Abstract
Background
The antimicrobial mechanisms of ɛ-polylysine (EPL) against Pseudomonas aeruginosa and Aspergillus fumigatus biofilm were investigated.
Material/Methods
We assessed the changes in electric conductivity of broth and total sugar concentration, as well as changes in phosphorous metabolism and protein expression, of the 2 organisms before and after treatment with EPL.
Results
The experimental results showed that EPL has antimicrobial activity against Pseudomonas aeruginosa and Aspergillus fumigatus, but the activity was much stronger for the former. After treatment with EPL, the electric conductivity and total sugar concentration of microbial broth increased, suggesting that EPL damages the cell membrane structure, which increases permeability of the cell membrane and release of cell components.
Conclusions
The consumption of phosphorous decreased in the EPL-treated organisms, which seriously affected the synthesis of important cell components such as nucleic acid and phospholipid, as well as energy metabolism.
MeSH Keywords: Aspergillus Fumigatus, Biofilms, Polylysine, Pseudomonas Aeruginosa
Background
ɛ-Polylysine, also known as ɛ-poly-L-lysine (EPL), was first separated from soil by Shima and Sakai in 1977 [1]. EPL is a lysine homopolymer typically produced as a homopolypeptide containing approximately 25–30 L-lysine residues. It can be completely digested and absorbed in vivo without any adverse effects. Previous research demonstrated that EPL can be adsorbed electrostatically to the cell surface of the bacteria, followed by stripping of the outer membrane [2–4]. This finally results in the abnormal distribution of the cytoplasm, causing damage to the bacterial cell, which is produced by bacterial fermentation [5,6]. The chemical structure of EPL is shown in Figure 1.
Figure 1.
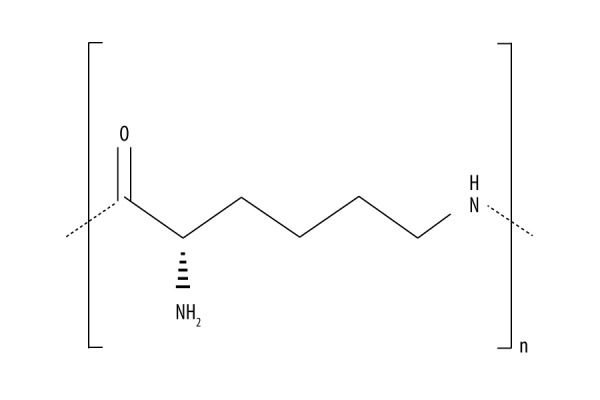
Chemical structure of EPL.
EPL can decompose into lysine in vivo, and lysine is one of 8 essential amino acids in the human body. Therefore, EPL is a nutrient-type antibacterial agent, and it is safer than other chemical preservatives. EPL has a broad antimicrobial spectrum, and it has obvious inhibition and killing effect on yeasts such as Candida albicans, red yeast, Pichia pastoris yeast, rose Sporobolomyces yeast; as well as on gram-positive bacteria such as heat-resistant bacillus, Bacillus coagulans, Bacillus subtilis, and gram-negative bacteria such as integrity-producing Bacillus and Escherichia coli [2–6].
Pseudomonas aeruginosa is a highly pathogenic strain of bacillus widely present in nature, human skin, and in the intestinal and respiratory tracts. Clinically, this bacterium is very common in wound infection and usually causes purulent lesions [7–10]. Aspergillus fumigatus is also widely present in nature. It is the most common opportunistic pathogenic fungi, especially in the patients with immune deficiency, organ transplantation, and medical treatment. Aspergillus fumigatus can adhere to implanted materials or tissue cells to form a typical biofilm structure, which is closely related to the refractory infection caused by the bacteria [8,9,11,13–17].
Recent studies have found that lysine and its derivatives have anti-fungal effects [12,14,15]. However, it is still unclear whether EPL has activity against Pseudomonas aeruginosa and Aspergillus fumigatus biofilm. In the present study, we assessed the effects of EPL on the biofilm of Pseudomonas aeruginosa and Aspergillus fumigatus in vitro, producing experimental evidence fundamental to research and development of new antimicrobial agents.
Material and Methods
Materials and reagents
Purified Aspergillus fumigatus and Pseudomonas aeruginosa bacteria were provided by the Clinical Laboratory Center of Gansu Provincial Hospital. EPL (analytically pure, MW ~5000 g/mol, PDI ~1.50) was purchased from Lanzhou Hong Rong Food Additives Co., Ltd. and nutrient agar and nutrient broth were purchased from Guangzhou Detgerm Microbiology Technology Co. Ltd. Other analytically pure chemical reagents were purchased from Lanzhou Zhonglian Chemical Reagent Co., Ltd.
Determination of EPL antimicrobial activity
Activated Aspergillus fumigatus and Pseudomonas aeruginosa were transferred into nutrient broth for culture at 35°C, then centrifuge at 3000 rpm for 15 min, after which the supernatant was removed and the bacteria were diluted to the concentration of 1×104 CFU/mL. EPL was added at a final concentration of 2.5 g/L. Bacterial fluid was incubated at room temperature for 5, 10, 20, 30, and 60 min, and 50-μL samples were taken. Samples underwent inverted culturing at 35°C for 24 h to observe colony growth. Sterile water was then used to replace EPL for experiments as blank control.
Determination of bacteria liquid conductivity
Aspergillus fumigatus and Pseudomonas aeruginosa bacteria grown to logarithmic phase were washed 3 times with 0.2 mol/L phosphate buffer (pH=7.4). Bacterium liquid concentration was adjusted to 1×106 CFU/mL, and 5 mL of sample was taken to mix with 1.5 g/L and 3 g/L of EPL with an equal volume. Conductivity was measured every 10 min. Sterile water was then used to replace EPL for experiments as blank control.
Determination of total bacterial soluble sugar
Aspergillus fumigatus and Pseudomonas aeruginosa bacteria (5 mL) grown to logarithmic phase were mixed with an equal volume of EPL (1 g/L and 2 g/L). Samples (1 mL) of mixed fluid were taken at 0, 2, 5, 8, 12, 18, and 24 h to centrifuge at 11 000 rpm for 10 min. Supernatant was diluted 6 times and 75 μL of liquid was transferred into a centrifuge tube to where anthrone reagent (100 μL) was added. The mixture was quickly placed in an ice bath to cool for 10 min, and then placed in a boiling water bath to boil for 20 min, then cooled and stored at room temperature for 30 min. The absorbance at 650 nm was determined by an enzyme-labelled instrument. A standard glucose solution was used to make the standard curve. Sterile water was then used to replace EPL for experiments as blank control.
Determination of phosphorus bacteria metabolism
The activated bacteria were transferred to nutrient liquid, cultured for 18 h at 35°C, followed by centrifugation at 3000 rpm for 20 min. Supernatant was discarded, the bacteria were diluted with 0.2 mol/L phosphate buffer (pH=7.4) to obtain the bacteria liquid at a concentration of 1×106 CFU/mL, then 0.2 mL bacteria liquid and 0.2 ml glucose solution (2 g/L) were transferred into a centrifuge tube. Phosphorus standard solution (100 μl) and EPL (100 μL, 3 g/L) were added and suspensions (0.5 mL) were taken at 0, 2, 5, 8, 12, 18, and 24 h. After treatment with trichloroacetic acid and ferrous sulfate, the absorbance of treated liquid at 650 nm was determined by an enzyme-labelled instrument. Sterile water was then used to replace EPL for experiments as blank control.
Polyacrylamide gel electrophoresis (SDS-PAGE) of total bacterial protein
Aspergillus fumigatus and Pseudomonas aeruginosa bacteria grown to logarithmic phase were washed with 0.2 mol/L phosphate buffer (pH=7.4), and then diluted to bacteria liquid with OD600=0.5. After mixing with an equal volume of EPL (2 g/L), the bacterial liquid samples (5 mL) taken at 1, 2, 3, 4, 5, and 6 h were centrifuged at 5000 rpm for 10 min. Sterile water (70 mL) and sample buffer (100 μL) were added into the precipitates, thoroughly mixed, and heated in a boiling water bath for 5 min. After centrifugation, supernatant (50 μL) was taken for SDS-PAGE electrophoresis. Sterile water was then used to replace EPL for experiments as blank control. The concentrations of stacking gel and separating gel were 6% and 18%, respectively. After electrophoresis, 0.1% Coomassie brilliant blue R-250 was used to stain the gels, and a methanol (34%) and acetic acid (9%) mixture was applied for decolorization.
Statistical analysis
All data are expressed using χ±s and the differences were compared using single-factor analysis of variance. SPSS 13 software was used for variance analysis.
Results
Antibacterial activity of EPL
The inhibitory effect of EPL on 2 strains of Aspergillus fumigatus and Pseudomonas aeruginosa with different interaction times is shown in Figure 2. The results show that EPL had an obvious antibacterial effect on both bacteria, and the inhibitory rate increased with increased interaction time. The antibacterial rate of Aspergillus fumigatus treated by EPL at 2 g/L for 30 min reached 62.4%, but the inhibitory rate of Pseudomonas aeruginosa was only 22.3%, and started to increase significantly (P<0.05) until 60 min. This suggests that the antimicrobial effect of EPL in gram-positive bacteria is stronger than in gram-negative bacteria. The antibacterial effect of EPL on Aspergillus fumigatus was in agreement with the previous results.
Figure 2.
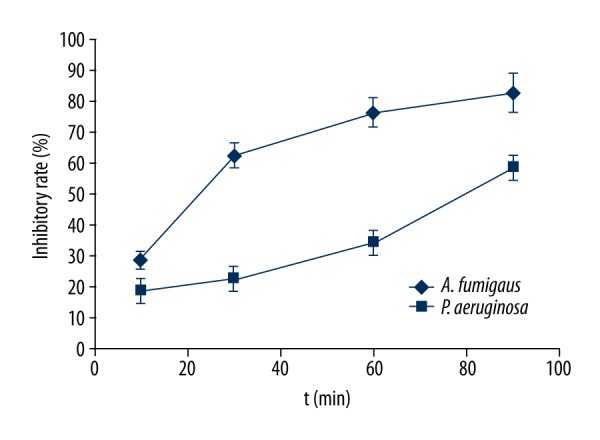
Antimicrobial activity of EPL against organisms.
Changes in electrical conductivity after EPL treatment
The cell membrane is a protection barrier of bacteria. When the bacterial cell membrane is destroyed due to exposure to a strong antibacterial agent, the protection barrier is broken, and the internal electrolyte will leak to outside the culture medium, and consequently the culture liquid conductivity will increase. Therefore, changes in bacteria liquid conductivity reflect the variety of bacterial cell membrane permeability. Figure 3 shows the change in conductivity of both bacteria after being treated by EPL at different times. When the EPL concentration was equal to 3 g/L, the conductivity of both bacterial liquids was higher than in the control group at different times of treatment, but Aspergillus fumigatus showed greater differences of conductivity compared to the control group, probably due to higher ionic leakage of Aspergillus fumigatus than Pseudomonas aeruginosa, suggesting that the degree of damage of EPL on Aspergillus fumigatus cell membrane was higher than that of Pseudomonas aeruginosa. On the other hand, when the concentration of EPL was equal to 1.5 g/L and the interaction time to Aspergillus fumigatus and Pseudomonas aeruginosa were less than 50 min and 60 min, respectively, the conductivity of both bacteria was lower than in the control group, probably because EPL did not destroy the cell membrane under these conditions but electrostatically bound to the external surface of the cell membrane to reduce the number of charged ions and decrease the conductivity of the medium. With an increase in interaction time, EPL gradually damaged the cell membrane, thereby gradually increasing the conductivity of both bacterial liquids. However, compared with Pseudomonas aeruginosa, the conductivity difference between Aspergillus fumigatus and the control group was much larger, further indicating that EPL has stronger antibacterial effect on Aspergillus fumigatus.
Figure 3.
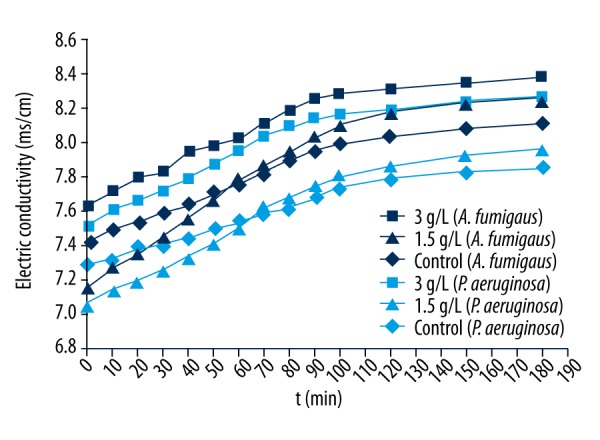
Changes in electric conductivity of Aspergillus fumigatus (dark-blue symbols) and Pseudomonas aeruginosa (light-blue symbols) treated by EPL.
Changes in total sugar concentration after EPL treatment
Carbohydrate is the primary carbon source and energy storage material for microorganisms. Bacteria at normal physiological state will absorb and utilize the external nutrients. However, when the membrane structure is destroyed, the contents of a cell, including sugars, will leak. The integrity of bacterial membrane structure can be investigated by determination of sugar concentrations in bacterial culture fluid. Figure 4 shows that the concentration of total sugar in culture liquid gradually increased with the extension of time after the interaction of the 2 bacteria and EPL at 3 g/L. This result indicates that the cell membrane structures of both bacteria were destroyed and intracellular carbohydrate gradually leaked to the outside of the cell. However, both bacteria control groups exhibited decreased total sugar concentration in the culture liquid, probably due to the absorption and utilization of carbohydrates in culture medium by normal bacteria during proliferation. It should be noted that the total sugar concentrations in the culture liquid and control groups were similar when the concentration of EPL was 1.5 g/L and the interaction time with the bacteria was 2 h, suggesting that the bacterial membrane was not completely destroyed at this time point, which is consistent with the conductivity results presented above.
Figure 4.
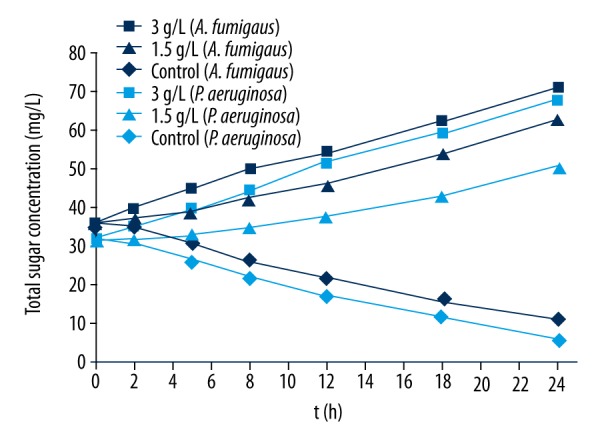
Changes in total sugar concentration of Aspergillus fumigatus (dark-blue symbols) and Pseudomonas aeruginosa (light-blue symbols) treated by EPL.
Effects of EPL on phosphorus metabolism of bacteria
Phosphorus is an essential trace element for all organisms, including microorganisms. It is an important component of the intermediate products of nucleic acids, phospholipids, and glucose metabolism, and plays a significant role in the energy metabolism of cells. Bacteria provide energy for growth and reproduction via utilization of glucose after a series of phosphoric acid reactions. Therefore, the overall metabolic function and growth state of a cell can be reflected by monitoring the consumption of phosphorus in bacterial metabolic activity. As shown in Figure 5, phosphorus consumption gradually decreased with the extension of time compared with the control group after the interaction of EPL and the 2 bacteria, indicating that their phosphorus metabolism was significantly affected by EPL, and suggesting that EPL not only damages the cell membrane, but also affects cell metabolism.
Figure 5.
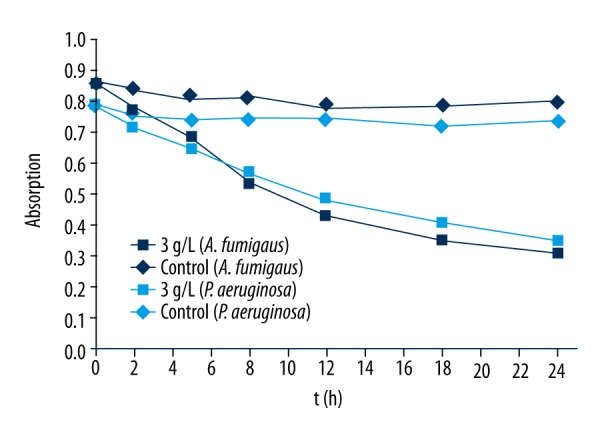
Changes in phosphorous metabolism (measured by absorption at 650 nm) of Aspergillus fumigatus (dark-blue symbols) and Pseudomonas aeruginosa (light-blue symbols) treated by EPL.
Discussion
The results of electrical conductivity and total sugar concentration for Aspergillus fumigatus and Pseudomonas aeruginosa cultures treated by EPL show that EPL first interacts with the surface of the bacterial membrane by static electricity at the initial stage of interaction between low concentration of EPL and the bacteria. The normal physiological functions of the bacteria are affected by EPL, and EPL gradually destroys the cell membrane to exert its antibacterial properties. However, higher concentrations of EPL can destroy cell membrane structure within a relatively short period of time to increase the permeability of the cells, which results in leakage of electrolytes. With the gradual leakage of carbohydrates from inside to outside the cell, the stability and energy metabolism of the cell structure are directly influenced, which finally cause cell death. These results are in agreement with previous studies [11], which have shown that tea polyphenols can increase the permeability of bacterial cell membranes and cause the leakage of intracellular proteins and carbohydrates. The phosphorus metabolism of bacteria suggests that EPL inhibits the synthesis of nucleic acid and phospholipid and affect the metabolism of sugar by reducing the phosphorus consumption of bacteria to further hinder the energy metabolism of cells.
Few published reports have explained how EPL inhibits bacterial protein expression, but it has been reported that EPL can inhibit the expression of human hepatocellular carcinoma cell line HepG2 apoptosis protein Bcl-2 [14]. It is well known that proteins are involved in the structural composition of microbial cells and in the form of enzymes to catalyze a variety of biochemical reactions in the cell.
EPL demonstrated a much stronger inhibitory effect on gram-positive Aspergillus fumigatus than on gram-negative Pseudomonas aeruginosa, possibly due to the different compositions of cell wall structure of the 2 bacteria. In general, the main components of the cell walls of gram-positive bacteria are peptidoglycan and a small amount of teichoic acid, through which sugars, amino acids, and most ions readily pass through. However, the structure of the cell wall of gram-negative bacteria is relatively complicated, consisting of the peptidoglycan layer and an outer membrane, and is not easily destroyed. Furthermore, the outer membrane has strong electronegativity to control the import and export of certain substances.
Conclusions
In this study, the antimicrobial mechanisms of EPL against Pseudomonas aeruginosa and Aspergillus fumigatus biofilm were investigated. EPL exhibits significant antimicrobial activity against both organisms, but the activity was much stronger for Pseudomonas aeruginosa. Electrical conductivity and total sugar concentration of microbial broth increased after EPL treatment, suggesting that EPL damages the cell membrane structure, resulting in increased cell membrane permeability and release of cell components.
Footnotes
Source of support: Departmental sources
References
- 1.Shima S, Sakai H. Polylysine produced by Streptomyces. Agricultural and Biological Chemistry. 1977;41:1807–9. [Google Scholar]
- 2.Geornaras I, Sofos JN. Activity of ɛ-polylysine against escherichia coli o157: h7, salmonella typhimurium, and listeria monocytogenes. Journal of Food Science. 2005;70(9):M404–8. doi: 10.1111/j.1750-3841.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 3.Wei N, Yuan X, Zhao J, et al. Rapidly in situ, forming chitosan/ɛ-polylysine hydrogels for adhesive sealants and hemostatic materials. Carbohydr Polym. 2013;96(1):342–48. doi: 10.1016/j.carbpol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Mclandsborough L, Mcclements DJ. Interactions of a cationic antimicrobial (ɛ-polylysine) with an anionic biopolymer (pectin): an isothermal titration calorimetry, microelectrophoresis, and turbidity study. J Agric Food Chem. 2011;59(10):5579–88. doi: 10.1021/jf104299q. [DOI] [PubMed] [Google Scholar]
- 5.Orive G, Tam SK, Pedraz JL, Hallé JP. Biocompatibility of alginate-poly-l-lysine microcapsules for cell therapy. Biomaterials. 2006;27(20):3691–700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Khattar V, Thottassery JV. Cks1 proteasomal turnover is a predominant mode of regulation in breast cancer cells: Role of key tyrosines and lysines. Int J Oncol. 2015;46(1):395–406. doi: 10.3892/ijo.2014.2728. [DOI] [PubMed] [Google Scholar]
- 7.Naraian R, Ram S, Kaistha SD, Srivastava J. Occurrence of plasmid linked multiple drug resistance in bacterial isolates of tannery effluent. Cell Mol Biol. 2012;58:134–41. [PubMed] [Google Scholar]
- 8.Rangel SM, Diaz MH, Knoten CA, et al. The Role of ExoS in dissemination of Pseudomonas aeruginosa during Pneumonia. PLoS Pathogens. 2015;11:e1004945. doi: 10.1371/journal.ppat.1004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray TS, Ledizet M, Kazmierczak BI. Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates. J Me Microbiol. 2010;59:511–20. doi: 10.1099/jmm.0.017715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman M, Bryan R, Rajan S, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tartor YH, Elnaenaeey EY. RT-PCR detection of exotoxin genes expression in multidrug resistant Pseudomonas aeruginosa. Cell Mol Biol. 2016;62:56–62. [PubMed] [Google Scholar]
- 12.Hayashi N, Nishizawa H, Kitao S, et al. Pseudomonas aeruginosa injects type III effector ExoS into epithelial cells through the function of type IV pili. FEBS Lett. 2015;589:890–96. doi: 10.1016/j.febslet.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathogens. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen HK, Høiby N, Pedersen SS. Experimental immunization with Pseudomonas aeruginosa alginate induces IgA and IgG antibody responses. APMIS. 1991;99:1061–68. [PubMed] [Google Scholar]
- 15.Johansen HK, Hougen HP, Cryz SJ, Jr, et al. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Respir Crit Care Med. 1995;152:1337–46. doi: 10.1164/ajrccm.152.4.7551392. [DOI] [PubMed] [Google Scholar]
- 16.Apodaca G, Bomsel M, Lindstedt R, et al. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–51. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohatami H. [Study on the pathogenetic role of alginate produced by mucoid Pseudomonas aerugiosa in diffuse panbronchiolitis]. Kansenshogaku Zasshi. 1995;69(5):553–67. doi: 10.11150/kansenshogakuzasshi1970.69.553. [DOI] [PubMed] [Google Scholar]


