Abstract
Objective
The Union for International Cancer Control (UICC) Node (N) classification is the most common used staging method for the prognosis of gastric cancer. It demands adequate, at least 16 lymph nodes (LNs) to be dissected; therefore different staging systems were invented.
Methods
Between March 2005 and March 2010, 164 patients were evaluated at the Department of General Surgery in the Kenézy Gyula Hospital and at the Department of General, Thoracic and Vascular Surgery in the Kaposi Mór Hospital. The 6th, 7th and 8th UICC N-staging systems, the number of examined LNs, the number of harvested negative LNs, the metastatic lymph node ratio (MLR) and the log odds of positive LNs (LODDS) were determined to measure their 5-year survival rates and to compare them to each other.
Results
The overall 5-year survival rate for all patients was 55.5% with a median overall survival time of 102 months. The tumor stage, gender, UICC N-stages, MLR and the LODDS were significant prognostic factors for the 5-year survival with univariate analysis. The 6th UICC N-stage did not follow the adequate risk in comparing N2 vs. N0 and N3 vs. N0 with multivariate investigation. Comparison of performances of the residual N classifications proved that the LODDS system was first in the prediction of prognosis during the evaluation of all patients and in cases with less than 16 harvested LNs. The MLR gave the best prognostic prediction when adequate (more than or equal to 16) lymphadenectomy was performed.
Conclusions
We suggest the application of LODDS system routinely in western patients and the usage of MLR classification in cases with extended lymphadenectomy.
Keywords: Gastric cancer, lymph node metastasis, prognosis, staging system, survival
Introduction
Gastric cancer is the fourth most common cancer in the world and the second leading cause of death due to cancer worldwide, with more than one million new cases estimated to arise each year (1). Most of them are diagnosed in China (2). The depth of tumor invasion (3,4), metastatic lymph node (LN) status and R0 resection are the most important independent prognostic factors for overall survival (OS) and disease free survival (DFS) (5,6). Furthermore, many investigators have demonstrated that LN metastasis is an independent risk factor for gastric cancer recurrence, as well as the time interval between radical gastrectomy and hepatic metastasis in patients following curative resection (7-9). Adjuvant chemotherapy and the patient’s prognosis are determined primarily by the Tumor-Node-Metastasis (TNM) stage. The TNM staging system in gastric cancer was introduced in 1974 (10) and has been modified periodically over time. This classification is the most common used staging method for prognosis in gastric cancer; however it demands at least 16 LNs to be dissected to avoid the stage migration phenomenon. A real problem is that the majority of western patients receive at most limited lymphadenectomy (D1) (11).
Due to this issue, different staging systems were invented to compensate for occasions where 16 LNs are not able to be dissected. Comparisons were made between Union for International Cancer Control (UICC) N-classification and other classifications where adequate dissections for (UICC) N-classification are not possible.
An ideal LN staging system should satisfy three conditions: decreased patient survival with increasing stage (monotonicity), similar survival within a group (homogeneity) and difference in survival between groups (distinctiveness) (12).
The aim of this bi-institutional study was to compare the next 7 different N-staging systems: 6th UICC N-stage, 7th UICC N-stage, 8th UICC N-stage, examined LN (eLN), the number of dissected negative LN (NLN), metastatic LN ratio (MLR) and the log odds of positive LNs (LODDS) foremost, in the Eastern European region.
Materials and methods
Patients
Between March 2005 and March 2010, 460 patients received operation on gastric cancer at the Department of General Surgery in the Kenézy Gyula Teaching Hospital, Debrecen and at the General, Thoracic and Vascular Surgery Department in the Kaposi Mór Teaching Hospital, Kaposvár. This study was performed in accordance with the Research Ethics Committee of the Kenézy Gyula Teaching Hospital. An informed consent form was signed by all patients, in compliance with the Helsinki Declaration of 1964.
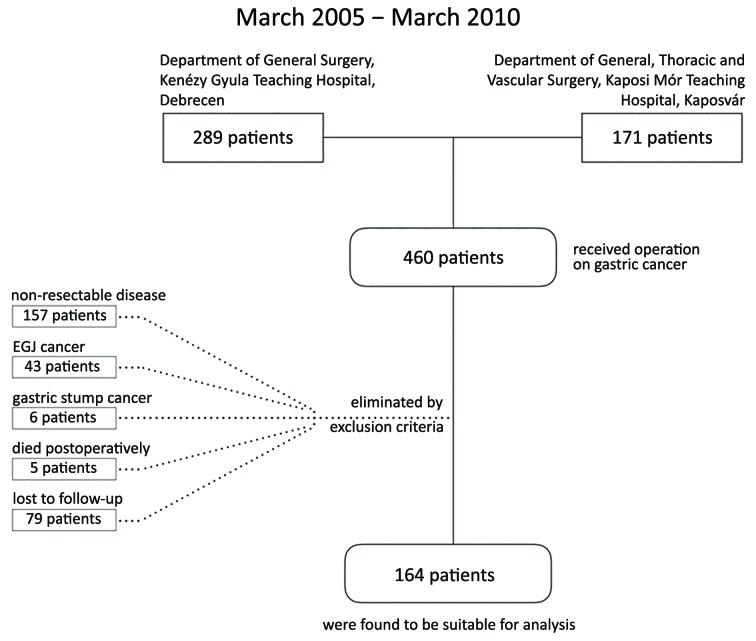
Eligibility criteria for inclusion were: 1) histologically proved gastric adenocarcinoma; 2) R0 resection; and 3) availability of complete follow-up data. The exclusion criteria were: 1) adenocarcinoma of esophago-gastric junction; 2) gastric stump cancer; 3) distant metastasis (visceral and/or peritoneal); 4) neoadjuvant oncological treatment; or 5) mortality due to postoperative complication. After the elimination, 164 patients were suitable for the analysis (Figure 1).
1.
Flow chart of patient selection.
All patients were followed up trimonthly in the first 2 years, every 6 months during the third to fifth years and annually thereafter. Physical examination, blood test, chest radiography, abdominal and pelvic computer tomography scan or abdominal ultrasound every 6 months and annual gastroscopy were performed during the follow-up period. Based on our chemotherapy protocols, only the patients with T3 and/or node positive gastric cancer had adjuvant treatment. The last follow-up was in December 2016. The median and mean follow-up time was 23.0 and 43.7 (range: 3–136) months, respectively.
Evaluated variables
Clinical data were collected for subsequent investigation, and included: sex (male or female), age at the time of surgery (≤60 or >60 years), size of primary tumor (≤50 or >50 mm), location of the tumor (upper-, middle-, or lower-third) and Borrmann classification (type I–IV) of cancer, and degree of differentiation (well, moderately or poorly differentiated carcinoma).
The extension of LN dissection (D1 or D2), the 6th UICC, the 7th UICC and the 8th UICC T- and N-staging systems were evaluated according to the 5-year survival rate. eLN (<16 or ≥16), NLN (0, 1–9, ≥10), MLR (0, 0.1%–20.0%, 20.1%–50.0%, ≥50.1%) and LODDS (< –1.125, –1.125 – –0.251, –0.250 – 0.749, ≥0.750) were determined to measure their 5-year survival data and to compare them to the above mentioned UICC N-staging systems and to each other. While the ranges of eLN, NLN and MLR classification were specified by the most common used data from literature. The LODDS’s values were figured by statistical analysis (Table 1). Finally we investigated the different N-staging systems according to the number of harvested LNs (<16 or ≥16).
1.
Performance of different LODDS cutoff sets
| No. | Top 3 ranked cutoff sets | Rank AIC | Rank LL | Rank c-index | Rank χ2 | Rank by product | Rank by sum | ||
| Cut 1 | Cut 2 | Cut 3 | |||||||
| LODDS, log odds of positive lymph nodes; AIC, Akaike information criterion, LL, log likelihood. | |||||||||
| 1 | –1.125 | –0.250 | 0.750 | 4 | 4 | 5 | 13 | 1 | 1 |
| 2 | –1.125 | –0.375 | 0.750 | 11 | 11 | 7 | 26 | 5 | 2 |
| 3 | –1.125 | –0.250 | 0.875 | 21 | 21 | 10 | 9 | 8 | 3 |
| 4 | –1.125 | –0.750 | –0.250 | 2 | 2 | 1 | 381 | 3 | 45 |
| 5 | –1.125 | –0.750 | –0.375 | 1 | 1 | 3 | 485 | 2 | 65 |
Statistical analysis
Log-rank tests were used to compare patient groups in terms of survival. Adjusted analysis was performed using Cox proportional hazards models. LODDS cutoff levels were based on using all possible cutoff triplets from –1.5 to 1 in steps of 0.125 to categorize LODDS. Cox models fitted using these categorized variants were ranked on Akaike information criterion (AIC) value, log likelihood, Harrell’s C coefficient, linear trend χ2 statistic and on the product as well as the sum of these ranks. The cutoff triplet associated with top rank based on the latter two was identified as optimal. The procedure was completed both with and without adjusting the models for age and sex.
Comparing the different staging systems, we evaluated discriminatory ability and monotonicity of gradient using the linear trend χ2 test. Furthermore, the AIC value from a Cox proportional hazard regression model was used to assess the discriminatory ability of each system. Receiver Operating Characteristic (ROC) Areas under Curves (AUC) were estimated by calculating Harrell’s C coefficient for each of the evaluated N-staging systems. The 5-year survival percentages were calculated by estimating the Kaplan-Meier survivor function at 60 months. All P values were based on a two-sided approach. The significance should be considered at P<0.05. Stata 2009 Statistical Software (Version 11; StataCorp LLC, College Station, TX, USA) (13) was used for data handling and analysis.
Results
A total of 164 patients with a median age of 66 (range: 35–90) years were evaluated. Sixty percent of patients were male and more than half of the patients (55%) had lower-third tumors. The average number of removed LNs was 10.48 (range: 1–38) per case. The mean number of metastatic LNs was 3.22 (range: 0–23). The overall 5-year survival rate for all patients was 55.5% with a median OS time of 102 months.
The 5-year survival rates and the results of the univariate analysis according to the patient’s and tumor’s characteristics were calculated and only the T-stage was a significant factor in this bi-institutional study (Table 2). The survival difference according to the tumor size was remarkable (65.8% vs. 49.3%); however it was not significant.
2.
Five-year survival rates and univariate analysis results according to patient and tumor characteristics
| Characteristics | n | 5-YSR (%) | P |
| 5-YSR, 5-year survival rate; UICC, Union for International Cancer Control. | |||
| Gender | 0.056 | ||
| Male | 99 | 46.4 | |
| Female | 65 | 70.2 | |
| Age (year) | 0.641 | ||
| <60 | 45 | 55.2 | |
| ≥60 | 119 | 55.3 | |
| Borrmann type | 0.279 | ||
| I | 2 | 50.0 | |
| II | 68 | 60.2 | |
| III | 72 | 50.5 | |
| IV | 4 | 25.0 | |
| Lymphadenectomy | 0.620 | ||
| D1 | 82 | 58.2 | |
| D2 | 82 | 53.0 | |
| Tumor size (mm) | 0.071 | ||
| <50 | 64 | 65.8 | |
| ≥50 | 100 | 49.3 | |
| Location (third) | 0.742 | ||
| Upper+middle | 73 | 52.9 | |
| Lower | 91 | 57.8 | |
| Histology | 0.467 | ||
| Poor | 86 | 59.0 | |
| Well | 78 | 52.2 | |
| 6th UICC T-stage | 0.037 | ||
| T1 | 25 | 82.5 | |
| T2 | 46 | 55.7 | |
| T3 | 82 | 47.7 | |
| T4 | 11 | 45.0 | |
| 7th, 8th UICC T-stage | 0.029 | ||
| T1 | 25 | 82.5 | |
| T2 | 20 | 69.1 | |
| T3 | 26 | 40.8 | |
| T4 | 93 | 47.5 | |
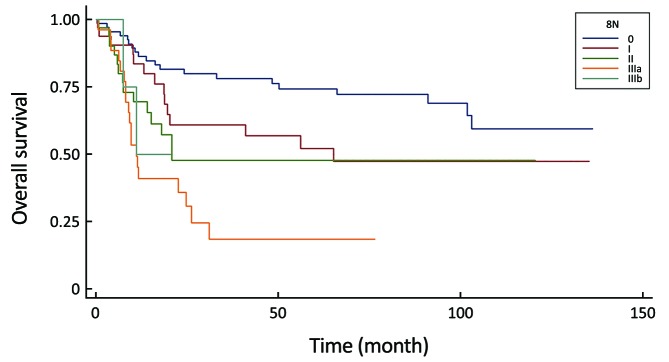
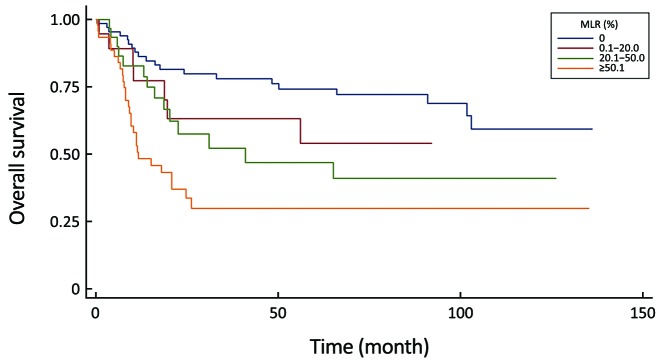
All of the evaluated N-staging classifications, excluding the eLN and NLN were significant prognostic factors for the 5-year survival with univariate analysis (Table 3). Figures 2–6 show the survival curves of these systems. Unfortunately, only 4 patients are representing the N3b group in the 8th staging system and their follow-up period was less than 5 years, so we could not calculate the survival rate.
3.
Five-year survival rates and statistical results of different N-staging systems
| Variables | n | 5-YSR (%) | P |
| 5-YSR, 5-year survival rates; UICC, Union for International Cancer Control; eLN, examined lymph nodes; NLN, number of retrieved negative lymph nodes; MLR, metastatic lymph node ratio; LODDS, log odds of positive lymph nodes. | |||
| 6th UICC N-stage | <0.0001 | ||
| N0 | 67 | 74.3 | |
| N1 | 66 | 49.3 | |
| N2 | 27 | 18.5 | |
| N3 | 4 | 0 | |
| 7th UICC N-stage | <0.0001 | ||
| N0 | 67 | 74.3 | |
| N1 | 33 | 52.1 | |
| N2 | 33 | 47.7 | |
| N3 | 31 | 19.0 | |
| 8th UICC N-stage | <0.0001 | ||
| N0 | 67 | 74.3 | |
| N1 | 33 | 52.1 | |
| N2 | 33 | 47.7 | |
| N3a | 27 | 18.5 | |
| N3b | 4 | N/A | |
| eLN | 0.581 | ||
| <16 | 127 | 54.2 | |
| ≥16 | 37 | 60.0 | |
| NLN | 0.209 | ||
| 0–9 | 117 | 52.2 | |
| 10–14 | 24 | 52.3 | |
| ≥15 | 23 | 73.1 | |
| MLR (%) | <0.0001 | ||
| 0 | 67 | 74.3 | |
| 0.1–20.0 | 19 | 54.2 | |
| 20.1–50.0 | 30 | 47.1 | |
| ≥50.1 | 48 | 30.0 | |
| LODDS | <0.0001 | ||
| < –1.125 | 29 | 81.9 | |
| –1.125 – –0.251 | 75 | 64.7 | |
| –0.250 – 0.749 | 43 | 31.0 | |
| ≥0.750 | 17 | 0 | |
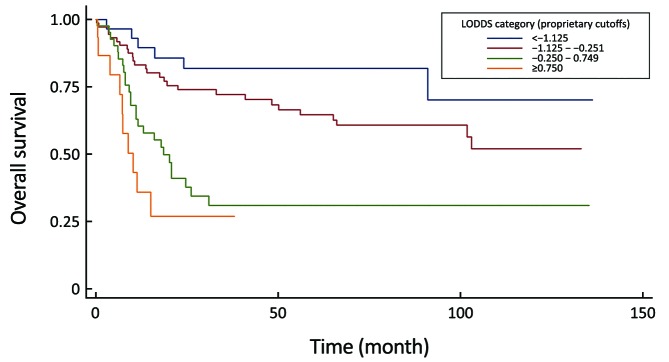
6.
Comparison of survival curves in the log odds of positive lymph nodes (LODDS) system (P<0.0001).
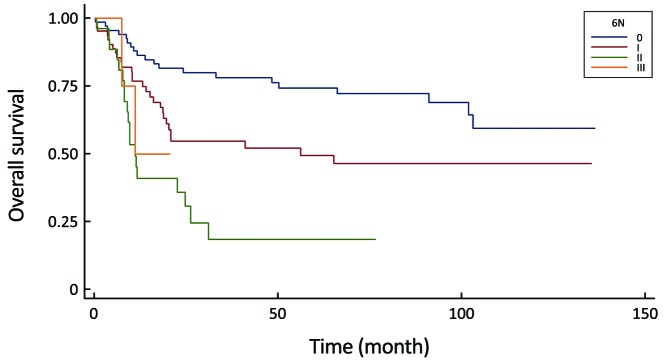
2.
Comparison of survival curves in the 6th UICC N-stage system (P<0.0001).
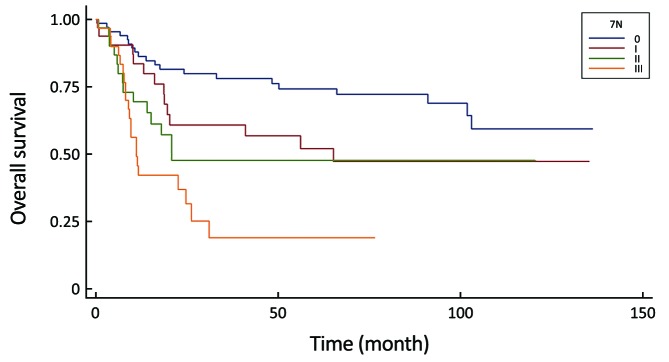
3.
Comparison of survival curves in the 7th UICC N-stage system (P<0.0001).
4.
Comparison of survival curves in the 8th UICC N-stage system (P<0.0001).
5.
Comparison of survival curves in the metastatic lymph node ratio (MLR) system (P<0.0001).
Meanwhile, our multivariate survival analysis found that all of the four significant classifications were an independent prognostic factor for survival, however during the investigation of hazard ratios, the monotonicity of gradient in the 6th and 8th UICC N-stage did not follow the adequate risk comparing N2 vs. N0 and N3 vs. N0 (HR: 4.97 and HR: 3.00) in the 6th staging system and comparing N3a vs. N0 and N3b vs. N0 (HR: 4.98 and HR: 3.02) in the 8th staging system (Table 4). The former system was excluded from further analysis, while the newest 8th system was evaluated keeping in mind that only 4 patients are representing the N3b group with an inadequate follow-up period.
4.
Multivariate analysis of significant N-stage systems
| Factor | Contrast | HR | 95% CI | P |
| UICC, Union for International Cancer Control; MLR, metastatic lymph node ratio; LODDS, log odds of positive lymph nodes; HR, hazard risk; 95% CI, 95% confidence interval. | ||||
| 6th UICC N-stage | I vs. 0 | 2.10 | 1.17–3.75 | 0.012 |
| II vs. 0 | 4.97 | 2.58–9.56 | <0.001 | |
| III vs. 0 | 3.00 | 0.68–13.19 | 0.144 | |
| 7th UICC N-stage | I vs. 0 | 1.83 | 0.92–3.64 | 0.084 |
| II vs. 0 | 2.48 | 1.24–4.95 | 0.010 | |
| III vs. 0 | 4.70 | 2.47–8.92 | <0.001 | |
| 8th UICC N-stage | I vs. 0 | 1.83 | 0.92–3.64 | 0.084 |
| II vs. 0 | 2.47 | 1.23–4.94 | 0.010 | |
| IIIa vs. 0 | 4.98 | 2.59–9.59 | <0.001 | |
| IIIb vs. 0 | 3.02 | 0.69–13.27 | 0.142 | |
| MLR (%) | 0.1–20.0 vs. 0 | 1.59 | 0.66–3.81 | 0.295 |
| 20.1–50.0 vs. 0 | 2.21 | 1.11–4.41 | 0.023 | |
| ≥50.1 vs. 0 | 3.89 | 2.14–7.08 | <0.001 | |
| LODDS | –1.125 – –0.251 vs. < –1.125 | 1.99 | 0.82–4.82 | 0.128 |
| –0.250 – 0.749 vs. < –1.125 | 4.83 | 1.97–11.87 | <0.001 | |
| ≥0.750 vs. < –1.125 | 9.17 | 3.25–25.87 | <0.001 | |
Comparison of performance of the residual N classifications (7th and 8th UICC N-stage, MLR, LODDS) proved that the LODDS system was the first in prognosis prediction during the evaluation of all patients (Table 5). Furthermore, the association between the number of retrieved LNs and survival rates of these N staging systems was examined. While the LODDS classification was the best predictor of survival in patients with less than 16 harvested LNs, the MLR showed the highest results when adequate (more than or equal to 16) LNs were examined (Table 6, 7).
5.
Comparison of performances of different staging systems
| System | AUC (c-index) | AIC | Linear trend χ2 |
| AUC, area under the curve; AIC, Akaike information criterion; LODDS, log odds of positive lymph nodes; UICC, Union for International Cancer Control; MLR, metastatic lymph node ratio. | |||
| LODDS | 0.700 | 618.880 | 12.777 |
| 8th UICC N-stage | 0.684 | 624.865 | 10.390 |
| 7th UICC N-stage | 0.683 | 623.370 | 10.979 |
| MLR | 0.672 | 625.084 | 9.712 |
6.
Comparison of performances of different staging systems in patients with <16 harvested lymph nodes
| System | AUC (c-index) | AIC | Linear trend χ2 |
| AUC, area under the curve; AIC, Akaike information criterion; LODDS, log odds of positive lymph nodes; UICC, Union for International Cancer Control; MLR, metastatic lymph node ratio. | |||
| LODDS | 0.711 | 471.976 | 9.437 |
| 7th UICC N-stage | 0.708 | 469.477 | 13.188 |
| 8th UICC N-stage | 0.708 | 469.477 | 13.188 |
| MLR | 0.663 | 477.084 | 8.928 |
7.
Comparison of performances of different staging systems in patients with ≥16 harvested lymph nodes
| System | AUC (c-index) | AIC | Linear trend χ2 |
| AUC, area under the curve; AIC, Akaike information criterion; UICC, Union for International Cancer Control; MLR, metastatic lymph node ratio, LODDS, log odds of positive lymph nodes. | |||
| MLR | 0.676 | 90.692 | 0.931 |
| LODDS | 0.664 | 89.446 | 2.507 |
| 8th UICC N-stage | 0.648 | 94.249 | 0.914 |
| 7th UICC N-stage | 0.644 | 92.252 | 0.824 |
Discussion
Effects of patient and tumor characteristics on 5-year survival
During the analysis of our results, we confirmed that the T-stage is a significant predictor for survival as well as the gender with univariate analysis. We found a noticeable variance in survival according to tumor size (66% vs. 49%); however it was not significant statistically. Furthermore, we could not prove significant survival differences in tumor location, Borrmann types of lesions and cancer differentiation as it was previously proved by Zhao et al., who analyzed 2,575 Chinese patients (14). In addition, we demonstrated that the degree of LN dissection (D1 vs. D2) was also a non-significant predictive factor for survival.
Different UICC N-stage systems
Several studies confirmed the superiority of the 7th UICC N-staging system to the 6th UICC N-staging classification in prognosis of OS (15-19). The main strength of the Asian studies was the use of data from multiple institutions, thereby reducing the risk of unique outcome due to single-institution bias. Our bi-institutional study resulted in superiority of the 7th UICC N-staging system to the 6th UICC N-staging classification. While our multivariate survival analysis found that, as an independent prognostic factor for survival, the 6th UICC N-stage did not follow the adequate clinical risk comparing N2 vs. N0 and N3 vs. N0, so it was unable to reproduce the real survival benefit of patients with a lower N stage. The investigation of 8th UICC N-stage system’s survival rates could not strengthen the results of Sano et al., who found a distinct prognosis in patients with pN3a and pN3b stages during the analysis of 25,441 patients from fifteen countries (20). However, in our study, only 4 patients were representing the N3b group with incomplete follow-up period, so we cannot conclude anything concerning the implementation of a new staging system with adaptation to the N3a and N3b subtypes separately. Adequate investigation of the 8th UICC N- Classification in prognosis requires a large scale study from a region with high incidence of gastric cancer, where more than 90% of patients have extended (more than 25 harvested LNs) LN dissection.
Role of removed and negative LNs in 5-year survival
The UICC N-staging system is criticized for the possibility of stage migration phenomenon and approximately half of the patients are misclassified by the latest version (21), so at least 16 harvested LNs are recommended for the accurate prediction.
Furthermore, Xu et al. demonstrated that it is necessary to examine at least 16 LNs for accurate pathological examination of gastric cancer, even in node-negative gastric cancer patients (22). Datta et al., who analyzed the data of more than 22,000 patients found that the examination of 15 or more LNs is a reproducible prognostic factor for gastric cancer outcomes in the United States and should continue to serve as a benchmark for the quality of care (23). Unfortunately, we did not find any survival benefit according to the total number of eLNs. It accounts for the results of ours and the latest Italian study comparing the impact of D1 vs. D2 lymphadenectomy on survival (24,25).
Huang and Deng et al. confirmed that the number of negative LNs is a good predictor for survival in patients with gastric cancer with a 10 and 15 cutoff levels (26,27). Based on their results, we created the next cutoff levels (0–9, 10–14, ≥15), but our study was not able to reproduce their data. Although the difference in 5-year survival rates was conspicuous, between more and less than 15 negative harvested LNs (73% vs. 52%), it was not significant (P=0.209).
MLR and LODDS, as alternative N-staging systems
In this current study we applied the most common used cutoff points (0; 0.20; 0.50) from the literature, which has the best statistical results (lowest AIC and highest C-index) (14,28-30), to avoid the unreproducible added value of our own calculated cutoff levels. Several papers showed the MLR’s superiority to the number-based (UICC) N-stage systems, because it is less influenced by the total number of harvested LNs (31-33). While Sun et al. demonstrated different prognoses in patients with the same MLR ratio (34), we verified this classification with uni- and multivariate analyses, as we proved its discriminatory ability and gradient monotonicity. However, in patients with incomplete lymphadenectomy, this classification had worse performances, as it was described earlier by Xu et al. (35).
Otherwise, this system was proved by our study as the best in prognosis prediction of patients with adequate (≥16) lymphadenectomy, as Liu et al. validated the superiority of it to the LODDS classification in patients with extended lymphadenectomy (36).
LODDS is defined as the log of the ratio between the probability of being a positive LN and the probability of being a negative LN when one LN is retrieved (34). The latest studies demonstrated that the LODDS classification is a better predictor of survival in contrast with UICC N-stage system or MLR, moreover it is not influenced by the number of less than 16 removed LNs (34,35,37-40). Especially, in cases with an MLR score of 0 or 1 (41), therefore a lot of Asian patients with early gastric cancer would not benefit from the MLR system owing to the pN0 status (37).
In our bi-institutional study, the LODDS staging had better discriminatory ability and monotonicity of the gradients with a smaller AIC value and a larger AUC under the ROC curve, than did the 6th, 7th and 8th edition UICC N-staging, or MLR systems. These results confirmed that the LODDS classification has the best prognostic stratification and the most precise prediction for survival in a region with low incidence of gastric cancer and high percentage of advanced, frequently non-resectable disease. Jian-Hui et al. justified when the number of retrieved LNs was insufficient (<15), the LODDS system had the best performance in homogeneity, discriminatory ability, monotonicity of gradients and accuracy of the prognosis evaluation (40). Our study strengthened it, the LODDS staging system was the best predictor of survival in gastric cancer during the analysis of all patients as well as, in patients with incomplete (<16) lymphadenectomy.
Conclusions
We demonstrated at first from the Eastern European region that the LODDS classification could determine the prognosis of gastric cancer more adequately than the other investigated N-staging systems in patients with less than 16 harvested LNs. Otherwise, the MLR system is an accurate classification for prediction of survival and could be used effectively in patient’s orientation when sufficient lymphadenectomy was performed.
Finally, we suggest the application of LODDS system routinely in western patients and the usage of MLR classification in cases with extended lymphadenectomy. However, to confirm our results with the above mentioned, calculated cutoff levels of LODDS classification, large-scale, multicenter, prospective trials would be recommended.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K, Ueyama T, Yao T, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030–7. doi: 10.1002/1097-0142(19920901)70:5<1030::AID-CNCR2820700504>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Adachi Y, Mori M, Maehara Y, et al. Long-term survival after resection for advanced gastric carcinoma. J Clin Gastroenterol. 1995;21:208–10. doi: 10.1097/00004836-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26:243–55. doi: 10.1053/ctrv.2000.0164. [DOI] [PubMed] [Google Scholar]
- 6.Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng JY, Liang H, Sun D, et al. Analysis of risk factors for the interval time, number and pattern of hepatic metastases from gastric cancer after radical gastrectomy. World J Gastroenterol. 2008;14:2440–7. doi: 10.3748/wjg.14.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K, Morisaki T, Sugitani A, et al. An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer. 1999;85:1500–5. doi: 10.1002/(SICI)1097-0142(19990401)85:7<1500::AID-CNCR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Kodera Y, Yamamura Y, Shimizu Y, et al. Lymph node status assessment for gastric carcinoma: is the number of metastatic lymph nodes really practical as a parameter for N categories in the TNM Classification? Tumor Node Metastasis. J Surg Oncol. 1998;69:15–20. doi: 10.1002/(SICI)1096-9098(199809)69:1<15::AID-JSO4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.International Union Against Cancer (UICC). TNM Classification of Malignant Tumours, 2nd edition. Geneva: UICC, 1974. Available online: http://www.uicc.org/
- 11.Schmidt B, Yoon SS. D1 versus D2 lymphadenectomy for gastric cancer. J Surg Oncol. 2013;107:259–64. doi: 10.1002/jso.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 13.Stata 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LLC.
- 14.Zhao LY, Li CC, Jia LY, et al. Superiority of lymph node ratio-based staging system for prognostic prediction in 2575 patients with gastric cancer: validation analysis in a large single center. Oncotarget. 2016;7:51069–81. doi: 10.18632/oncotarget.9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikken JL, van de Velde CJ, Gönen M, et al. The New American Joint Committee on Cancer/International Union Against Cancer staging system for adenocarcinoma of the stomach: increased complexity without clear improvement in predictive accuracy. Ann Surg Oncol. 2012;19:2443–51. doi: 10.1245/s10434-012-2403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592–8. doi: 10.1002/cncr.25550. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Sun XW, Li CF, et al. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1,503 patients. Ann Surg Oncol. 2011;18:1060–7. doi: 10.1245/s10434-010-1424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchet A, Mocellin S, Ambrosi A, et al. Validation of the new AJCC TNM staging system for gastric cancer in a large cohort of patients (n=2,155): focus on the T category. Eur J Surg Oncol. 2011;37:779–85. doi: 10.1016/j.ejso.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer. 2011;14:166–71. doi: 10.1007/s10120-011-0024-6. [DOI] [PubMed] [Google Scholar]
- 20.Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–25. doi: 10.1007/s10120-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HK, Yang HK, Kim WH, et al. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88:1408–12. doi: 10.1046/j.0007-1323.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Huang Y, Geng Q, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PLoS One. 2012;7:e38681. doi: 10.1371/journal.pone.0038681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta J, Lewis RS Jr, Mamtani R, et al. Implications of inadequate lymph node staging in resectable gastric cancer: a contemporary analysis using the National Cancer Data Base. Cancer. 2014;120:2855–65. doi: 10.1002/cncr.28780. [DOI] [PubMed] [Google Scholar]
- 24.Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 25.Tóth D, Bíró A, Kincses Z, et al. Role of a computer program in gastric cancer surgery — beyond the evidence. Magy Seb (in Hungarian) 2017;70:48–55. doi: 10.1556/1046.70.2017.1.7. [DOI] [PubMed] [Google Scholar]
- 26.Huang CM, Lin JX, Zheng CH, et al. Effect of negative lymph node count on survival for gastric cancer after curative distal gastrectomy. Eur J Surg Oncol. 2011;37:481–7. doi: 10.1016/j.ejso.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Deng J, Zhang R, Wu L, et al. Superiority of the ratio between negative and positive lymph nodes for predicting the prognosis for patients with gastric cancer. Ann Surg Oncol. 2015;22:1258–66. doi: 10.1245/s10434-014-4121-8. [DOI] [PubMed] [Google Scholar]
- 28.Kutlu OC, Watchell M, Dissanaike S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg Oncol. 2015;24:84–8. doi: 10.1016/j.suronc.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Zhang J, Cao S, et al. The evaluation of metastatic lymph node ratio staging system in gastric cancer. Gastric Cancer. 2013;16:309–17. doi: 10.1007/s10120-012-0190-1. [DOI] [PubMed] [Google Scholar]
- 30.Wong J, Rahman S, Saeed N, et al. Prognostic impact of lymph node retrieval and ratio in gastric cancer: a U.S. single center experience. J Gastrointest Surg. 2013;17:2059–66. doi: 10.1007/s11605-013-2380-5. [DOI] [PubMed] [Google Scholar]
- 31.Xiao LB, Yu JX, Wu WH, et al. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer. World J Gastroenterol. 2011;17:5123–30. doi: 10.3748/wjg.v17.i46.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alatengbaolide , Lin D, Li Y, et al. Lymph node ratio is an independent prognostic factor in gastric cancer after curative resection (R0) regardless of the examined number of lymph nodes. Am J Clin Oncol. 2013;36:325–30. doi: 10.1097/COC.0b013e318246b4e9. [DOI] [PubMed] [Google Scholar]
- 33.Xu DZ, Geng QR, Long ZJ, et al. Positive lymph node ratio is an independent prognostic factor in gastric cancer after D2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16:319–26. doi: 10.1245/s10434-008-0240-4. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Xu Y, Li de M, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116:2571–80. doi: 10.1002/cncr.24989. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Bian YH, Jin X, et al. Prognostic assessment of different metastatic lymph node staging methods for gastric cancer after D2 resection. World J Gastroenterol. 2013;19:1975–83. doi: 10.3748/wjg.v19.i12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Deng J, Zhang R, et al. The RML of lymph node metastasis was superior to the LODDS for evaluating the prognosis of gastric cancer. Int J Surg. 2013;11:419–24. doi: 10.1016/j.ijsu.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Qiu MZ, Qiu HJ, Wang ZQ, et al. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLos One. 2012;7:e31736. doi: 10.1371/journal.pone.0031736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Appleby DH, Zhang X, et al. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br J Surg. 2013;100:505–14. doi: 10.1002/bjs.9014. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Deng J, Zhang R, et al. The RML of lymph node metastasis was superior to the LODDS for evaluating the prognosis of gastric cancer. Int J Surg. 2013;11:419–24. doi: 10.1016/j.ijsu.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Aurello P, Petrucciani N, Nigri GR, et al. Log odds of positive lymph nodes (LODDS): what are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg. 2014;18:1254–60. doi: 10.1007/s11605-014-2539-8. [DOI] [PubMed] [Google Scholar]
- 41.Jian-Hui C, Shi-Rong C, Hui W, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol. 2016;37:11105–13. doi: 10.1007/s13277-015-4191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]