Abstract
Sex differences in the expression of social behavior are typically apparent in adolescent and adult rats. While the neurobiology underlying juvenile social play behavior has been well characterized, less is known about discrete brain regions involved in adult responsiveness to a same sex peer. Furthermore, whether adult males and females differ in their responsiveness to a social interaction in terms of neuronal activation indexed via immediate early gene (IEG) expression remains to be determined. Thus, the present study was designed to identify key sites relevant to the processing of sensory stimuli (generally) or social stimuli (specifically) after brief exposure to a same-sex social partner by assessing IEG expression. Four-month-old male and female Fisher (F) 344 rats (N = 38; n = 5–8/group) were either left undisturbed in their home cage as controls (HCC), exposed to a testing context alone for 30 min (CXT), or were placed in the context for 20 min and then allowed to socially interact (SI) with a sex-matched conspecific for 10 min. Females demonstrated greater levels of social behavior, relative to males. Analysis of c-Fos induction revealed that females exhibited greater c-Fos expression in the prefrontal cortex, regardless of condition. In many brain regions, induction was similar in the CXT and SI groups. However, in the bed nucleus of the stria terminalis (BNST), females exhibited greater c-Fos induction in response to the social interaction relative to their male counterparts, indicating a sex difference in responsivity to social stimuli. Taken together, these data suggest that the BNST is a sexually dimorphic region in terms of activation in response to social stimuli.
Keywords: social interaction, Fischer 344, sex differences, c-Fos
1.0 Introduction
Interactions with peers play a crucial role in mammalian development across the entire lifespan. Social deprivation during critical periods (e.g., post-weaning) leads to abnormal social behavior in adulthood (Fone and Porkess, 2008; Hermes et al., 2011; Toth et al., 2011). In rodents, social behavior has been studied extensively in juveniles, owing to the high levels of social play exhibited by rats at this age. Indeed, juvenile social play behavior and its underlying neurobiology, have been well characterized (Pellis and Pellis, 1998; Siviy and Panksepp, 2011; Trezza et al., 2010; Vanderschuren et al., 2016). There are sex differences in juvenile play behavior: frequency of social play, generally, is higher in males, although females respond more quickly when approached by a conspecific (Pellis et al., 1997). Rats from different strains also differ in terms of their play behavior. For instance, female Sprague Dawley rats show comparable or sometimes higher, rates of playful attacks than males, whereas Long Evans females engage in less play fighting than males (Pellis et al., 1997).
Using c-Fos expression as a reporter of relative differences in neuronal activation (Kovács, 2008) it has been demonstrated that the dorsal striatum (Gordon et al., 2002; van Kerkhof et al., 2014), ventral striatum (Gordon et al., 2002; van Kerkhof et al., 2014), medial prefrontal cortex (mPFC) (Cheng et al., 2008; van Kerkhof et al., 2014), bed nucleus of the stria terminalis (BNST) (Cheng et al., 2008; van Kerkhof et al., 2014), and dorsal raphe (van Kerkhof et al., 2014) are involved in juvenile social play. Not surprisingly, several brain regions known to mediate motivation/reward are implicated in the regulation of social play, a behavior that is highly rewarding in young rats (Vanderschuren et al., 2016). However, much less is known about the neurobiology of adult responding to a same sex conspecific. Compared to adolescents, adults exhibit reduced levels of social behavior (Perkins et al., 2016). Adolescents also exhibit greater induction of c-Fos protein in response to a social partner in the amygdala (AMG) and BNST, whereas adults exhibit less c-Fos induction following a social interaction in the PFC, nucleus accumbens (NAC), and brainstem relative to their counterparts placed alone in the context (Varlinskaya et al., 2013).
Sex differences in the expression of social behavior have been observed in adult rats. For instance, adult male Lister rats spend more time interacting with a familiar age-matched conspecific (Johnston and File, 1991) and adult male Sprague-Dawley rats (Stack et al., 2010; Varlinskaya et al., 2014) and Wistar rats (Dumais et al., 2013) engage in higher levels of active social interaction with a novel adult or juvenile conspecific (Dumais et al., 2013), relative to their female counterparts. We have shown previously that 3-month old male and female Fisher 344 (F344) rats exhibit similar levels of social investigation of novel and familiar social partners, with both sexes demonstrating a significant decline in social behavior during late aging (i.e., 18 months and beyond) (Perkins et al., 2016). Although F344 rats exhibit lower levels of social behavior relative to other strains (e.g. Sprague-Dawley, Wistars, etc.), we sought to assess characteristics of adult social behavior in this strain and examine patterns of neuronal activation. F344 rats are one of three strains (F344, Brown Norway, F344/BN) provided by the National Institute of Aging for use in aging research, and since our long-term goal is to delineate neural mechanisms driving declines in social behavior associated with late aging, we first wanted to establish whether there were sex differences in adult social behavior, per se, and determine if these differences were also associated with altered patterns of neuronal activation.
To our knowledge, very few studies have compared neuronal activation following a social interaction in adult males and females. For instance, male Sprague-Dawley rats exhibit greater expression of the IEG zif268 in the dorsal mPFC, prelimbic cortex (PL), infralimbic cortex (IL), and striatum, an effect that was likely due to greater levels of social interaction in adult males relative to adult females. No sex differences were observed in zif268 expression in the NAC, AMG, or hippocampus (HPC) (Stack et al., 2010). However, in the study by Stack et al. (2010), neuronal activation was not assessed in a non-socially tested control group, making it difficult to determine whether there are sex differences in neuronal activation in response to behavioral testing in general, or social interaction, specifically. Thus, we conducted two experiments in which rats were either behaviorally-naïve, exposed to a testing apparatus alone, or exposed to a novel social partner (Figure 1A). In the first experiment, neuronal activation in adult male F344 rats was assessed by examining the expression of three IEGs: c-Fos, Egr-1, and c-Jun, all of which are inducible transcription factors whose expression is increased in response to activation of intracellular signaling cascades (Hughes and Dragunow, 1995). In addition, expression of IEGs is associated with activation of downstream transcription factors that are involved in synaptic plasticity. Although c-Fos expression is by far the most common tool used to assess neuronal activation, we chose to expand our analyses and to include two other immediate early genes: Egr-1 (previously referred to as Zif268) and c-Jun (see Herdegen and Leah, 1998 for a comprehensive review of c-Fos and c-Jun). Since the first experiment supported c-Fos as the most sensitive marker of neuronal activation using these testing parameters, in the second experiment, we compared c-Fos mRNA expression in response to behavioral testing (generally) and social interaction (specifically) in male and female F344 rats.
Figure 1.
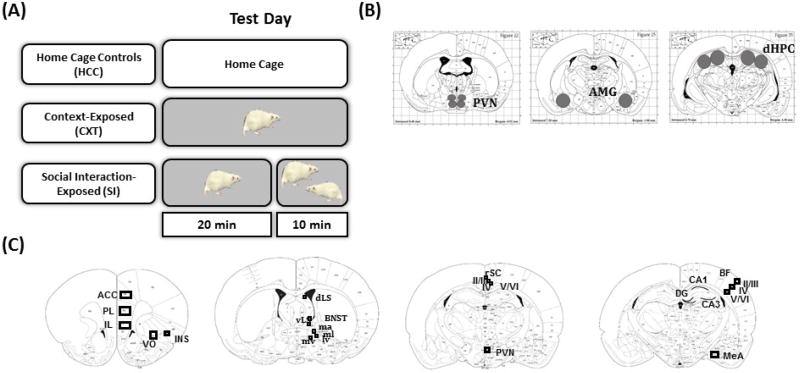
(A) Animals were randomly assigned to one of three conditions, home cage controls (HCC), context-exposed (CXT), or social interaction-exposed (SI) for the assessment of c-Fos expression. HCC animals remained in their home cage throughout testing. CXT animals were exposed alone to a behavioral testing apparatus for 30 min. SI animals were placed alone into the apparatus for 20 min, after which a non-familiar same sex and age conspecific was placed into the apparatus for a 10-min social interaction test. Rats were returned to their home cage after testing for 30 min prior to tissue collection. (B) Regions of interest for analysis of IEG gene expression with RT-PCR in male F344 rats. (C) Regions of interest for analysis of c-Fos mRNA expression with in situ hybridization in male and female F344 rats.
2.0 Results
2.1 Experiment 1
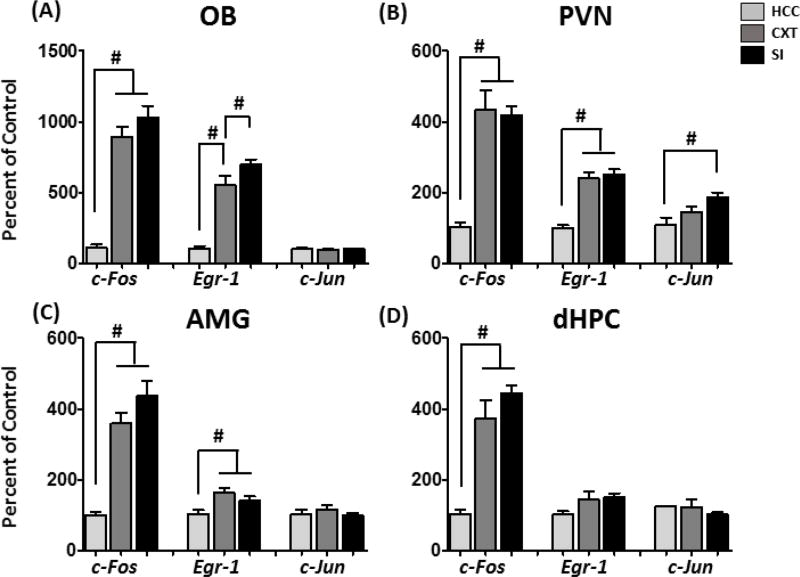
Expression of the immediate early genes c-Fos, Egr-1, and c-Jun was assessed in the olfactory bulb (OB), paraventricular nucleus of the hypothalamus (PVN), amygdala (AMG), and dorsal hippocampus (dHPC) using RT-PCR. Regions of interest were isolated using a tissue punching procedure. There were main effects of Condition on c-Fos expression in all regions of interest: OB [F(2,18) = 45.77, p < 0.0001], PVN [F(2,18) = 28.58, p < 0.0001], AMG [F(2,18) = 22.71, p < 0.001] and dHPC [F(2,18) = 30.44, p < 0.0001]. Post-hoc tests indicated similar levels of c-Fos induction in the CXT and SI groups (Figure 2A–D). In the OB, although CXT rats had a significant increase in Egr-1, compared to HCC, SI rats exhibited higher Egr-1, over and above what was observed in CXT rats [main effect of Condition: F(2,18) = 50.83, p < 0.00001], indicating sensitivity to the social interaction. Increased Egr-1 expression was observed in the CXT and SI groups in the PVN [main effect of Condition: F(2,18) = 35.91, p < 0.0001] and AMG [main effect of Condition: F(2,18) = 4.90, p < 0.05], although no differences were observed in dHPC. c-Jun, however, was not a very sensitive reporter of neuronal activation under these testing conditions. In fact, the only region in which changes in c-Jun were observed was the PVN: SI rats exhibited increased expression of c-Jun, relative to HCC [main effect of Condition: F(2,18) = 6.77, p < 0.01]. Results of Exp. 1 supported the use of c-Fos as a reporter of neuronal activation under these testing conditions, due to high levels of expression observed in the CXT and SI groups. However, we did not observe differences between the CXT and SI groups, which may be a result of the tissue punching procedure that may not have sufficient anatomical resolution. Thus, in Exp. 2, we used in situ hybridization to assess c-Fos expression with better anatomical specificity in males and females.
Figure 2.
IEG expression in male F344 rats who were home cage controls (HCC), context-exposed (CXT), or social interaction-exposed (SI). Testing was 30 min total, after which CXT and SI rats were returned to their home cage for 30 min prior to tissue collection in (A) OB, (B) PVN, (C) AMG, (D) dHPC. C-Fos expression in all regions was elevated following behavioral testing (CXT and SI), relative to HCC. Egr-1 expression was elevated in CXT and SI rats in the OB, PVN, and AMG, although in the OB, SI rats exhibited increased Egr-1 expression, relative to CXT rats. C-Jun expression was only increased in the PVN in response to the SI condition. # indicates significant differences between conditions.
2.2 Experiment 2
2.2a Behavioral Results
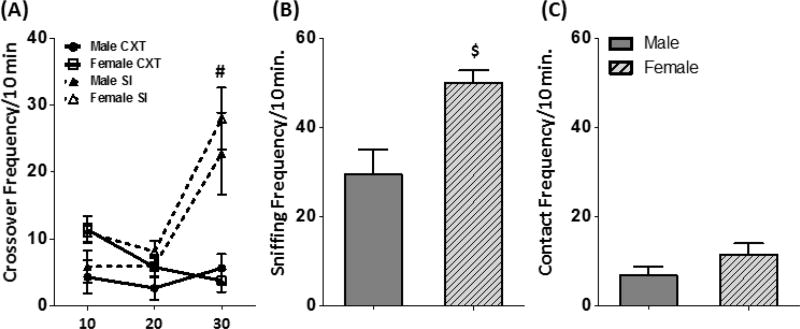
Crossovers exhibited during the testing session were used as a measure of total locomotor activity. There were no differences between groups in crossovers exhibited during the first two 10-min time bins (Figure 3A). As expected, when a social partner was introduced, both male and female SI rats exhibited a significant increase in crossovers [Time Bin × Condition interaction: F(2,46) = 16.00, p < 0.0001]. Females showed higher levels of social investigation [t(14) = 3.24, p < 0.01], although no sex differences were evident for social contact behavior [t(14) = 1.40, p = 0.18] (Figure 3B–C).
Figure 3.
(A) Total crossovers (mean ± SEM) exhibited by male and female CXT and SI rats on test day. Rats in the SI group, regardless of sex, exhibited an increase in crossovers upon introduction of a social partner. Females exhibited greater levels of social investigation (mean ± SEM) (B), but not contact (mean ± SEM) (C), than males. # indicates a main effect of Condition; $ indicates a main effect of sex.
2.2b C-Fos Induction: Optical Density
Analysis of c-Fos expression revealed several interesting patterns of neuronal activation in response to behavioral testing. All data (mean optical density ± SEM) and statistical analyses can be found in Tables 2 and 3. A MANOVA including all ROIs revealed significant main effects of condition [F(44,20) = 3.09, p < 0.01] and sex [F(22,10) = 2.94, p < 0.05]. Due to sex differences in c-Fos induction, separate one-way ANOVAs were conducted for males and females.
Table 2.
Average c-Fos mRNA (mean optical density ± SEM) expression in adult male F344 rats.
| Region | Subregion | HCC | CXT | SI | ANOVA RESULTS |
|---|---|---|---|---|---|
| Prefrontal Cortex | ACC | 0.023 ± 0.011 | 0.076 ± 0.016* | 0.092 ± 0.009* | F(2,16) = 8.79, p < 0.01 |
| PL | 0.021 ± 0.008 | 0.082 ± 0.018* | 0.096 ± 0.011* | F(2,16) = 9.52, p < 0.01 | |
| IL | 0.0014 ± 0.006 | 0.069 ± 0.014* | 0.079 ± 0.009* | F(2,16) = 11.85, p < 0.001 | |
| VO | 0.045 ± 0.016 | 0.130 ± 0.026* | 0.128 ± 0.010* | F(2,16) = 6.96, p < 0.01 | |
| INS | 0.015 ± 0.004 | 0.015 ± 0.003 | 0.012 ± 0.002 | F(2,16) = 0.46, p = 0.64 | |
| Bed Nucleus of the Stria Terminalis | MA | −0.004 ± 0.001 | 0.027 ± 0.009* | 0.015 ± 0.004* | F(2,17) = 7.37, p < 0.01 |
| LD | −0.004 ± 0.001 | 0.018 ± 0.007* | 0.006 ± 0.004 | F(2,17) = 5.17, p < 0.05 | |
| MV | −0.003± 0.001 | 0.022 ± 0.008* | 0.013 ± 0.003* | F ( 2,17) = 7.04, p < 0.01 | |
| LV | −0.001 ± 0.003 | 0.014 ± 0.003* | 0.006 ± 0.003 | F(2,17) = 5.37, p < 0.05 | |
| Lateral Septum | DORSAL | −0.003 ± 0.001 | 0.014 ± 0.006 | 0.016 ± 0.008 | F(2,17) = 2.56, p = 0.11 |
| VENTRAL | −0.003 ± 0.002 | 0.027 ± 0.010* | 0.026 ± 0.006* | F(2,17) = 5.95, p < 0.05 | |
| Rostral Splenial Cortex | LAYER II/III | 0.060 ± 0.012 | 0.189 ± 0.046* | 0.179 ± 0.016* | F(2,16) = 6.44, p < 0.01 |
| LAYER IV | 0.030 ± 0.006 | 0.115 ± 0.029* | 0.117 ± 0.011* | F(2,16) = 7.88, p < 0.01 | |
| LAYER V/VI | 0.020 ± 0.003 | 0.108 ± 0.034* | 0.111± 0.013* | F(2,16) = 5.87, p < 0.05 | |
| Barrel Field Cortex | LAYER II/III | 0.060 ± 0.012 | 0.189 ± 0.046* | 0.179 ± 0.016* | F(2,16) = 11.58, p < 0.001 |
| LAYER IV | 0.054 ± 0.013 | 0.300 ± 0.068* | 0.364 ± 0.046* | F(2,16) = 12.68, p < 0.001 | |
| LAYER V/VI | 0.035 ± 0.006 | 0.219 ± 0.063* | 0.236 ± 0.045* | F(2,16) = 6.51, p < 0.01 | |
| MeA | 0.015 ± 0.004 | 0.097 ± 0.032* | 0.088 ± 0.014* | F(2,16) = 5.35, p < 0.05 | |
| PVN | 0.032 ± 0.013 | 0.088 ± 0.025 | 0.097 ± 0.020 | F(2,16) = 3.06, p = 0.08 | |
| Hippocampus | CA1 | 0.019 ± 0.008 | 0.136 ± 0.048* | 0.149 ± 0.033* | F(2,16) = 4.73, p < 0.05 |
| CA3 | 0.03 ± 0.009 | 0.131 ± 0.037* | 0.144 ± 0.030* | F(2,16) = 4.81, p < 0.05 | |
| DG | 0.025 ± 0.010 | 0.122 ± 0.035* | 0.116 ± 0.021* | F(2,16) = 5.60, p < 0.05 |
Note: All values represent optical density–background labeling.
indicates a significant difference from HCC
indicates a significant difference from CXT.
Table 3.
Average c-Fos mRNA (mean optical density ± SEM) expression in adult female F344 rats.
| Region | Subregion | HCC | CXT | SI | ANOVA RESULTS |
|---|---|---|---|---|---|
| Prefrontal Cortex | ACC | 0.054 ± 0.014 | 0.103 ± 0.025 | 0.163 ± 0.038* | F(2,17) =3.42, p = 0.06 |
| PL | 0.063 ± 0.018 | 0.105 ± 0.027 | 0.174 ± 0.043* | F(2,17) = 2.74, p = 0.09 | |
| IL | 0.050 ± 0.014 | 0.114 ± 0.037 | 0.155 ± 0.027* | F(2,17) = 2.99, p = 0.08 | |
| VO | 0.097 ± 0.027 | 0.179 ± 0.045 | 0.239 ± 0.051 | F(2,17) = 2.59, p = 0.10 | |
| INS | 0.022 ± 0.005 | 0.018 ± 0.003 | 0.035 ± 0.010 | F(2,17) = 1.29, p = 0.30 | |
| Bed Nucleus of the Stria Terminalis | MA | −0.008 ± 0.001 | 0.011 ± 0.004* | 0.026 ± 0.007* | F(2,17) = 10.11, p < 0.01 |
| LD | −0.006 ± 0.00 | 0.008 ± 0.003* | 0.018 ± 0.006* | F(2,17) = 8.49, p < 0.05 | |
| MV | −0.006 ± 0.002 | 0.009 ± 0.004 | 0.044 ± 0.013*^ | F ( 2,14) = 4.21, p < 0.01 | |
| LV | −0.004 ± 0.002 | 0.005 ± 0.001 | 0.024 ± 0.007*^ | F(2,17) = 9.24, p < 0.05 | |
| Lateral Septum | DORSAL | −0.004 ± 0.002 | 0.012 ± 0.003* | 0.015 ± 0.004* | F(2,17) = 7.06, p < 0.01 |
| VENTRAL | −0.005 ± 0.001 | 0.024 ± 0.009* | 0.038 ± 0.010* | F(2,17) = 6.54, p < 0.01 | |
| Rostral Splenial Cortex | LAYER II/III | 0.089 ± 0.023 | 0.111 ± 0.014 | 0.227 ± 0.053* | F(2,17) = 3.80, p < 0.05 |
| LAYER IV | 0.048 ± 0.014 | 0.067 ± 0.009 | 0.155 ± 0.039*^ | F(2,17) = 4.29, p < 0.05 | |
| LAYER V/VI | 0.044 ± 0.015 | 0.060 ± 0.006 | 0.151 ± 0.036*^ | F(2,17) = 5.06, p < 0.05 | |
| Barrel Field Cortex | LAYER II/III | 0.049 ± 0.020 | 0.127 ± 0.018 | 0.342 ± 0.041*^ | F(2,17) = 23.82, p < 0.001 |
| LAYER IV | 0.070 ± 0.023 | 0.178 ± 0.023* | 0.385 ± 0.041*^ | F(2,17) = 24.54, p < 0.001 | |
| LAYER V/VI | 0.048 ± 0.019 | 0.118 ± 0.019 | 0.270 ± 0.043*^ | F(2,17) = 12.25, p < 0.001 | |
| MeA | 0.020 ± 0.006 | 0.069 ± 0.013* | 0.122 ± 0.015*^ | F(2,17) = 16.40, p < 0.001 | |
| PVN | 0.033 ± 0.010 | 0.037 ± 0.005 | 0.087 ± 0.031 | F(2,17) = 1.88, p = 0.18 | |
| Hippocampus | CA1 | 0.015 ± 0.007 | 0.064 ± 0.010 | 0.158 ± 0.027*^ | F(2,17) = 13.81, p < 0.01 |
| CA3 | 0.045 ± 0.018 | 0.078 ± 0.013 | 0.157 ± 0.027*^ | F(2,17) = 7.20, p < 0.01 | |
| DG | 0.015 ± 0.006 | 0.055 ± 0.010 | 0.128 ± 0.021*^ | F(2,17) = 13.44, p < 0.01 |
Note: All values represent optical density–background labeling.
indicates a significant difference from HCC
indicates a significant difference from CXT.
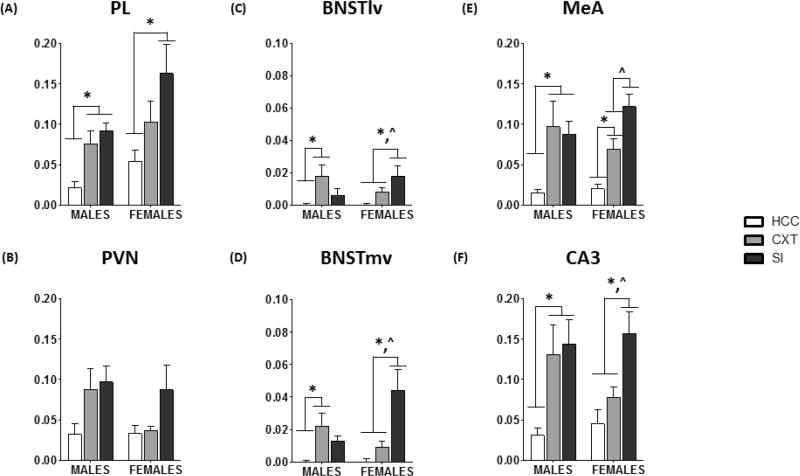
In males, there was a very consistent pattern of c-Fos induction in which there was a similar level of c-Fos expression in the CXT and SI groups. For example, there was a main effect of Condition in the PL [F(2,16) = 9.52, p < 0.01] (Figure 4A). Post-hoc analyses revealed a significant increase in c-Fos expression in the CXT and SI groups, relative to HCC, but CXT and SI animals were not different from one another. This pattern was also evident in the ACC, IL, VO, VLS, BNSTma, BNSTmv, MEA (Figure 4E), BF II/III, BF IV, BF V/VI, rSC II/III, rSC IV, rSC V/VI, CA1, CA3 (Figure 4F), and DG (see caption for Figure 4 for a list of abbreviations and their definitions). On the other hand, in some BNST subregions, the only significant differences were between CXT and HCC males [BNSTld: F(2,17) = 5.17, p < 0.05; BNSTlv: F(2,17) = 5.37, p < 0.05] (Figure 4C, D). Finally, no significant group differences were observed in the INS, DLS, or PVN (Figure 4B). Thus, in males, there were very few brain regions in which social interaction led to a specific induction of c-Fos.
Figure 4.
c-Fos mRNA expression (optical density; mean ± SEM) in home cage controls (HCC), context-exposed (CXT), or social interaction-exposed (SI). Testing was 30 min total, after which CXT and SI rats were returned to their home cage for 30 min prior to tissue collection in (A) PL, (B) PVN, (C) BNSTlv, (D) BNSTmv, (E) MeA, and (F) CA3. In PL, MeA, and CA3, males in the CXT and SI groups exhibited similar levels of c-Fos expression. In BNST, males in the CXT group exhibited increased c-Fos, relative to HCC. Females in the SI group exhibited increased c-Fos relative to both HCC and CXT in PL, BNSTlv, BNSTmv, and CA3. In MeA, CXT females exhibited increased c-Fos expression relative to HCC, whereas SI females exhibited increased c-Fos expression relative to CXT and HCC females. PL: prelimbic cortex, PVN: paraventricular nucleus of the hypothalamus, BNSTlv: bed nucleus of the stria terminalis, ventrolateral division, BNSTmv: bed nucleus of the stria terminalis medial division, anterior part, MeA: medial amygdala, CA3: Cornu Ammonis 3. * indicates a significant difference from HCC; ^ indicates a significant difference from CXT.
Although there were similar main effects of Condition in females, post-hoc analyses revealed that the patterns of c-Fos expression were significantly different than what was observed in males. For example, within the PFC [ACC, PL, IL], there were trends for main effects of Condition (p’s < 0.10) and examination of the mean optical density suggests a difference between SI and HCC females, but no difference between HCC and CXT females (Table 3). In several ROIs, SI females were significantly different from both HCC and CXT females, in contrast to what was observed in males. These included the BNSTmv (Figure 4D), BNSTlv (Figure 4C), BF II/III, BF V/VI, rSC IV, rSC V/VI, CA1, CA3 (Figure 4F), and DG. In sum, in females, there were several brain regions in which neuronal activation was specific to social interaction, or otherwise larger than what was observed in those that were context-exposed.
3.0 Discussion
The goal of these experiments was to (i) test several common IEGs to determine which would be most sensitive under these testing parameters; (ii) examine whether sex differences in the expression of social behavior are evident in adult F344 rats; and (iii) assess neuronal activation in response to a context or social interaction in male and female F344 rats. In Exp. 1, we examined the expression of three IEGs: c-Fos, Egr-1, and c-Jun, to determine which would be a more sensitive reporter of neuronal activation under these testing circumstances. Not surprisingly, c-Fos was the best, with behavioral testing inducing a robust (e.g., 2–5-fold) c-Fos mRNA increase in response to both context and social interaction relative to the HCC group. In contrast, modest (e.g., 1–3-fold) changes were observed in Egr-1 expression, whereas c-Jun mRNA was only elevated in the PVN of the SI rats.
Surprisingly, there were very few brain region differences in neuronal activation to a social stimulus per se assessed via IEG expression, with, in general, similar gene expression evident in the CXT and SI groups. These findings suggest that gene expression was associated, to a large extent, with placement of a rat in a relatively novel and, hence, anxiogenic environment rather than with responding to a conspecific. However, SI rats had greater Egr-1 expression than CXT rats, in the OB, an effect that is likely the result of olfactory input from the social partner. Indeed, olfaction plays a substantial role in social behavior. For instance, odor signatures emitted by a conspecific can provide a wealth of information about health status, reproductive status, and familiarity (Arakawa et al., 2011). In addition, detection of olfactory cues is required for conspecific recognition, which is mediated, in part, by the actions of the neuropeptides oxytocin (OT) and arginine vasopressin (AVP), as well as gonadal steroids (Choleris et al., 2009; Gabor et al., 2012; Young, 2002).
In Exp. 2, the same testing parameters were used to assess whether responsiveness to the social stimulus could produce more pronounced c-Fos expression than exposure to the context alone. In addition, Exp. 2 used in situ hybridization, which had the added benefit of providing increased anatomical specificity, compared to the nonspecific tissue punch procedure used in Exp. 1. We expected that analysis of more discrete brain regions would yield areas in which SI produced a more robust c-Fos response. However, we observed no significant differences between the CXT and SI groups in males, consistent with the results with RT-PCR. In fact, c-Fos induction in males in the CXT and SI groups was remarkably similar in most brain regions examined, indicative of a general activation in response to behavioral testing that is not specifically related to social behavior. Whether a longer social interaction exposure would produce a separation between CXT and SI males remains to be determined. However, in females, we observed a substantially different pattern. In almost all ROIs, c-Fos induction was larger in the SI group, relative to HCC and CXT groups. Thus, females exhibited robust neuronal activation that was specific to exposure to a conspecific.
In the BNST, there were considerable differences between the patterns of c-Fos expression observed in males and females. For example, in the BNSTlv, males in the CXT group had increased c-Fos relative to both the HCC and SI groups. In contrast, c-Fos expression was higher in SI females, relative to both CXT and SI females. A similar pattern was observed in the BNSTma and BNSTmv, suggesting that the BNST as a whole exhibits a sexually dimorphic response to social stimuli. The BNST receives substantial OT projections from the PVN that are thought to play a role in the processing of chemosignals during sexual and/or social behavior (Petrulis, 2013). Although the phenotype of the neurons activated under these conditions is unknown, it is likely that a subpopulation of those activated by social interaction are OT or AVP neurons, since the BNST receives extensive OT and AVP innervation (de Vries and Buijs, 1983; Petrulis, 2013). Importantly, sex differences are observed in the AVP system in the BNST, with males exhibiting more AVP fibers in LS that originate from AVP-producing cells in the BNST (de Vries and Buijs, 1983). If social interaction produced a robust increase in AVP, we would have expected greater c-Fos induction in the BNST of males than females, owing to higher levels of AVP in males (de Vries and Buijs, 1983). On the contrary, we observed greater c-Fos induction in female SI rats, arguing against these being AVP neurons. On the other hand, OT is a well-known modulator of social behavior. Some studies have shown that males and female rats do not differ in OT mRNA expression and OT-receptor (OT-R) binding (Dumais and Veenema, 2016), although increased OT-R binding in male Wistar rats in the BNST has been observed (Dumais et al., 2013). To our knowledge, OT-R binding density has not been examined in male and female F344 rats, and it is possible that in this strain, OT-R binding density is higher in females than males, owing to the higher levels of social interaction exhibited by females. Perhaps social interaction produced a robust increase in OT production leading to increased activation of neurons containing OT-R within the BNST and MEA. Indeed, SI females exhibited robust activation of neurons within the BSNT and MEA, although future studies utilizing double-labeling for OT-R and Fos are needed to determine if this is the case.
Another goal of this study was to examine sex differences in the expression of social behavior in F344 rats. Adult male Sprague Dawley rats exhibit greater levels of social interaction with a novel conspecific, compared to their female counterparts (Stack et al., 2010). In a study examining sex differences in social investigation as a function of partner familiarity, Johnston & File (1991) demonstrated that although adult male Lister rats exhibit greater levels of social investigation than females, this effect was more pronounced when the social partner was familiar. When presented with a novel age-matched social partner, we observed greater levels of social interaction in F344 females than in their male counterparts. Although levels of social behavior are lower in F344 rats, relative to other strains such as Sprague Dawley rats, sex differences in social behavior were evident in adults. Interestingly, female F344 rats exhibit greater levels of social behavior relative to males, which is in contrast with Sprague-Dawley rats. Thus, F344 rats may be a unique strain in which to examine mechanisms driving sex differences in social behavior. In addition, in the current study, rats were single-housed, whereas in previous studies, rats were pair-housed (Stack et al., 2010). It is well known that housing condition significantly influences the expression of social behavior (Varlinskaya et al., 1999; Varlinskaya and Spear, 2008) as well as rewarding properties of social (Douglas et al., 2004) and other natural stimuli (Douglas et al., 2003). For instance, social isolation eliminated novel object-induced CPP in Sprague Dawley females, with males being insensitive to these effects of social isolation (Douglas et al., 2003). Clearly, future studies should systematically examine the effects of social isolation on social interaction in male and female F344 rats.
Within the PFC, males had lower c-Fos induction relative to their female counterparts, regardless of testing condition. Although there are sex differences in the timing of neuronal loss (Willing and Juraska, 2015) and dendritic complexity (Markham et al., 2013) in adolescence, less is known about sex differences in neuron number in adulthood that could contribute to the changes observed in the PFC. Previous studies have reported no sex differences in the number of neurons in the mPFC in adult Long Evans rats (Koss et al., 2012; Wise et al., 2016). In addition, Sprague Dawley females (P62) exhibit fewer and shorter branches in apical, but not basilar, dendrites in the mPFC, compared to their male counterparts (Garrett and Wellman, 2009). On the other hand, the PFC is sensitive to stress. In fact, Sprague Dawley males (P51–59 at stress onset) are more susceptible to the effects of stress (7 d) on dendritic complexity in the PFC (Garrett and Wellman, 2009). Perhaps social isolation (a potential stressor) influences neuronal number in a sexually dimorphic manner, such that social isolation decreases neuronal number in males, but not females, although future studies would be needed to confirm whether this is indeed the case.
As with any project, the present studies have some minor interpretational limitations as well. For instance, we elected to assess a single time point (30 min post-testing) for the assessment of IEGs in both experiments. This time point was selected based on prior studies demonstrating that c-Fos gene induction evinces an apparent peak in expression at this time point, though it should be noted that the time course of c-Fos expression can vary across brain sites (e.g. (Pace et al., 2005; Weinberg et al., 2007). Second, the present studies assessed gene expression changes, which may not be perfectly predictive of changes in functional protein. However, gene expression analysis allows for greater temporal resolution when the goal is to discriminate between highly similar experiences such as context exposure and social interaction. Nevertheless, these minor limitations (single time point, gene expression analysis) should be considered in interpretation of the present findings. It is noteworthy that in female rats only 10 min of social interaction in the novel context was sufficient to produce significantly greater c-fos mRNA levels as assessed 30 min later than was present in rats that were exposed to the novel context but lacked that final 10 min of social interaction. Although we incorporated a non-socially tested control, future studies should consider introducing a novel object to the CXT group to more closely mimic the introduction of a novel social partner in the SI group. In sum, the use of context-exposed controls should be considered a major strength of the present studies since few other published works have attempted to isolate IEG responses specifically to the social aspect of the task.
In summary, we demonstrated that social investigation is elevated in adult F344 females, compared to their male counterparts. In males, c-Fos induction was observed in the CXT and SI groups at comparable levels, suggesting a generalized activation in response to behavioral testing. Within the BNST, a sexually dimorphic pattern of activation was observed, wherein SI males exhibited c-Fos induction that was similar in magnitude to that observed in male and female CXT rats. However, SI females exhibited robust activation, particularly within the BNSTmv, highlighting the BNST as a target region to examine mechanisms that drive sex differences in social behavior. Future studies will assess activation of OT neurons within the PVN and OTR in the BNST in response to a social partner. Altogether, these data contribute to the growing literature on sex differences in social behavior, and provide an important step in understanding the mechanism(s) that drive sex differences in social behavior regulation. Furthermore, these findings can be used as a basis for designing future studies to assess neural responses to social stimuli during late aging in the F344 rat strain.
4.0 Experimental Procedures
4.1 Subjects
Experiment 1 utilized male F344 rats (3 months; n = 6–9/group), whereas Experiment 2 used male and female F344 rats (4 months; n = 6–8/group). Subjects were obtained from the National Institute of Aging (NIA) colony maintained by Charles River Laboratories and given at least 1 week to acclimate to the colony before the onset of experimentation. Social partners were age- and sex-matched F344 rats. Colony conditions were maintained at 22 ± 1°C with 12:12 light:dark cycle (lights on 0700h). Rats were single-housed and provided ad libitum access to food and water. On the day prior to the onset of behavioral testing, rats were handled for 3 min. At all times, rats were maintained and treated in accordance with the guidelines set forth by the Institute of Laboratory Rat Resources, (1996) and in accordance with the protocol approved by the IACUC at Binghamton University.
4.2 Experimental Procedures
4.2a Apparatus
Testing was conducted in rooms with dim lighting (10–20 lux) between 0800 and 1200 with no experimenter present. Males and females were tested in separate rooms. Rats were placed in Plexiglas chambers (Binghamton Plate Glass, Binghamton, NY) 45 × 30 × 30 cm. Clean pine shavings lined the bottoms of the apparatuses. Each test chamber was divided into two compartments that were equal in size by a clear Plexiglas partition containing an aperture (9 × 7 cm) that allowed for movement of rats between the compartments. After testing of each subject, the soiled wood shavings were removed, chambers were cleaned with water, and clean shavings were added for the next subject. Behavior of the rats was recorded by a camcorder (Panasonic model AF-X8, Secaucus, NJ). At the end of each day, chambers were wiped down with a 3% hydrogen peroxide solution.
4.2b Experimental Conditions
There were three experimental conditions: home cage controls (HCC), context-exposed (CXT), and social interaction-exposed (SI). HCC rats were handled prior to test day, but did not undergo any behavioral testing. CXT rats were placed into the testing apparatus for 30 min. SI rats were placed into the apparatus for 20 min, after which a novel age- and sex-matched social partner was introduced for a 10-min social interaction test (see Figure 1A). Rats were returned to their home cage after testing for 30 min prior to tissue collection.
4.2c Behavioral Measures
The total number of crossovers (movement through the aperture) demonstrated by the experimental subjects (CXT and SI groups) was recorded for each 10-min time bin of testing and was used as an index of locomotor activity. For rats in the SI group, social investigation was defined as the sniffing of any part of the body of the partner, whereas frequency of contact behavior was scored as the sum of crawling over and under the partner and social grooming.
4.3 Methods for Experiment 1
The goal of Exp. 1 was to determine which IEG would be best suited to probe responsiveness to a social stimulus. RT-PCR was used as a high-throughput method to assess several IEGs (c-Fos, Egr-1, and c-Jun) in brain structures known to be involved in the regulation of social behavior: olfactory bulb (OB), paraventricular nucleus of the hypothalamus (PVN), amygdala (AMG), and dorsal hippocampus (dHPC). Rats were rapidly decapitated (unanesthetized) 30 min after the end of testing (CXT and SI), or were taken directly from their home cages (HCC). This time point was chosen based on previous research indicating peak expression of c-Fos, Egr-1, and c-Jun mRNA at 30–60 min following acute stress exposure (Cullinan et al., 1995; Pace et al., 2005). Brains were removed and flash-frozen in methylbutane (EMD Millipore, Darmstadt, Germany; cat. no. MX0760-1) on dry ice and stored at −80° C until processing. OB were collected into 2.0 mL RNase-free tubes (Eppendorf; Cat. No. 022363352) for later RNA extraction.
Brains were sectioned on a cryostat and structures of interest were microdissected with biopsy punches according to the stereotaxic atlas of the rat brain (Paxinos and Watson, 1998) and placed into 2.0 mL RNase-free tubes (Figure 1B). 500 µL of Trizol® RNA reagent (Invitrogen, Grand Island, NY; Cat. No. 15596018) and a 5 mm stainless steel bead were added to each tube. Tissue was homogenized using a TissueLyser (Qiagen, Velencia, CA) for 2 mins at 20 Hz. Chloroform (100 µL; Cat. No. CX1055-2; EMD, Darmstadt, Germany) was added to each sample containing Trizol, and samples were centrifuged for 15 mins at 12,000g (4° C). RNA was extracted using an RNeasy Mini Kit (Qiagen; Cat. No. 74106). RNA quantity and quality were assessed using a Nanodrop spectrophotometer (Nanodrop 2000, Thermo Scientific, Wilmington, DE), after which samples were stored at −80° C. cDNA was synthesized on 0.1–1.0 µg of total RNA from each sample using a QuantiTect® Reverse Transcription Kit (Qiagen, Valencia, CA; Cat. No. 205313) and stored at −20° C.
Amplification of cDNA was achieved using 5 µL of IQ SYBR Green Supermix (Bio-Rat, Hercules, CA; Cat. No. 170–8882), 0.5 µL primer (final concentration 250 nM), 0.5 µL cDNA, and 4 µL RNase-free water, for a total reaction volume of 10 µL. Primer sequences can be found in Table 1. Samples were run in triplicate in a 384 well plate (BioRad; Cat. No. HSP-3805) and the reaction was captured in real-time with a PCR detection system (BioRad, Model No. CFX384) (Doremus-Fitzwater et al., 2015). The first step in the PCR reaction was a 3-min hot start (95° C), followed by denaturation (30 s at 95° C), annealing (30 s at 60° C), and finally an extension step (30 s at 72° C) for 50 cycles. A final denaturation (1 min at 95° C) and annealing cycle (1 min at 55° C) was used to ensure that PCR product was properly aligned prior to melt curve analysis. Melt curve analysis was accomplished through 0.5 ° C changes every 15 s (55° C to 95° C). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene in all analyses. Expression was calculated using the 2ΔΔCT method (Livak and Schmittgen, 2001). HCC rats were used as the ultimate control to which all data were compared.
Table 1.
RT-PCR primer sequences used in Experiment 1.
| Gene target |
Accession number | RT-PCR primer sequence | Function |
|---|---|---|---|
| C-Fos | NM_022197.2 | Forward: CCAAGCGGAGACAGATCAAC Reverse: AAGTCCAGGGAGGTCACAGA |
Immediate early gene used as a reporter of cellular activity. Basal expression of c-fos is very low across many brain structures (Herdegen & Leah, 1998). |
| Egr-1a | NM_012551 | Forward: ACAACTACCCCAAACTGGAGG Reverse: CGATGTCAGAAAAGGACTCTGTG |
Immediate early gene used as a reporter of cellular activity. |
| C-Jun | NM_021835 | Forward: CCAACCAACGTGAGTGCAAG Reverse: CGTCCCCGCTTCAGTAACAA |
Immediate early gene used as a reporter of cellular activity. C-Jun is expressed at higher basal levels than c-fos (Herdegen & Leah, 1998). |
| GAPDHb | NM_017008 | Forward: GTGCCAGCCTCGTCTCATAG Reverse: AGAGAAGGCAGCCCTGGTAA |
GAPDH is critical for cellular metabolic processes. It was used as a stable reference gene for assessment of target gene expression. |
Early growth response gene 1; also referred to as zif268.
Glyceraldehyde 3-phosphate dehydrogenase
4.4 Methods for Experiment 2
Based on results from Exp. 1 demonstrating that c-Fos was the most sensitive reporter of neuronal activation under these testing conditions, in a second study c-Fos mRNA expression was assessed with in situ hybridization. This technique allowed for the analysis of c-Fos induction across several regions of interest (ROIs) with enhanced anatomical specificity relative to the tissue punching method used in Exp. 1. Rats were rapidly decapitated (unanesthetized) 30 mins after the end of testing (CXT and SI), or were taken directly from their home cages (HCC). Brains were removed and flash-frozen in methylbutane (EMD Millipore, Darmstadt, Germany; cat. no. MX0760-1) on dry ice and stored at −80° C until processing.
Sections (12 µm) were cut on a cryostat (Leica model 1850), thaw-mounted on Colorfrost® plus microscope slides (VWR, Radnor, PA) and stored at −80 °C. Sections were collected that contained the following regions of interest (ROIs) according to Paxinos & Watson (1986; Figures 1C): anterior cingulate cortex (ACC), prelimbic cortex (PL), infralimbic cortex (IL), insula (INS), ventral orbital cortex (VO), dorsal lateral septum (dLS), ventral lateral septum (vLS), bed nucleus of the stria terminalis (BNST) medial division (BNSTmd), anterior part (BNSTma), BNST lateral division, dorsal part (BNSTld), ventromedial BNST (BNSTmv), ventrolateral BNST (BNSTlv), retrosplenial cortex (rSC) layers II/III (rSC II/III), IV (rSC IV), and V/VI (rSC V/VI), PVN, barrel field cortex (BF) layers II/III (BF II/III), IV (BF IV), and V/VI (BF V/VI), CA1, CA3, dentate gyrus (DG), and medial amygdala (MeA).
The methods used to assess c-Fos mRNA expression have been described previously (Newsom et al., 2012). Briefly, sections were fixed in 4% paraformaldehyde for 1 hour, followed by a 10 min acetylation step in 0.1 M triethanolamine with 0.25% acetic anhydride. Then, sections were dehydrated using graded alcohols. Sections were hybridized overnight at 55°C with a [35S]- UTP-labeled riboprobe against c-Fos mRNA (680 mer), diluted in hybridization buffer [50% formamide, 10% dextran sulfate, 2 × saline sodium citrate (SSC), 50 mM PBS (pH 7.4), 1 × Denhardt’s solution and 0.1 mg/ml yeast tRNA]. The next day, sections were incubated with RNase A (200 µg/ml) at 37 °C for 1 hour. Finally, sections were washed to a final stringency of 0.1 × SSC (65 °C) for 1 hour. Dehydrated sections were exposed to X-ray film (BioMax MR; Eastman Kodak, Rochester, NY) for structure-appropriate times (1–3 weeks), after which film images were digitized using a Northern Light lightbox (model B95, Imaging Res, Inc), a Sony CCD video camera (model XC-ST70) fitted with a Navitar 7000 zoom lens connected to a LG3-01 frame grabber (Scion Corp) inside a Dell Dimension 500, and captured with a Scion Image (beta release 4.0.2). Structures chosen for c-fos mRNA analysis include regions with high levels of neuronal activity (as indicated by induction of c-fos mRNA) in response to acute loud noise stress (Burow et al., 2005). Densitometry measurements were obtained using ImageJ software 1.46r (National Institutes of Health). Uncalibrated optical density from left and right hemispheres on 4–6 sections per brain, per ROI was performed by an individual blind to the treatment condition. Optical density values were averaged across each of the tissue sections/hemispheres for each brain to yield a single value for that brain. Background labeling was subtracted from optical density for all data used in analyses.
4.4 Data Analysis
Data were analyzed using Statistica Version 12 (StatSoft, Inc, Tulsa, OK). Gene expression was analyzed with a one-way ANOVA with 3 groups (HCC, CXT, and SI). For Exp. 2, the total number of crossovers during behavioral testing was examined with a 2 × 2 × 3 [Sex × Condition × Time Bin] mixed-factor ANOVA, with Time Bin treated as a repeated measure. Social behavior was analyzed with independent samples t-tests. c-Fos expression was first analyzed across all ROIs with a multivariate ANOVA to account for increased Type I error associated with several independent ANOVAs. Then, c-Fos expression in each ROI was analyzed using separate 2 × 3 [Sex × Condition] ANOVAs. Outliers, defined as any values greater than 2 standard deviations from the mean, were removed from analyses, leaving samples sizes of 5–8/group. For all analyses, Fisher’s Least Significant Difference (LSD) post-hoc tests were used to isolate significant pairwise group differences in the event of significant main effects or interactions.
Highlights.
Adult females exhibited greater levels of social behavior relative to adult males
C-Fos induction was similar in context- and social-interaction exposed males
The BNST was a site of sexually dimorphic responses to social interaction
Acknowledgments
Supported by NIH grant number R01AG043467 to T.D. and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- Arakawa H, Cruz S, Deak T. From models to mechanisms: Odorant communication as a key determinant of social behavior in rodents during illness-associated states. Neurosci. Biobehav. Rev. 2011;35:1916–1928. doi: 10.1016/j.neubiorev.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156:247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front. Neuroendocrinol. 2009;30:442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- de Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE, Deak T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol. Behav. 2015;148:131–144. doi: 10.1016/j.physbeh.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol. Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: Correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: Sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057.Chronic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res. Bull. 2002;57:651–659. doi: 10.1016/S0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/S0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol. Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system.pdf. Pharmacol. Rev. 1995:47. [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex Differences in Animal Tests of Anxiety. Physiol. Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska J. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.pestbp.2011.02.012.Investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács KJ. Measurement of immediate-early gene activation- c-fos and beyond. J. Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative. PCR and Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, Koenig JI. Peri-adolescent maturation of the prefrontal cortex is sex-specific and disrupted by prenatal stress. J. Comp. Neurol. 2013;521:1828–1843. doi: 10.1002/cne.23262.Peri-adolescent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom RJ, Osterlund C, Masini CV, Day HE, Spencer RL. Cannabinoid receptor type 1 antagonism significantly modulates basal and loud noise induced neural and HPA axis responses in male Sprague Dawley rats. Neuroscience. 2012;204:64–73. doi: 10.1016/j.neuroscience.2011.11.043.Cannabinoid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur. J. Neurosci. 2005;22:1679–1690. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego, CA: 1998. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci. Biobehav. Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play fighting of rats in comparative perspective: A schema for neurobehavioral analyses. Neurosci. Biobehav. Rev. 1998;23:87–101. doi: 10.1016/S0149-7634(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Perkins AE, Doremus-Fitzwater TL, Spencer RL, Varlinskaya EI, Conti MM, Bishop C, Deak T. A working model for the assessment of disruptions in social behavior among aged rats: The role of sex differences, social recognition, and sensorimotor processes. Exp. Gerontol. 2016;76:46–57. doi: 10.1016/j.exger.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A. Chemosignals and hormones in the neural control of mammalian sexual behavior. Front. Neuroendocrinol. 2013;34:255–267. doi: 10.1016/j.yfrne.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci. Biobehav. Rev. 2011;35:1821–1830. doi: 10.1016/j.neubiorev.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex Differences in Social Interaction in Rats: Role of the Immediate-Early Gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Mikics E, Tulogdi A, Aliczki M, Haller J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm. Behav. 2011;60:28–36. doi: 10.1016/j.yhbeh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof LWM, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJMJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Struct. Funct. 2014;219:1181–1211. doi: 10.1007/s00429-013-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Achterberg EJM, Trezza V. The neurobiology of social play behavior and its rewarding value in rats. Neurosci. Biobehav. Rev. 2016;70:86–105. doi: 10.1016/S0149-7634(96)00020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya E, Spear L, Spear N. Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol. Behav. Behav. 1999;67:475–482. doi: 10.1016/S0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: Impact of social deprivation and test context familiarity. Behav. Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024.Social. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 2014;45:433–444. doi: 10.1016/j.pestbp.2011.02.012.Investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt Ba, Spear LP. Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Dev. Psychobiol. 2013;55:684–697. doi: 10.1002/dev.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Girotti M, Spencer RL. Restraint-induced fra-2 and c-fos expression in the rat forebrain: Relationship to stress duration. Neuroscience. 2007;150:478–486. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska J. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LM, Sadowski RN, Kim T, Willing J, Juraska JM. Long-term effects of adolescent exposure to bisphenol A on neuron and glia number in the rat prefrontal cortex: Differences between the sexes and cell type. Neurotoxicology. 2016;53:186–192. doi: 10.1016/j.neuro.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol. Psychiatry. 2002;51:18–26. doi: 10.1016/S0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]