Abstract
Cryopreservation has become an important and accepted tool for long-term germplasm conservation of animals and plants. To protect genetic resources, repositories have been developed with national and international cooperation. For a repository to be effective, the genetic material submitted must be of good quality and comparable to other submissions. However, due to a variety of reasons, including constraints in knowledge and available resources, cryopreservation methods for aquatic species vary widely across user groups which reduces reproducibility and weakens quality control. Herein we describe a standardizable freezing device produced using 3-dimensional (3-D) printing and introduce the concept of network sharing to achieve aggregate high-throughput cryopreservation for aquatic species. The objectives were to: 1) adapt widely available polystyrene foam products that would be inexpensive, portable, and provide adequate work space; 2) develop a design suitable for 3-D printing that could provide multiple configurations, be inexpensive, and easy to use, and 3) evaluate various configurations to attain freezing rates suitable for various common cryopreservation containers. Through this approach, identical components can be accessed globally, and we demonstrated that 3-D printers can be used to fabricate parts for standardizable freezing devices yielding relevant and reproducible cooling rates across users. With standardized devices for freezing, methods and samples can harmonize into an aggregated high-throughput pathway not currently available for aquatic species repository development.
Keywords: 3-D printing, Cryopreservation, High-throughput, Standardized freezing devices, Freezing rates, Aquatic
1. Introduction
Environmental changes, pollution, and overharvesting caused by human activities continue to threaten and reduce the diversity of natural fish and shellfish populations [13,27]. At the same time, medical research is generating thousands of new transgenic and mutant lines of model species such as Zebrafish [10,16]. Cryopreservation of germplasm is a powerful tool to preserve genetic resources such as these for conservation, stock enhancement, biomedical research, and aquaculture [13,25]. It has been applied around the world for numerous aquatic species [6,24]. However, if cryopreservation is going to be an effective tool, reproducibility must be improved [9], and protocols and equipment need to be standardized and harmonized across user groups.
Although there are published studies on more than 200 aquatic species demonstrating the success of cryopreservation [15,34], there is little uniformity among those reports, and the ability to repeat or compare research is low [31]. Among the wide variety of methods, there are published reports demonstrating the capability of freezing spermatozoa in nitrogen vapor using a portable polystyrene foam container [3,22,26], which enables application in the field and in small-scale production. The use of a polystyrene foam box also provides a low-cost alternative for cryopreservation compared to a programmable freezer. However, most of these studies fail to report important information such as cooling curves and the dimensions of the box, depth of liquid nitrogen, or height above the nitrogen at which the samples were frozen [11,14,17]. Because the vapor-phase temperature gradient inside a polystyrene foam box can be influenced by factors such as wall thickness, volume proportion of liquid nitrogen to air space, and sample distance above the liquid nitrogen [2,29], the lack of these measurements and cooling curves makes comparisons problematic and often disallows reproducibility among studies.
Indeed, many “standard protocols” in cryopreservation of aquatic species often prove to be non-transferrable due to lack of access to specific information, tools, or equipment [30,37]. With the advent of 3-dimensional (3-D) printing technology, variations among devices can be removed as a bottleneck that prevents the merging of cryopreserved products from different sources under a uniform standard. Thermoplastics such as polylactic acid (PLA) are well suited to use for cryogenic activities [33] and are routinely used in 3-D printers. In addition, the philosophy behind open-source 3-D printing could be used in forming a genetic resource community to share and improve cryopreservation experiences with the use of standardized or harmonized devices and protocols. As such, our goal was to develop an approach that could assist small-scale cryopreservation in achieving standardizable aggregate high-throughput. This aggregate pathway is an approach to support repository development by combining defined-quality samples from multiple locations into a unified repository system. The objectives were to: 1) adapt widely available polystyrene foam insulating containers that would be inexpensive, portable, and provide adequate work space; 2) develop a design suitable for 3-D printing that could provide multiple configurations, be inexpensive, and easy to use, and 3) evaluate various configurations to attain freezing rates suitable for various common cryopreservation containers. In this study, we introduce a 3-D printed freezing device to serve as a conceptual model to encourage development of similar devices providing a range of cooling rates based on commonly used containers for aquatic species cryopreservation, and introduce the prospect of using 3-D printing to enhance standardization and harmonization among members of the developing aquatic cryopreservation community.
2. Materials and methods
2.1. Insulating boxes
Products from a large commercial supplier (Polar Tech Industries, Genoa, IL) were selected to provide insulation during cryopreservation due to their wide availability and low cost. Sufficient insulation was achieved by inserting the Insulated Bio Foam Container (Item # 214F) with 31.8-mm wall thickness into a Nestable Insulated Shipper (Item # NS-9KD) with 25.4-mm wall thickness. The combination provided a 57.2-mm total wall thickness and a 12.7-mm layer of air between the two boxes. An outer cardboard carton was used to protect the boxes from punctures and to facilitate shipping. The Bio Foam container had a height of 178 mm and held ~5 L of liquid nitrogen when filled half-way. The liquid nitrogen level inside dropped at a rate of ~10 mm per hr when held at room temperature (23 °C) with both boxes covered with the provided lids.
The temperature profile within the insulating boxes was recorded using 4 type-T thermocouples (Omega Engineering, Stamford, CT) and a multichannel temperature data logger (UEi DT304, UEi Test Instruments, Beaverton, OR). The inner Bio Foam container was filled with liquid nitrogen to a depth of 102 mm. Thermocouples were positioned at 19 mm, 38 mm, 57 mm, and 76 mm above the liquid nitrogen surface and temperatures were recorded for 30 min with the polystyrene lids in place.
2.2. Device design
The major components of the freezing device were two horizontal platforms, two sets of connecting columns of different heights (40 mm and 50 mm), an extruded (closed-cell) polystyrene foam raft, a lifter, and the two nested expanded polystyrene (e.g., Styrofoam) foam boxes described above (Fig. 1). All freezing device components were designed using the free version software Sketchup 8 (Trimble Navigation, Sunnyvale, CA, version 8.0.16846) and were fabricated by 3-D printing using polylactic acid (PLA) filament (Makerbot Industries, Brooklyn, NY) with a fused deposition modeling (FDM) MakerBot Replicator 2 (Makerbot Industries, Brooklyn, NY) 3-D printer. This PLA filament was chosen over acrylonitrile butadiene styrene (ABS) filament because of our observations of its better properties at cryogenic temperatures (−196 °C) [33]. The polystyrene raft was cut from a 12.7-mm thick (R-3) faced extruded polystyrene foam insulation board (STYRO-FOAM™ Brand DURAMATE™ Plus, Dow Chemical Company, Midland, MI) available at home improvement stores. A 127-mm × 127-mm centered square was cut in the raft that could be removed or replaced as needed (Fig. 1). Removal of the inner raft square decreased the total surface area of the polystyrene raft from 542 cm2 to 155 cm2. This allowed for more interaction of nitrogen vapor with the containers increasing the cooling rate for use with French straws. Cryovials and Bank-it tubes only used the full sized 542 cm2 raft. This design provided a standardized means to suspend samples over liquid nitrogen to provide reproducible cooling rates that could be adjusted easily by changing the device configurations.
Fig. 1.
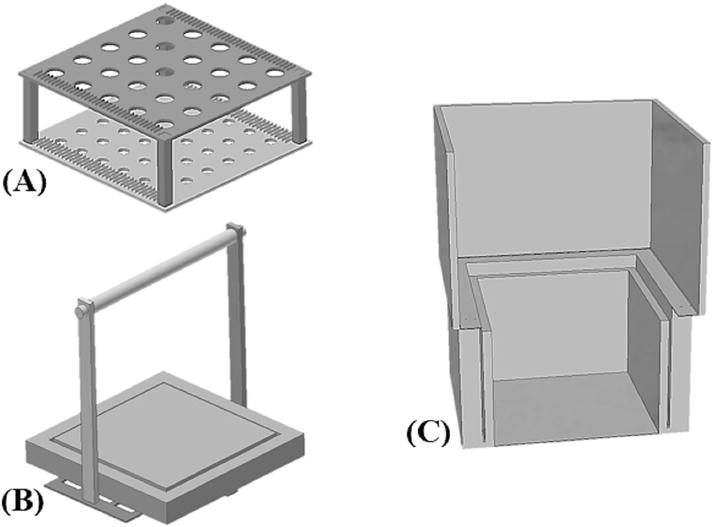
Diagram of the components of a prototype 3-D printed freezing device. A: Upper and lower freezing platforms (140 × 140 mm, L × W) with 50-columns (40-mm columns not shown). B: Raft lifter with polystyrene raft (152 × 152 mm × 13, L × W × D). The raft had a center-cut square that could be inserted or removed depending on desired freezing rates. C: Cut-away view of the nestable insulated shipper with insulated bio foam container.
For use, liquid nitrogen was added to the inner Bio Foam container at a depth of about 100 mm (95–105 mm, about 5 L). The depth of liquid nitrogen was determined by slowly inserting a black ruler to the bottom, removing it, and viewing the frozen condensate. In general, a lowering of the liquid nitrogen level by 5–10 mm per freezing run did not affect performance. After each run, the liquid nitrogen level was readjusted. The raft lifter and raft were lowered into the liquid nitrogen for pre-cooling and both polystyrene boxes were covered and allowed to equilibrate for a minimum of 5 min. This stabilized nitrogen vapor levels inside the freezing chamber and cooled the outer container layer. If the lifter and raft were not pre-cooled, the liquid nitrogen would boil when lowered with samples and cause improper cooling. After equilibration, all polystyrene boxes remained covered until freezing began. The sample containers were placed on the freezing platform and held at 4 °C. For our trials, the freezing platform was fully loaded with the specific container types being tested. To begin freezing, the lifter with raft were carefully raised from the liquid nitrogen, the freezing platform was placed on top of the raft and the entire apparatus was lowered onto the surface of the liquid nitrogen. The lifter sank into the liquid nitrogen, while the freezing platform remained on the surface, providing a reproducible cooling configuration for all 6 combinations of platform and raft options using four different container types (Table 1). The two boxes were covered until the full freezing profile was achieved (according to cooling rate, usually no more than 20 min). After freezing, the samples were plunged into liquid nitrogen while containers were sorted for storage in dewars.
Table 1.
Average cooling rates (°C/min from 4 °C to −80 °C) (mean ± SD, n = 3) of French straws, Cryovial and Nunc Bank-it tubes for each design configuration. Column height represents which columns were used to raise the freezing platform above liquid nitrogen. If none, only the freezing platform was used. Sample size represents the sample size loaded in the tubes during freezing. The two raft sizes represent the maximum-area raft (542 cm2) and with the center square removed (155 cm2).
| Container | Raft size | Sample size | Column height
|
||
|---|---|---|---|---|---|
| None | 40 mm | 50 mm | |||
| 0.25-mL French straw | 542 cm2 raft | 250 μL | 21.8 ± 7.3 | <4 °C | <4 °C |
| 155 cm2 raft | 250 μL | 31.1 ± 4.8 | 9.5 ± 0.4 | 7.7 ± 0.1 | |
| 0.5-mL French straw | 542 cm2 raft | 500 μL | 11.0 ± 5.8 | <4 °C | <4 °C |
| 155 cm2 raft | 500 μL | 31.2 ± 12.2 | 6.2 ± 0.5 | 5.3 ± 0.4 | |
| 2-mL Cryovial | 542 cm2 raft | 50 μL | −a | 15.9 ± 1.8 | 17.1 ± 3.6 |
| 100 μL | −a | 11.4 ± 1.8 | 16.0 ± 4.0 | ||
| 500 μL | −a | 6.2 ± 0.8 | 4.9 ± 0.2 | ||
| 0.5-mL Nunc Bank-it | 542 cm2 raft | 100 μL | −a | 24.2 ± 9.7 | 14.6 ± 2.1 |
| 500 μL | −a | 9.5 ± 2.4 | 6.6 ± 0.6 | ||
| 1000 μL | −a | 6.9 ± 1.3 | 5.0 ± 0.6 | ||
Not applicable, container must use columns.
2.3. Performance testing
Comprehensive tests were performed using 4 type-T thermocouples and a multichannel temperature data logger to record the temperature profiles (measured to 0.1 °C) of the different combinations of variables. The tests were performed indoors at room temperature of 22–25 °C. Thermocouples were inserted into containers filled with Hanks’ balanced salt solution, a commonly used extender solution for cryopreservation of fish sperm [35], at an osmolality of 300 mOsmol/kg (HBSS300: 0.137 M NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, and 5.55 mM glucose, pH 7.2) [32] and were placed at specified positions across the platform (Fig. 2). Each freezing run used the maximum number of specific containers allowed by the design of the platform.
Fig. 2.
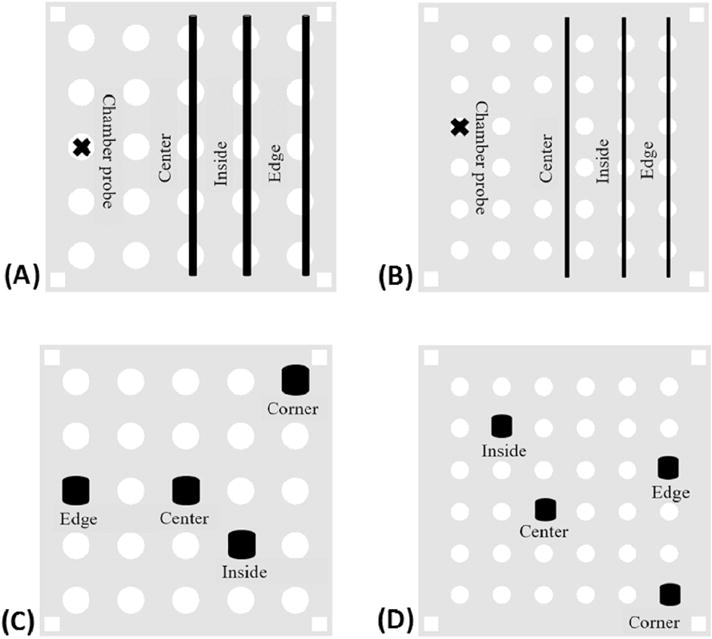
The positioning of data logger thermocouples used during freezing curve tests. All thermocouples were inserted into containers filled with Hanks’ balanced salt solution (300 mOsmol/kg). A: 0.5-ml French straws; B: 0.25-ml French straws; C: 2-ml Cryovials; D: 0.5-ml Nunc Bank-it tubes. A thermocouple (X) was used to record the ambient positional temperature profiles for A and B.
Different combinations of height (no columns, 40-mm columns, or 50-mm columns) and raft surface area (542 cm2 or 155 cm2) were tested. Ambient positional temperature profiles were recorded during the testing of 0.25-mL and 0.5-ml French straws. Cooling curves were recorded from 4 °C to −80 °C and average cooling rate was calculated as the temperature change over time from 4 °C to −80 °C. To obtain consistent data, each test was performed with thermocouples in the same position for each container and with the maximum number of containers filled with HBSS300 (i.e., maximum thermal mass to standardize heat). We focused attention on producing a range of cooling rates from 4 °C to 40 °C per min, which are the rates used for most aquatic species protocols. Each container type with each freezing combination was tested at least 3 times. Cooling rates were calculated based on average values using Microsoft Excel.
3. Results
3.1. Temperature profile of insulating boxes
Static temperatures at specific heights above the surface of the liquid nitrogen were recorded after thermal stabilization. The maximum temperatures measured ranged from −167 °C (19 mm) to −79 °C (76 mm) (Fig. 3). All containers were held at < 76 mm above the liquid nitrogen for freezing.
Fig. 3.
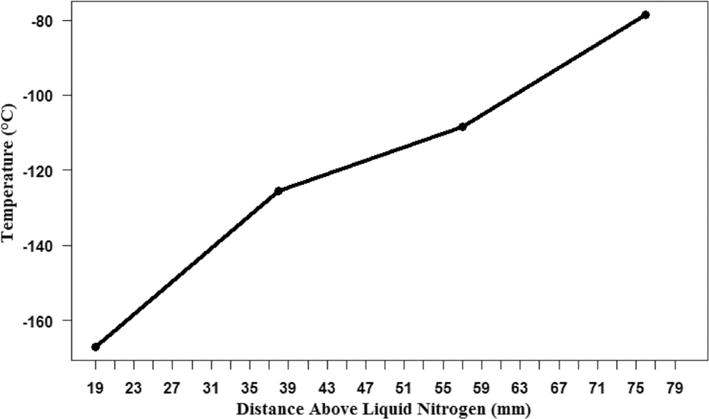
Maximum temperatures recorded after 30 min for specific heights above liquid nitrogen within the double polystyrene boxes. These heights were below the highest position of the tallest device configuration. Therefore, all samples were exposed to positional temperatures at or below −80 °C.
3.2. Freezing device
Multiple freezing configurations were achieved by adjusting the height of the upper platform above the liquid nitrogen surface with the use of 40-mm columns, 50-mm columns, or no columns and changing the surface area of the polystyrene raft from 542 cm2 to 155 cm2 by removing the center square. Use of a floating platform in the open-top polystyrene boxes provided greater working space during freezing and sorting than did freezing in the neck of standard shipping dewars (a traditional means for on-site freezing) [40]. The upper platforms were designed to hold specific numbers of four commonly used containers for sperm cryopreservation: 33 0.5-mL French straws (IMV International Corp., Minneapolis, MN), 34 0.25-mL French straws (IMV International Corp.), 25 2-mL Corning Cryovials (Corning, Tewksbury, MA), and 36 0.5-mL Nunc Bank-it tubes (Thermo Fisher Scientific, Waltham, WA). Removal of the inner raft square reduced the floatation capability of the raft. Thus, full racks of Cryovial or Bank-it tubes could not be supported reliably using the 155 cm2 raft, but full racks of 0.25-mL and 0.5-mL French straws could be supported. The material (PLA) used for the printed components collectively weighed 129 g. Most common brands of filament (e.g., Makerbot, Hatchbox) range from US$18 to US$60 for a 1 kg spool of PLA resulting in a unit material cost of US$2.30 to US$7.70 per device.
3.3. Container performance
Multiple configurations of the raft and height above the liquid nitrogen surface were used to produce a range of cooling rates typically used for fish sperm (i.e., from 4 °C/min to 40 °C/min) (Fig. 4). Four freezing rates were recorded for the 0.25-mL and 0.5-mL straws (Table 1). The fastest rates were produced by using only the freezing platform and 155 cm2 raft. The slowest rates were produced by using 50-mm columns and 542 cm2 raft. The Cryovials and Bank-it tubes were also tested with three different sample volumes each (from 50 μL to 1000 μL). Two freezing rates were attained for each sample volume (Table 1). The freezing rates for the Cryovials and Bank-it tubes were produced by using the 542 cm2 raft and the 40-mm and 50-mm columns. Columns were used with the Cryovials and Bank-it tubes because they were positioned vertically and protruded below the upper freezing platform unlike the French straws which were positioned horizontally on top of the freezing platform.
Fig. 4.
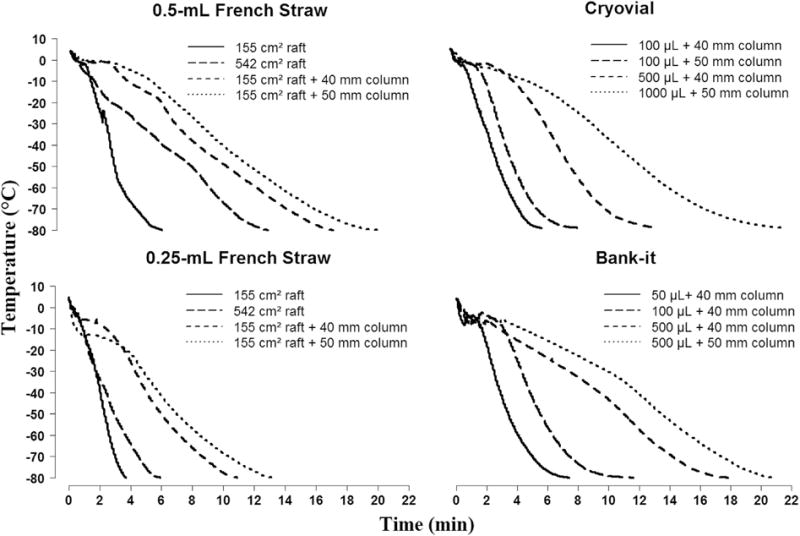
Representation of cooling curves for different container configurations. The freezing device was designed to provide a range of reproducible cooling rates for four containers: 0.5-ml French straws, 0.25-ml French straws, 2-ml Cryovial, and 0.5-ml Nunc Bank-it tubes. A range of cooling rates was produced by various combinations of rafts area (155 cm2 and 542 cm2) and column height (40 mm, 50 mm, or none). Such cooling curves could be used for reference among different users. See Table 1 for average cooling rates.
4. Discussion
There are a variety of freezing devices used in aquatic cryopreservation that range from inexpensive and portable to complex and expensive. Freezing samples in nitrogen vapor using an inexpensive polystyrene foam box is a commonly used method [4,17]. The lightweight characteristics of a polystyrene box facilitates the use of this design in field settings [12]. Through the years there have been different levels of design sophistication. The simplest design used only a polystyrene raft which the samples were placed on and floated on the liquid nitrogen surface [23,28]. Other designs included the use of metal frames and adjustable freezing heights [5,21]. The use of a shipping dewar is another technique used to freeze samples in nitrogen vapor. Shipping dewars can be expensive, but this technique is simple and multiple freezing rates can be achieved by the different height placement of samples in the dewar. The simplest method used goblets positioned at the desired height on aluminum canes which were placed inside the dewar [39]. Other methods used a custom polystyrene foam container inside a shipping dewar to control cooling rates or adapted a mammalian embryo freezer to hold French straws that were positioned inside the dewar at different heights [19,38]. The most complex and expensive device is the programmable freezer. Programmable freezers can cost between US$15,000 and US$60,000 and typically have a freezing capacity of hundreds of samples or more. These freezers can meet high-throughput needs (hundreds to thousands of samples per day) of commercial activities, but can be too costly for low-throughput research efforts (tens to hundreds of samples per day). The high cost of commercially available freezing devices and use of non-standardized custom freezing devices is a bottleneck that continues to prevent new user groups from joining the field.
4.1. Standardization and file sharing
The first step towards aggregate high-throughput is the standardization of freezing protocols. Reproducing an object implies reformation of the same materials with the same structural information. The traditional path of replicating cryopreservation protocols starts from individual laboratories developing protocols through experiments. Peer-reviewed publications are written to share the information with the scientific community, and other laboratories interpret the publications and attempt to reestablish similar conditions within their understanding and availability of resources. Unfortunately, for most aquatic species cryopreservation research, much of the relevant information is typically not recorded, controlled, or reported which provides fundamental challenges for reproducibility [35].
To contribute to solving these chronic problems, evolving technologies that are now becoming available were utilized which provide the basis of a networking community. Access to 3-D printing is becoming easier with print shops, libraries, and companies (e.g., United Parcel Service and Federal Express) now equipped with 3-D printers. Manufacturers are targeting markets with everyday consumers for home use making this technology available and cost effective. These FDM printers can range from US$350 to US$4,000, but after this initial expense, individual printed objects often cost less than 1 US$ each, and had an actual per unit cost in this project of ~5 US$ based on the filament we used [7]. As such, 3-D files, along with an instruction manual, can be shared through online community resources (e.g., Thingiverse, Grabcad) and be reproduced anywhere. A separate online resource could also be created to share these 3-D files, tutorials, and educational materials among members of the cryobiology community. The US National Institutes of Health have recently created a 3-D Printing Exchange portal (http://3Dprint.nih.gov) that is a hub for downloading and sharing models [8]. It contains over 2500 3-D files related to biomolecules, chemicals, organisms, laboratory ware, and medical applications such as prosthetic devices. This direct method of file sharing and instruction minimizes information loss, allowing results from multiple users to be comparable.
The next step towards high-throughput would be to create and standardize the channels of quality assessment used to aggregate the products. This approach evolves naturally from a standardization and quality assurance approach traditionally used for high-throughput mass production in industry [18]. Individual cryopreservation processes can be standardized or harmonized by systematically reducing the variation at each step. Although most users may not have the necessary tools to evaluate product quality, comprehensive facilities or institutions with reliable quality assessment capabilities could provide such a service. Quality assessment assays, such as post-thaw motility or assessment of membrane integrity [36], could be used to generate and reinforce global standards, and with standardization across users, germplasm repositories can be efficiently developed and expanded with reproducible results [9]. Although individual freezing devices such as that described herein can only produce tens or hundreds of samples per day, utilization of standardized aggregate high-throughput pathways could link hundreds or thousands of users and additively expand production. Further studies could focus on evaluation of 3-D printed freezing devices for different species, applications, and adaption of existing cryopreservation protocols for use with them.
It has been shown previously with open-source file sharing that users of initial designs often take the liberty to make changes based on their knowledge and resources [20]. Although this phenomenon is part of the concept of commons-based peer production [1], it should be considered carefully in this particular case when standardized designs become accepted. Using identical printed parts and procedures will help in achieving standardization. If, however, pieces are redesigned, the use of standardized calculations for freezing rates when using liquid nitrogen vapor, and comprehensive standardized reporting (e.g., of temperature profiles in addition to averaged rates) could ensure harmonization of methods across users while still allowing improvements to be made. As stated above, PLA thermoplastic is especially well suited for cryogenic applications [33]. In our experience, it does not appreciably shrink or warp when cooled, does not become brittle, and can be safely handled immediately after removal from liquid nitrogen. As such, it provides a useful starting material for 3-D printing of cryobiological devices.
5. Conclusions
In summary, we have provided a new option by use of 3-D printing to address the current wide variation in cryopreservation of freezing devices used around the world. The use of 3-D printing allows for rapid prototyping of devices and creates new opportunities for users to join the cryopreservation community. The design of freezing devices has features of portability, adjustable cooling rates, reproducibility, and ease of use. The design files of this prototype freezing device describe herein are available (www.aquaticgermplasm.com) and can be printed with shared stereolithography (STL) files. An approach such as this could provide cryopreservation capability to users who do not currently have access to expensive freezing devices. An interactive community based on comparing and sharing standardized (or at least harmonized) cryopreservation protocols and samples could be established around such devices and concepts, and the results and frozen products could ultimately be integrated into an aggregate high-throughput pathway of repository development for germplasm and genetic resources of aquatic species. Such as approach could also be developed and applied for other user groups outside of aquatic species.
Acknowledgments
We thank N. Novelo, A. Guitreau, and B. Ho for technical assistance. This work was supported in part by funding from the National Institutes of Health, Office of Research Infrastructure Programs (R24-RR023998 and R24-OD011120), with additional support provided by the National Institute of Food and Agriculture, United States Department of Agriculture (Hatch project LAB94231). This report was approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 2016-241-30597.
References
- 1.Benkler Y, Nissenbaum H. Commons-based peer production and virtue*. J Political Philos. 2006;14:394–419. [Google Scholar]
- 2.Blundell DJ, Ricketson BW. The temperature of liquid nitrogen in cryostat dewars. Cryogenics. 1979;19:33–36. [Google Scholar]
- 3.Bye VJ, Ponniah AG. Application of genetics in aquaculture. CMFRI Spec Publ. 1983;13:1–90. [Google Scholar]
- 4.Cabrita E, Engrola S, Conceição LEC, Pousão-Ferreira P, Dinis MT. Successful cryopreservation of sperm from sex-reversed dusky grouper. Epinephelus marginatus, Aquaculture. 2009;287:152–157. [Google Scholar]
- 5.Cabrita E, Robles V, Alvarez R, Herráez M. Cryopreservation of rainbow trout sperm in large volume straws: application to large scale fertilization. Aquaculture. 2001;201:301–314. [Google Scholar]
- 6.Cabrita E, Sarasquete C, Martínez-Parámo S, Robles V, Beirao J, Pérez-Cerezales S, Herraez M. Cryopreservation of fish sperm: applications and perspectives. J Appl Ichthyol. 2010;26:623–635. [Google Scholar]
- 7.Coakley MF, Hurt DE. 3D Printing in the laboratory: maximize time and funds with customized and open-source labware. J Lab Autom. 2016;21:489–495. doi: 10.1177/2211068216649578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coakley MF, Hurt DE, Weber N, Mtingwa M, Fincher EC, Alekseyev V, Chen DT, Yun A, Gizaw M, Swan J. The NIH 3D print exchange: a public resource for bioscientific and biomedical 3D prints. 3D Print Addit Manuf. 2014;1:137–140. doi: 10.1089/3dp.2014.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins FS, Tabak LA. NIH plans to enhance reproducibility. Nature. 2014;505:612. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detrich HW, Westerfield M, Zon LI. The Zebrafish: Genetics, Genomics and Informatics. Academic Press; San Diego: 2004. [Google Scholar]
- 11.Glogowski J, Kolman R, Szczepkowski M, Horváth Á, Urbányi B, Sieczyński P, Rzemieniecki A, Domagała J, Demianowicz W, Kowalski R. Fertilization rate of Siberian sturgeon (Acipenser baeri, Brandt) milt cryopreserved with methanol. Aquaculture. 2002;211:367–373. [Google Scholar]
- 12.Gopalakrishnan A, Thakur K. Cryopreservation of brown trout (Salmo trutta fario) sperm: the influence of extender composition and fertilization procedure. Fish Technol. 1999;36:104–109. [Google Scholar]
- 13.Guan M, Rawson DM, Zhang T. Cryopreservation of zebrafish (Danio rerio) oocytes using improved controlled slow cooling protocols. Cryobiology. 2008;56:204–208. doi: 10.1016/j.cryobiol.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Gwo JC. Cryopreservation of yellowfin seabream (Acanthopagrus latus) spermatozoa (teleost, perciformes, sparidae) Theriogenology. 1994;41:989–1004. doi: 10.1016/s0093-691x(05)80022-6. [DOI] [PubMed] [Google Scholar]
- 15.Gwo JC. Cryopreservation of aquatic invertebrate semen: a review. Aquac Res. 2000;31:259–271. [Google Scholar]
- 16.Hagedorn M, Ricker J, McCarthy M, Meyers S, Tiersch T, Varga Z, Kleinhans F. Biophysics of zebrafish (Danio rerio) sperm. Cryobiology. 2009;58:12–19. doi: 10.1016/j.cryobiol.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horváth Á, Wayman WR, Urbányi B, Ware KM, Dean JC, Tiersch TR. The relationship of the cryoprotectants methanol and dimethyl sulfoxide and hyperosmotic extenders on sperm cryopreservation of two North-American sturgeon species. Aquaculture. 2005;247:243–251. [Google Scholar]
- 18.Hu E, Yang H, Tiersch TR. High-throughput cryopreservation of spermatozoa of blue catfish (Ictalurus furcatus): establishment of an approach for commercial-scale processing. Cryobiology. 2011;62:74–82. doi: 10.1016/j.cryobiol.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh ICC, Tanaka D, Itagane T, Tsuji M, Tsuchihashi Y, Ohta H. Dry shipper cryopreservation of seven-band grouper (Epinephelus septemfasciatus Thunberg) spermatozoa. Aquac Res. 2012;44:59–66. [Google Scholar]
- 20.Kostakis V, Fountouklis M, Drechsler W. Peer production and desktop manufacturing: the case of the Helix_T wind turbine project. Sci Technol Hum Values. 2013;38:773–800. [Google Scholar]
- 21.Lahnsteiner F, Weismann T, Patzner R. Cryopreservation of semen of the grayling (Thymallus thymallus) and the Danube salmon (Hucho hucho) Aquaculture. 1996;144:265–274. [Google Scholar]
- 22.Leibo S, Michael Kubisch H, Dee Schramm R, Harrison RM, VandeVoort CA. Male-to-male differences in post-thaw motility of rhesus spermatozoa after cryopreservation of replicate ejaculates. J Med Primatol. 2007;36:151–163. doi: 10.1111/j.1600-0684.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Liu Y, Liu S, Xu T, Liu Q, Li X. Cryopreservation of strip spawned sperm using non-programmable freezing technique in the blue mussel. Mytilus galloprovincialis, Aquac Res. 2015:1–11. [Google Scholar]
- 24.Mounib MS. Cryogenic preservation of fish and mammalian spermatozoa. J Reprod Fertil. 1978;53:13–18. doi: 10.1530/jrf.0.0530013. [DOI] [PubMed] [Google Scholar]
- 25.Mudrak VA, Looney GL. Risk considerations in the application of cryopreservation techniques for aquatic species. In: Tiersch TR, Green CC, editors. Cryopreservation in Aquatic Species. second. World Aquaculture Society; Baton Rouge: 2011. pp. 931–938. [Google Scholar]
- 26.Patil R, Lakra WS. Effect of cryoprotectants, equilibration periods and freezing rates on cryopreservation of spermatozoa of mahseer, Tor khudree (Sykes) and T. putitora (Hamilton) Aquac Res. 2005;36:1465–1472. [Google Scholar]
- 27.Pimentel D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ. 2005;52:273–288. [Google Scholar]
- 28.Richardson GF, Wilson CE, Crim LW, Yao Z. Cryopreservation of yellowtail flounder (Pleuronectes ferrugineus) semen in large straws. Aquaculture. 1999;174:89–94. [Google Scholar]
- 29.Santos MV, Sansinena M, Zaritzky N, Chirife J. Mathematical prediction of freezing times of bovine semen in straws placed in static vapor over liquid nitrogen. Cryobiology. 2013;66:30–37. doi: 10.1016/j.cryobiol.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Suquet M, Dreanno C, Fauvel C, Cosson J, Billard R. Cryopreservation of sperm in marine fish. Aquac Res. 2000;31:231–243. [Google Scholar]
- 31.Tiersch TR. Process pathways for cryopreservation research, application and commercialization. In: Tiersch TR, Green CC, editors. Cryopreservation in Aquatic Species. second. World Aquaculture Society; Baton Rouge: 2011. pp. 646–671. [Google Scholar]
- 32.Tiersch TR, Goudie CA, Carmichael GJ. Cryopreservation of channel catfish sperm: storage in cryoprotectants, fertilization trials, and growth of channel catfish produced with cryopreserved sperm. Trans Am Fish Soc. 1994;123:580–586. [Google Scholar]
- 33.Tiersch TR, Monroe WT. Three-dimensional printing with polylactic acid (PLA) thermoplasic offers new opportunities for cryobiology. Cryobiology. 2016 doi: 10.1016/j.cryobiol.2016.10.005. http://dx.doi.org/10.1016/j.cryobiol.2016.1010.1005. [DOI] [PMC free article] [PubMed]
- 34.Tiersch TR, Yang H, Jenkins JA, Dong Q. Sperm cryopreservation in fish and shellfish. Soc Reprod Fertil Suppl. 2007;65:493–508. [PubMed] [Google Scholar]
- 35.Torres L, Hu E, Tiersch TR. Cryopreservation in fish: current status and pathways to quality assurance and quality control in repository development. Reprod Fertil Dev. 2016;28:1105–1115. doi: 10.1071/RD15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres L, Tiersch TR. Amine reactive dyes: an alternative to estimate membrane integrity in fish sperm cells. Aquaculture. 2016;463:71–78. doi: 10.1016/j.aquaculture.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viveiros A, Orfão L, Maria A, Allaman I. A simple, inexpensive and successful freezing method for curimba Prochilodus lineatus (Characiformes) semen. Animal Reprod Sci. 2009;112:293–300. doi: 10.1016/j.anireprosci.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Wayman WR, Thomas RG, Tiersch TR. Cryopreservation of sperm of spotted seatrout (Cynoscion nebulosus) Gulf Res Rep. 1996;9:183–188. [Google Scholar]
- 39.Wayman WR, Thomas RG, Tiersch TR. Refrigerated storage and cryopreservation of black drum (Pogonias cromis) spermatozoa. Theriogenology. 1997;47:1519–1529. doi: 10.1016/s0093-691x(97)00158-1. [DOI] [PubMed] [Google Scholar]
- 40.Wayman WR, Tiersch TR. Research methods for cryopreservation of sperm. In: Tiersch TR, Green CC, editors. Cryopreservation in Aquatic Species. second. World Aquaculture Society; Baton Rouge: 2011. pp. 672–683. [Google Scholar]


