Abstract
Objective
We retrospectively analyzed the clinical prognostic value of the 8th edition of the American Joint Committee on Cancer (AJCC) staging system for luminal A breast cancer.
Methods
Using both the anatomic and prognostic staging in the 8th edition of AJCC cancer staging system, we restaged patients with luminal A breast cancer treated at the Breast Disease Center, Peking University First Hospital from 2008 to 2014. Follow-up data including 5-year disease free survival (DFS), overall survival (OS) and other clinic-pathological data were collected to analyze the differences between the two staging subgroups.
Results
This study included 421 patients with luminal A breast cancer (median follow-up, 61 months). The 5-year DFS and OS rates were 98.3% and 99.3%, respectively. Significant differences in 5-year DFS but not OS were observed between different anatomic disease stages. Significant differences were observed in both 5-year DFS and OS between different prognostic stages. Application of the prognostic staging system resulted in assignment of 175 of 421 patients (41.6%) to a different group compared to their original anatomic stages. In total, 102 of 103 patients with anatomic stage IIA changed to prognostic stage IB, and 24 of 52 patients with anatomic stage IIB changed to prognostic stage IB, while 1 changed to prognostic stage IIIB. Twenty-two of 33 patients with anatomic stage IIIA were down-staged to IIA when staged by prognostic staging system, and the other 11 patients were down-staged to IIB. Two patients with anatomic stage IIIB were down-staged to IIIA. Among seven patients with anatomic stage IIIC cancer, two were down-staged to IIIA and four were down-staged to stage IIIB.
Conclusions
The 8th edition of AJCC prognostic staging system is an important supplement to the breast cancer staging system. More clinical trials are needed to prove its ability to guide selection of proper systemic therapy and predict prognosis of breast cancer.
Keywords: AJCC cancer staging, anatomic stage, breast cancer, luminal A, prognostic stage, prognosis, systemic therapy
Introduction
Breast cancer is the most common malignant tumor in women worldwide (1-3). Considering its wide morphological spectrum, clinical presentation and response to therapy, breast cancer is thought to be a highly heterogeneous malignancy. Cancer staging and establishment of molecular subtypes are important for physicians to develop treatment strategies (4). In 2011, attendees of the 12th St. Gallen Consensus Meeting (5) suggested that breast cancer should be divided into four subtypes, luminal A type, luminal B type, human epidermal growth factor receptor 2 (HER2) positive and triple negative breast cancer. The use of these subtypes promoted the treatment of breast cancer from group therapy to classification therapy.
The American Joint Committee on Cancer (AJCC) cancer staging system is used by physicians and health care professionals worldwide to facilitate the uniform description and reporting of neoplastic diseases. The newest edition, the 8th edition of the AJCC cancer staging system (6) will be implemented on January 1st, 2018. Proper classification and staging of cancer is essential for physicians to administer proper treatment and evaluate the results of patient management and clinical trials. Classification and staging also serve as the standard for local, regional, and international reporting on the incidence and outcome of cancer.
In this retrospective study, we analyzed the clinical significance of the prognostic staging system of the 8th edition of AJCC cancer staging system for luminal A breast cancer. Follow-up data including 5-year disease free survival (DFS) and overall survival (OS) as well as other clinic-pathological data were collected to analyze the differences between the new prognostic staging subgroup and the traditional anatomic staging subgroup.
Materials and methods
Patient cohort
The study cohort comprised patients diagnosed with luminal A breast cancer and treated at the Breast Disease Center, Peking University First Hospital from January 2008 to December 2014. This study was approved by the Ethics Committee of Peking University First Hospital.
Molecular subtypes of breast cancer
The molecular subtypes evaluated in this study are based on the 2011 St. Gallen Consensus and are shown in Table 1 .
1.
Molecular subtypes of breast cancer
| Intrinsic subtype | Clinic-pathological definition |
| ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2. | |
| Luminal A | ER and/or PR positive, HER-2 negative, Ki67<14% |
| Luminal B | |
| Luminal B (HER-2 negative) | ER and/or PR positive, HER-2 negative, Ki67≥14% |
| Luminal B (HER-2 positive) | ER and/or PR positive, HER-2 positive, any Ki67 |
| HER-2 overexpression | HER-2 overexpression, ER and PR absent |
| Triple negative | ER and PR absent, HER-2 negative |
Histopathological grading and immunohistochemical examination of breast cancer
Each surgical specimen was immunohistochemically examined, and the results were assessed according to the American Society of Clinical Oncology/College of American Pathologists clinical practice guidelines (7,8). Estrogen receptor (ER) and progesterone receptor (PR) positivity were defined as any level of nuclear positive (≥1%). HER2 positive was defined as immunohistochemical positivity (3+) or fluorescence in situ hybridization (FISH) overexpression. Ki67 positivity was defined in terms of its nuclear staining. The Ki67 labeling index was calculated as the percentage of MIB-1 positive cells among 1,000 malignant cells observed at high-power magnification (×400) (9).
The histopathological grade was determined using the modified Scarff-Bloom-Richardson grading system (Nottingham Combined Histologic Grade) (10).
Anatomic and prognostic staging of breast cancer
Anatomic and prognostic staging was based on the 8th edition of AJCC cancer staging system (6).
Statistical analysis
Survival data associated with different cancer stages and other clinicpathological data were analyzed with IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA). The DFS period was defined as the interval from the date of surgery to the date on which disease recurrence, either loco-regional or distant metastasis, was first observed, or the date of last follow-up without any evidence of recurrence. OS was calculated from the date of diagnosis of the primary breast cancer to the date of death or last follow-up. The Kaplan-Meier method was used to describe the DFS and OS and log-rank test was used to compare the differences between clinicpathological subgroups. The Breslow pairwise comparison was used to find the differences between any two subgroups of anatomic or prognostic stages in DFS and OS. P<0.05 (two-sided) was considered statistically significant.
Results
Patient characteristics
In total, 2,171 patients with primary invasive breast cancer were diagnosed and treated from January 1st, 2008 to December 31st, 2014 at the Breast Disease Center, Peking University First Hospital. Among them, 446 (20.54%) patients had the luminal A breast cancer subtype. After exclusion of patients with incomplete clinic-pathological and/or follow-up data, 421 patients with luminal A breast cancer were enrolled in this study. All patients were female, and their median age was 56 (range, 24–91) years. A total of 166 (39.4%) patients were pre-menopausal and 255 (60.6%) were post-menopausal. The median follow-up time was 61 (range, 7–100) months. The 5-year DFS is 98.3%, and the 5-year OS is 99.3%. The other clinic-pathological data are shown in Table 2 .
2.
Clinic-pathological characteristics of luminal A breast cancer patients (N=421)
| Factors | n (%) | Case of metastasis | 5-year DFS | Case of death | 5-year OS | ||||
| DFS (%)* | χ2** | P** | OS (%)* | χ2** | P** | ||||
| DFS, disease-free survival; OS, overall survival; BCS, breast conserving surgery; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy; ET, endocrine therapy; *, DFS and OS are analyzed by Kaplan-Meier survival analysis; **, univariate analysis by log-rank test. | |||||||||
| Age (year) | 3.501 | 0.174 | 12.872 | 0.002 | |||||
| <35 | 8 (1.9) | 0 | 100 | 0 | 100 | ||||
| 35–65 | 293 (69.6) | 7 | 97.5 | 3 | 98.7 | ||||
| >65 | 120 (28.5) | 7 | 93.1 | 9 | 94.2 | ||||
| Menstrual status | 3.762 | 0.520 | 2.426 | 0.119 | |||||
| Premenopausal | 166 (39.4) | 2 | 98.6 | 2 | 98.3 | ||||
| Postmenopausal | 255 (60.6) | 12 | 94.9 | 10 | 96.8 | ||||
| Lymph-vascular invasion | 14.092 | <0.001 | 5.957 | 0.015 | |||||
| Yes | 18 (4.3) | 3 | 80.2 | 2 | 87.1 | ||||
| No | 403 (95.7) | 11 | 97.0 | 10 | 97.9 | ||||
| Breast surgery | 0.974 | 0.324 | 1.677 | 0.195 | |||||
| BCS | 106 (25.2) | 2 | 97.9 | 1 | 98.7 | ||||
| Mastectomy | 315 (74.8) | 12 | 95.7 | 11 | 97.0 | ||||
| Tumor size | 11.521 | 0.009 | 23.626 | <0.001 | |||||
| T1 | 263 (62.5) | 3 | 98.6 | 3 | 99.1 | ||||
| T2 | 140 (33.3) | 11 | 92.1 | 9 | 95.0 | ||||
| T3 | 15 (3.6) | 0 | 100 | 0 | 100 | ||||
| T4 | 3 (0.7) | 0 | 100 | 1 | 50.0 | ||||
| Lymph node status | 20.532 | <0.001 | 14.234 | 0.003 | |||||
| N0 | 284 (67.5) | 5 | 99.2 | 7 | 97.9 | ||||
| N1 | 100 (23.8) | 7 | 90.7 | 3 | 97.4 | ||||
| N2 | 29 (6.9) | 0 | 100 | 0 | 100 | ||||
| N3 | 8 (1.9) | 2 | 83.3 | 2 | 72.9 | ||||
| Grade | 2.550 | 0.279 | 2.144 | 0.342 | |||||
| I | 290 (68.9) | 7 | 97.4 | 6 | 97.9 | ||||
| II | 124 (29.5) | 7 | 93.8 | 6 | 96.1 | ||||
| III | 7 (1.7) | 0 | 100 | 0 | 100 | ||||
| Treatment | 4.287 | 0.117 | 6.179 | 0.046 | |||||
| NAC+ET | 52 (12.4) | 5 | 93.8 | 5 | 94.0 | ||||
| AC+ET | 102 (24.2) | 2 | 97.0 | 1 | 98.0 | ||||
| ET | 267 (63.4) | 7 | 96.7 | 6 | 97.2 | ||||
Differences between the 7th and 8th editions of AJCC cancer staging system for breast cancer
The anatomic staging system is identical between the 7th and 8th editions of AJCC cancer staging system for breast cancer. However, the prognostic staging system of breast cancer is totally new in the 8th edition. This prognostic staging system is characterized by the addition of several important biologic factors — grade, hormone receptor (ER, PR) expression, HER2 overexpression/amplification, and genomic panels [e.g., the Oncotype DX® multigene panel, which produces a specific recurrence score (11,12), et al.].
Patients staged by the 8th edition of AJCC anatomic staging system
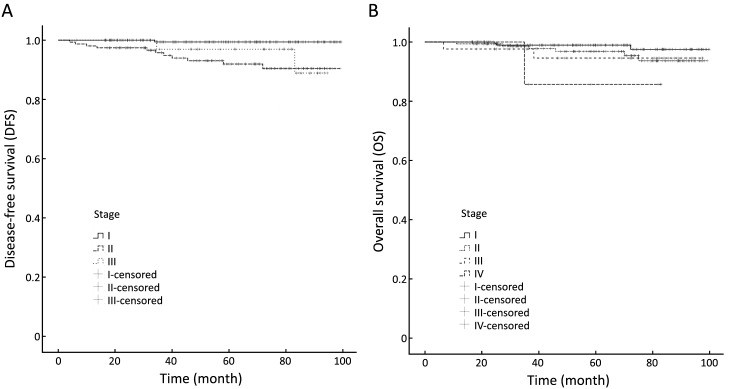
Among the 421 enrolled patients with luminal A breast cancer, 216 (51.3%) had stage I cancer, 213 stage IA and 3 stage IB. One hundred and fifty-five (36.8%) had stage II cancer, 103 stage IIA, 52 stage IIB. Forty-two (10.0%) had stage III cancer, 33 stage IIIA, 2 stage IIIB, 7 stage IIIC. Eight patients (1.9%) had de novo stage IV cancer. Analysis by the log-rank test revealed statistically significant differences in 5-year DFS (Plog-rank=0.003) between different disease stages, but no significant differences in 5-year OS (Plog-rank=0.203). Table 3 and Figure 1 show the differences in DFS and OS in different stage groups among patients staged by the 8th edition of the AJCC anatomic staging system.
3.
Comparison of DFS and OS using the 8th edition of AJCC anatomic and prognostic staging system of luminal A breast cancer (N=421)
| Stage | Anatomic stage groups | Prognostic stage groups | |||||||||
| n | 5-year DFS (%)* | P** | 5-year OS (%)* | P** | n | 5-year DFS (%)* | P** | 5-year OS (%)* | P** | ||
| DFS, disease-free survival; OS, overall survival; *, DFS and OS are analyzed by Kaplan- Meier survival analysis; **, log-rank test. | |||||||||||
| 0.003 | 0.203 | 0.012 | 0.006 | ||||||||
| I | 216 | 99.4 | 98.9 | 342 | 96.9 | 98.7 | |||||
| II | 155 | 91.9 | 96.8 | 61 | 94.6 | 95.9 | |||||
| III | 42 | 97.0 | 94.6 | 10 | 87.5 | 78.8 | |||||
| IV | 8 | 85.7 | 8 | 85.7 | |||||||
1.
Disease-free survival (A) and overall survival (B) analyses within different disease stages by the 8th edition of the American Joint Committee on Cancer (AJCC) anatomic staging system.
Patients staged by the 8th edition of AJCC prognostic staging system
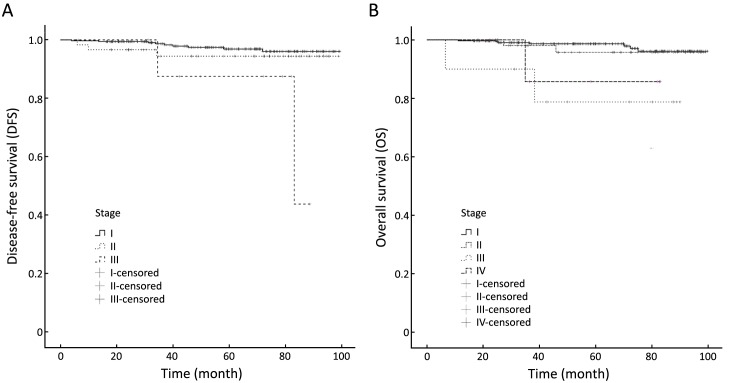
After staging by the 8th edition of the AJCC prognostic staging system, some patients’ disease stage differed from their original anatomic stage. Among the 421 enrolled patients with luminal A breast cancer, 342 (81.2%) had prognostic stage I cancer, 212 IA and 130 IB. Sixty-one (14.5%) had prognostic stage II cancer, 23 IIA, 38 IIB. Ten (2.4%) had prognostic stage III cancer, 4 IIIA, 5 IIIB, 1 IIIC. Eight (1.9%) had de novo stage IV cancer. Analysis by the log-rank test showed statistically significant differences in both 5-year DFS (Plog-rank=0.012) and 5-year OS (Plog-rank=0.006) between different prognostic stages. Table 3 and Figure 2 show the differences in DFS and OS in different stage groups among patients staged by the 8th edition of the AJCC prognostic staging system.
2.
Disease-free survival (A) and overall survival (B) analyses within different disease stages by the 8th edition of the American Joint Committee on Cancer (AJCC) prognostic staging system.
Pairwise comparisons of differences of DFS and OS between each sub-stage within anatomic and prognostic groups
The differences of 5-year DFS and OS between each sub-stage within anatomic and prognostic groups are shown in Table 4 and Table 5 . There were statistic differences between anatomic stage I and II in 5-year DFS (P=0.001), but there were no statistic differences between each sub-stage in 5-year OS. There were statistic differences between prognostic stage I, II and III in 5-year OS (P=0.000 and P=0.028), but no statistic differences between stage III and IV. There were no differences between each prognostic sub-stage in 5-year DFS.
4.
Pairwise comparisons of differences of DFS and OS between each sub-stage within anatomic groups
| Anatomic stage groups | P value | ||||||||
| DFS | OS | ||||||||
| I | II | III | IV | I | II | III | IV | ||
| DFS, disease-free survival; OS, overall survival. Breslow pairwise comparison was applied, and P<0.05 was statistically significant. | |||||||||
| I | 0.001 | 0.101 | 0.503 | 0.109 | 0.021 | ||||
| II | 0.001 | 0.316 | 0.503 | 0.620 | 0.220 | ||||
| III | 0.101 | 0.316 | 0.109 | 0.620 | 0.438 | ||||
| IV | 0.021 | 0.220 | 0.438 | ||||||
5.
Pairwise comparisons of differences of DFS and OS between each sub-stage within prognostic groups
| Prognostic stage groups | P value | ||||||||
| DFS | OS | ||||||||
| I | II | III | IV | I | II | III | IV | ||
| DFS, disease-free survival; OS, overall survival. Breslow pairwise comparison was applied, and P<0.05 was statistically significant. | |||||||||
| I | 0.190 | 0.061 | 0.503 | 0.000 | 0.039 | ||||
| II | 0.190 | 0.398 | 0.503 | 0.028 | 0.238 | ||||
| III | 0.061 | 0.398 | 0.000 | 0.028 | 0.706 | ||||
| IV | 0.039 | 0.238 | 0.706 | ||||||
Changes from anatomic stage groups to prognostic stage groups
Compared to the anatomic stage groups, the application of the prognostic staging system resulted in assignment of 175 of 421 (41.6%) patients to a different group. Most of them down-staged to better prognostic groups, only one patient with anatomic stage IIB cancer changed to prognostic stage IIIB. Table 6 shows the changes in disease stages from anatomic stage groups to prognostic stage groups.
6.
Changes in disease stages from anatomic stage groups to prognostic stage groups (N=421)
| Anatomic stage groups | Change to | Prognostic stage groups | ||
| Stage | n | Stage | n | |
| IA | 213 | IB | 4 | |
| IA | 209 | |||
| IB | 3 | IA | 3 | |
| IIA | 103 | IB | 102 | |
| IIA | 1 | |||
| IIB | 52 | IB | 24 | |
| IIIB | 1 | |||
| IIB | 27 | |||
| IIIA | 33 | IIB | 11 | |
| IIA | 22 | |||
| IIIB | 2 | IIIA | 2 | |
| IIIC | 7 | IIIA | 2 | |
| IIIB | 4 | |||
| IIIC | 1 | |||
| IV | 8 | IV | 8 | |
Changes from anatomic stage I to other disease stages
Four of 213 patients with anatomic stage IA cancer changed to prognostic stage IB, and 3 of 3 patients with anatomic stage IB cancer changed to prognostic stage IA.
Changes from anatomic stage II to other disease stages
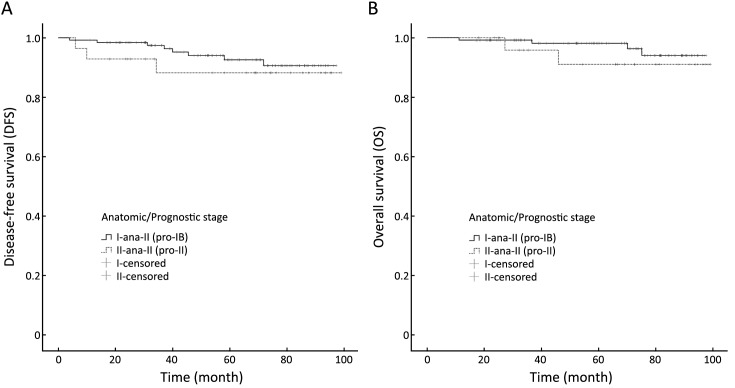
One hundred and two of 103 patients with anatomic stage IIA cancer changed to prognostic stage IB, and 24 of 52 patients with anatomic stage IIB cancer changed to prognostic stage IB, while one changed to prognostic stage IIIB. No significant statistic differences were observed in DFS (log-rank=0.542, P=0.462) or OS (log-rank=0.823, P=0.364) between patients whose anatomic stage II cancer was down-staged to prognostic stage IB [ana-II (pro-IB) group] and patients whose anatomic stage II cancer remained prognostic stage II [ana-II (pro-II) group]. Figure 3 shows the results of the DFS and OS analysis between ana-II (pro-IB) group and ana-II (pro-II) group.
3.
Disease-free survival (A) and overall survival (B) analyses between patients whose anatomic stage II cancer was down-staged to prognostic stage IB [ana-II (pro-IB) group] and patients whose anatomic stage II cancer remained prognostic stage II [ana-II (pro-II) group].
Changes from anatomic stage III to other disease stages
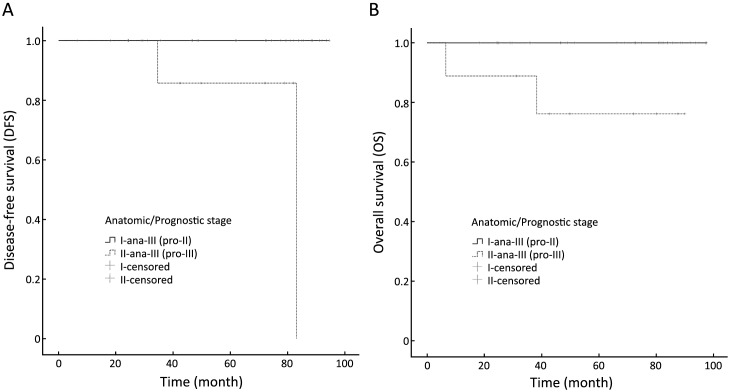
Twenty-two of 33 patients with anatomic stage IIIA cancer changed to prognostic stage IIA, and the remaining 11 patients were down-staged to prognostic stage IIB. Two patients with anatomic stage IIIB cancer were down-staged to prognostic stage IIIA. Two of 7 patients with anatomic stage IIIC cancer changed to prognostic stage IIIA, and four patients changed to stage IIIB. Significant differences in DFS (log-rank=11.931, P=0.001) and OS (log-rank=7.237, P=0.007) were observed between patients whose anatomic stage III cancer were down-staged to prognostic stage II [ana-III (pro-II) group] and patients whose anatomic stage III cancer remained prognostic stage III [ana-III (pro-III) group]. Figure 4 shows the DFS and OS analysis between the ana-III (pro-II) group and ana-III (pro-III) group.
4.
Disease-free survival (A) and overall survival (B) analyses between patients whose anatomic stage III cancer was down-staged to prognostic stage II [ana-III (pro-II) group] and patients whose anatomic stage III cancer remained prognostic stage III [ana-III (pro-III) group].
Discussion
Four decades have passed since publication of the 1st edition of the AJCC staging manual in 1977. This staging system has become the most effective cancer classification and prognosis evaluation system worldwide. Cancers are classified into different stages depending on the TNM scoring system: tumor size, lymph nodes affected, and metastases. The 7th edition of the AJCC cancer staging system published in 2010 is still based on clinic-pathological information (13). The 8th edition of the AJCC breast cancer anatomic staging is also based on the TNM system, however, the evolving knowledge of breast cancer biology and the increased validation of various biomarkers including genomic profiling have made the anatomic staging alone less sufficient to show the differences in the molecular characteristics of breast cancer (14,15). Breast cancers with different molecular characteristics have different prognoses, patterns of recurrence, and dissimilarities of sensitivities to systemic therapies (16). Thus, the most substantial change in the 8th edition of the AJCC staging system is its brand-new prognostic staging system, and this is the biggest highlight of the revision of this edition, the Breast Cancer Expert Panel recommends prioritizing the use of this prognostic staging system in patients with breast cancer.
Taken into consideration of its heterogeneity, breast cancer has different response to therapy and prognoses between each subtype (17). Because of ER and PR positive, HER2 negative, low ki67 expression, and often low Oncotype DX® recurrence score (18), luminal A breast cancer is associated with better DFS and OS than other molecular subtypes (19). In order to study the differences between the 8th edition of the AJCC anatomic staging system and prognostic staging system for breast cancer, we retrospectively classified 421 patients with luminal A breast cancer treated in the Breast Disease Center, Peking University First Hospital using both the anatomic and prognostic staging systems. The overall 5-year DFS and OS rates were 98.3% and 99.3%, respectively. In the anatomic stage groups, we observed significant statistic differences in 5-year DFS (log-rank=11.933, P=0.003) between different disease stages, but no significant statistic differences in 5-year OS (log-rank=4.606, P=0.203). In the prognostic stage groups, we observed significant statistic differences in both 5-year DFS (log-rank=8.816, P=0.012) and 5-year OS (log-rank=12.581, P=0.006) between different prognostic stages. This might be a proof that the prognostic stage can predict the OS better than the anatomic stage, even in patients with luminal A breast cancer, which had a very high 5-year OS of 99.3% in the present study.
By adding important biologic factors [grade, hormone receptor (ER, PR) expression, HER2 overexpression/amplification and genomic panels] to the anatomic TNM staging system, the 8th edition of the AJCC prognostic staging system has caused some patients’ disease stages to differ from their original anatomic stage. In the present study, the application of the prognostic stage groups resulted in the assignment of 175 of 421 (41.6%) patients to a different group; most of them were down-staged to better prognostic stages. In total, 102 of 103 patients with anatomic stage IIA cancer changed to prognostic stage IB, and 24 of 52 patients with anatomic stage IIB cancer changed to prognostic stage IB, while one changed to prognostic stage IIIB. Additionally, 22 of 33 patients with anatomic stage IIIA cancer changed to prognostic stage IIA, and the remaining 11 patients were down-staged to prognostic stage IIB. Two patients with anatomic stage IIIB cancer were down-staged to prognostic stage IIIA. Two of seven patients with anatomic stage IIIC cancer changed to prognostic stage IIIA, and four patients changed to stage IIIB. These changes in disease stage resulted in changes in DFS and OS, especially in patients with stage III cancer. In the anatomic stage groups, the 5-year DFS and OS rates were 91.9% and 96.8% in stage II, 97.0%, and 94.6% in stage III. In the prognostic stage groups, however, 5-year DFS and OS rates changed dramatically to 87.5% and 78.8%, respectively, in prognostic stage III, with almost the same 5-year DFS and OS in prognostic stage II as those in the anatomic stage II. Thus, we can draw the conclusion that prognostic staging system can classify breast cancer more precisely and predict the prognosis more accurately than the anatomic staging system. Patients with luminal A breast cancer are both ER and PR positive and HER2 negative, thus, grade is considered to be the leading cause of the difference between the prognostic stage and the anatomic stage in the same patient. The 8th AJCC Breast Cancer Expert Panel recommends the determination of histopathological grade using the Nottingham combined histologic grade.
Considering the differences in pathological characteristics, risk of relapse, and sensitivities to available therapies among individual patients with breast cancer, cancer staging is so important that it should not only provide information on the biological features of the breast cancer, but also reflect the molecular characteristics of the breast cancer. A personalized-medicine approach to breast cancer also requires more precise cancer staging and molecular profiling of breast cancer (20,21). Based on all the currently available knowledge including both the biological and molecular characteristics of breast cancer (22-24), the evidence-based anatomic TNM staging system is supplemented, as appropriate, by selected molecular markers and newly acquired insights into the molecular underpinnings of cancer, the 8th edition of the AJCC cancer staging system has developed prognostic staging system, which serves as a bridge from a population-based to a more personalized approach to cancer staging (25). In the present study, more than 40% of patients with luminal A breast cancer were down-staged to better prognostic cancer stages than their anatomic stages. With these adjustments of cancer stages, patients with breast cancer might have the opportunity to avoid excessive treatment without affecting their prognosis.
This was a single-center retrospective study with a relatively small number of patients. More multi-center prospective studies should be performed to more fully determine the clinical value of the prognostic staging system in patients with breast cancer. We believe that prognostic staging system will become another gold standard cancer classification system along with the anatomic staging system or an even more effective system as a guide to select whether to apply systemic therapy in patients with breast cancer.
Conclusions
The 8th edition of the AJCC prognostic staging system is an important supplement to the current breast cancer staging system. More clinical trials are needed to prove its significance in guiding the selection of proper systemic therapy and predicting the prognosis of breast cancer.
Acknowledgements
This study was supported by research grants from the Beijing Municipal Commission of Health and Family Planning (No. 2009-1011), the Beijing Municipal Science and Technology Commission (No. D090507043409010 and Z131107002213007), and the Precision Medicine Special Project of National Key Research and Development Program (No. 2016YFC0901302).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan X, Han R, Zhou J, et al. Incidence, mortality and survival of female breast cancer during 2003-2011 in Jiangsu province, China. Chin J Cancer Res. 2016;28:321–9. doi: 10.21147/j.issn.1000-9604.2016.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HL, Ding A, Wang FW. Prognostic effect analysis of molecular subtype on young breast cancer patients. Chin J Cancer Res. 2015;27:428–36. doi: 10.3978/j.issn.1000-9604.2015.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes — dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th edition. New York: Springer, 2016.
- 7.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 9.Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer: experience form a large study with long-term follow- up. Histopathology. 2002;41:154–61. [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. V. 2. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 12.Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33:2695–704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th edition. New York: Springer, 2010.
- 14.Zhou B, Ji K, Xin L, et al. Updates and interpretations of the 8th edition of AJCC breast cancer staging system. Chin J Prac Surg (in Chinese) 2017;37:10–14. [Google Scholar]
- 15.Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 16.Wei S, Siegal GP. Metastatic organotropism: an intrinsic property of breast cancer molecular subtypes. Adv Anat Pathol. 2017;24:78–81. doi: 10.1097/PAP.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 17.Koren S, Bentires-Alj M. Breast tumor heterogeneity: source of fitness, hurdle for therapy. Mol Cell. 2015;60:537–46. doi: 10.1016/j.molcel.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Ademuyiwa FO, Thorat MA, Jain RK, et al. Expression of Forkhead-box protein A1, a marker of luminal A type breast cancer, parallels low Oncotype DX 21-gene recurrence scores. Mod Pathol. 2010;23:270–5. doi: 10.1038/modpathol.2009.172. [DOI] [PubMed] [Google Scholar]
- 19.Puig-Vives M, Sánchez MJ, Sánchez-Cantalejo J, et al. Distribution and prognosis of molecular breast cancer subtypes defined by immunohistochemical biomarkers in a Spanish population-based study. Gynecol Oncol. 2013;130:609–14. doi: 10.1016/j.ygyno.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Z. Decision making for breast cancer: From the individualized treatment to precision medicine plan. Zhongguo Shi Yong Wai Ke Za Zhi (in Chinese) 2015;35:697–700. doi: 10.7504/CJPS.ISSN1005-2208.2015.07.02. [DOI] [Google Scholar]
- 21.De Abreu FB, Schwartz GN, Wells WA, et al. Personalized therapy for breast cancer. Clin Genet. 2014;86:62–7. doi: 10.1111/cge.12381. [DOI] [PubMed] [Google Scholar]
- 22.Kos Z, Dabbs DJ. Biomarker assessment and molecular testing for prognostication in breast cancer. Histopathology. 2016;68:70–85. doi: 10.1111/his.12795. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Li Z, Yang J, et al. Cell-specific biomarkers and targeted biopharmaceuticals for breast cancer treatment. Cell Prolif. 2016;49:409–20. doi: 10.1111/cpr.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toss A, Venturelli M, Peterle C, et al. Molecular biomarkers for prediction of targeted therapy response in metastatic breast cancer: trick or treat? Int J Mol Sci. 2017;18:pii: E85. doi: 10.3390/ijms18010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more " personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]