Evaluation, and Management of Von Willebrand Disease”).
FVIII, coagulation factor VIII activity; VWD, von Willebrand disease; VWF, von Willebrand factor; VWF:Ag, VWF antigen; VWF:RCo, VWF ristocetin cofactor activity.
These values represent prototypical cases. Exceptions occur, and repeat testing may be necessary.
Until more laboratories clearly define a reference range, the VWF:RCo/VWF:Ag ratio of <0.5–0.7 is recommended to distinguish Type 1 vs. Type 2 VWD variants (A, B, or M).
30 IU/dL (or 30%) is recommended as the “cut-off” for the definite diagnosis of VWD for the following reasons: (1) There is a high frequency of blood type O in the United States, which is associated with “low” VWF levels; (2) Bleeding symptoms are reported by a significant proportion of normal individuals; (3) No abnormality in the VWF gene has been identified in many individuals who have mildly to moderately low VWF:RCo levels. This does not preclude the diagnosis of VWD in patients with VWF:RCo of 30–50 IU/dL if there is supporting clinical and/or family evidence of VWD, nor does this preclude the use of agents to increase VWF levels in those who have VWF:RCo of 30–50 IU/dL and who may be at risk for bleeding.
↓ Refers to a decrease in function or in the test result compared with the laboratory reference range.
Summary of the Clinical and Laboratory Diagnosis of VWD
Clinical and laboratory evaluation for VWD or AVWS, or for other bleeding disorders, is relatively complex and there is no single laboratory test that can screen for the presence of VWD or AVWS. The 2008 NHLBI guidelines suggest an algorithmic approach to the clinical and laboratory diagnosis of these conditions.
Footnotes
Authors disclosing potential conflicts of interest are Dr. Andra James (speaking and consulting fees, and research support from CSL Behring, and honoraria from Grifols), Dr. Marilyn Manco-Johnson (speaking and consulting fees, and research support from CSL Behring), Dr. Robert Montgomery (consultant for Baxter, CSL Behring, GTI-Diagnostics, and Astra Zeneca; CSL Behring fellowship for research support), Dr. William Nichols (CSL Behring Humate-P surgical trial study, supervision of “central laboratory” activities at the Mayo Special Coagulation Laboratory, via contract through Mayo Clinical Trials Services), Dr. Thomas Ortel (speaking fee from CSL Behring), and Dr. J. Evan Sadler (consultant and Clinical Advisory Board member for Baxter). Drs. Margaret Rick and Mark Weinstein reported no potential conflicts of interest and contributed to this manuscript in their private capacities, and no official endorsement or support by the National Institutes of Health or the Food and Drug Administration is intended or should be inferred. All other authors reported no potential conflicts of interest.
References
- 1.The National Heart, Lung, and Blood Institute. The Diagnosis, Evaluation, and Management of Von Willebrand Disease. Bethesda, MD: National Institutes of Health Publication 08-5832; 2007. Available at: http://www.nhlbi.nih.gov/guidelines/vwd. [Google Scholar]
- 2.The National Heart, Lung, and Blood Institute. A Pocket Guide to The Diagnosis, Evaluation, and Management of Von Willebrand Disease. Bethesda, MD: National Institutes of Health Publication 08-5833; 2008. Available at: http://www.nhlbi.nih.gov/guidelines/vwd/vwd_pocket-gde.htm. [Google Scholar]
- 3.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): Evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 4.Chee YL, Crawford YL, Watson HG, et al. British Committee for Standards in Haematology. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504. doi: 10.1111/j.1365-2141.2007.06968.x. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Hematology, Practice Committee. 2008 Clinical Practice Guideline on the Evaluation and Management of von Willebrand Disease. Washington, DC: 2008. Available at: http://www.hematology.org/policy/resources/guidelines/VWD_Quick_Reference(rf).pdf. [Google Scholar]
- 6.Sadler JE, Budde U, Eikenboom JCJ, et al. Update on the pathophysiology and classification of von Willebrand disease: A report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 7.Veyradier A, Balian A, Wolf M, et al. Abnormal von Willebrand factor in bleeding angiodysplasias of the digestive tract. Gastroenterology. 2001;120:346–353. doi: 10.1053/gast.2001.21204. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu M, Masai H, Miwa Y. Occult gastrointestinal bleeding due to acquired von Willebrand syndrome in a patient with hypertrophic obstructive cardiomyopathy. Intern Med. 2007;46:481–485. doi: 10.2169/internalmedicine.46.6026. [DOI] [PubMed] [Google Scholar]
- 9.Le Tourneau T, Susen S, Caron C, et al. Functional impairment of von Wille-brand factor in hypertrophic cardiomyopathy. Circulation. 2008;118:1550–1557. doi: 10.1161/CIRCULATIONAHA.108.786681. [DOI] [PubMed] [Google Scholar]
- 10.Tiede A, Priesack J, Werwitzke S, et al. Diagnostic workup of patients with acquired von Willebrand syndrome: A retrospective single-centre cohort study. J Thromb Haemost. 2008;6:569–576. doi: 10.1111/j.1538-7836.2008.02909.x. [DOI] [PubMed] [Google Scholar]
- 11.Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]