Abstract
Objectives
To describe the longitudinal changes in hepatic function among HIV-infected tuberculosis (TB) patients receiving once-daily nevirapine (NVP) or efavirenz (EFV)-based antiretroviral treatment (ART) along with rifampin-containing anti-TB treatment.
Methods
This was a nested study within a randomized clinical trial, taking place between May 2006 and June 2008 at the National Institute for Research in Tuberculosis, Chennai, India. Antiretroviral-naïve HIV-infected TB patients were initiated on an intermittent short-course regimen and randomized to receive didanosine and lamivudine with either NVP (400 mg) or EFV (600 mg) once-daily. Blood was analyzed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum alkaline phosphatase (SAP), and bilirubin at baseline, at ART initiation, fortnightly after ART initiation until 2 months, then monthly until 6 months and 6-monthly thereafter.
Results
Of the 168 patients included (79% men, median CD4 count 93 cells/mm3, median viral load 242 000 copies/ml), 104 were on EFV-based ART and 64 on NVP-based ART. There was a small but statistically significant elevation in ALT and SAP at 2 weeks and AST at 6 weeks after ART initiation. The proportion of patients with rate-limiting toxicity of liver enzymes was small. None had treatment terminated because of hepatotoxicity.
Conclusion
Hepatotoxicity is not a major concern when HIV-infected TB patients, with normal baseline liver function initiate treatment for both infections simultaneously.
1. Introduction
It is estimated that there are approximately 1.1 million adults co-infected with HIV and tuberculosis (TB) globally.1 The World Health Organization (WHO) recommends initiating antiretroviral treatment (ART) for all HIV–TB co-infected patients within a few weeks of TB treatment, regardless of CD4 cell count, to reduce all-cause mortality and improve TB treatment outcomes.2,3 In a public health approach, recommended ART regimens include combinations of two nucleoside reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (NNRTI), with nevirapine (NVP) or efavirenz (EFV) being the preferred NNRTIs.2 For the treatment of TB, regimens containing isoniazid and a rifamycin throughout are recommended, as they have better outcomes in terms of lower failure and recurrence rates.4,5
Although studies have reported clinical and virological efficacy with the concurrent use of NNRTI and rifamycin-based TB treatment,6,7 few reports have been published on the safety of the concomitant use of these regimens. Isoniazid, rifampin, NVP, and EFV are all associated with hepatotoxicity, and little is known about the relative rates of hepatotoxicity with either NVP or EFV in the setting of rifampin-based TB treatment.8 Further, there is no information on rates of hepatotoxicity among HIV–TB co-infected patients treated with an intermittent (three times weekly) regimen. Studies in HIV-uninfected populations suggest that toxicity rates tend to be lower in patients given intermittent compared to daily chemotherapy.9
2. Methods
This study was part of a prospective randomized controlled clinical trial “Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy when co-administered with rifampin-based antitubercular therapy”, the details of which have been described elsewhere.10 In brief, between May 2006 and June 2008, HIV-1-infected adults with active TB and a CD4 count 250 cells/mm3 were enrolled in a study at the National Institute for Research in Tuberculosis (formerly the Tuberculosis Research Centre). Patients with prior ART or ATT, an HIV-2 infection, or serum aminotransferases >2.5 times the upper limit of normal (ULN) were excluded. The ‘once-daily ART’ controlled clinical trial collected detailed clinical and laboratory data to monitor the safety and efficacy of administering different ART regimens in combination with ATT. Therefore, it was possible to monitor the development of hepatotoxicity at various time points in the trial.
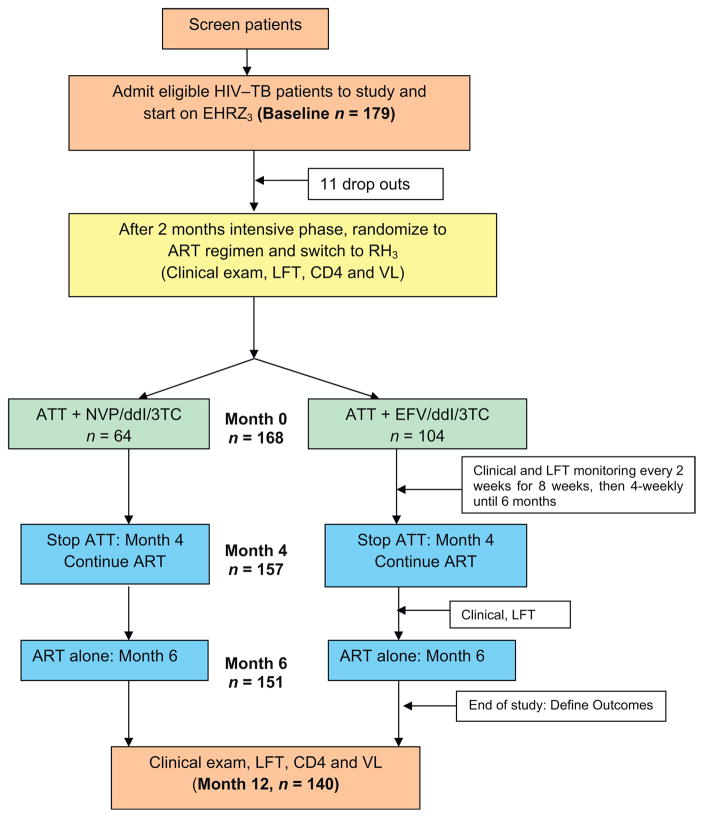
All patients enrolled in the ‘once-daily ART’ study initiated a standard TB treatment with four drugs: isoniazid (600 mg), rifampin (450/600 mg based on body weight <60 or >=60 kg), ethambutol (1200 mg), and pyrazinamide (1500 mg) for the first 2 months; two drugs, isoniazid and rifampin, were used for the subsequent 4 months. All drugs were administered three times weekly (2EHRZ3/4RH3) under direct observation, in accordance with national guidelines. After 2 months of TB treatment, participants were randomized to the once-daily ART regimen (month 0) with either NVP (400 mg, after a lead-in period of 200 mg once-daily) or EFV (600 mg per day), along with didanosine (250/400 mg for body weight <60 or 60 kg) and lamivudine (300 mg).
The time of ATT initiation was defined as ‘baseline’ and the time of ART initiation (i.e., 2 months after ATT initiation) as month 0. Subsequent weeks/months are chronological with reference to month 0 throughout this article. ART was administered under direct supervision 3 days per week and supplied to the patient for self-administration on the remaining days. ATT was stopped at 6 months and ART was continued (Figure 1).
Figure 1.
Schematic diagram of the ‘once-daily ART’ study design.
Liver function tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum alkaline phosphatase (SAP), and bilirubin were measured using an automated analyzer (Olympus AU400, Japan) at baseline (month 2), month 0, then every 2 weeks until week 8 (month 2) and then once every 8 weeks until 6 months. Hepatotoxicity grades were defined in accordance with the AIDS Clinical Trials Group (ACTG) criteria: grade I, 1.25–2.5 times ULN; grade II, 2.5–5.0 times ULN; grade III, 5–10 times ULN; grade IV, >10 times ULN.11 Mild hepatotoxicity was defined as ACTG grades I and II, and severe toxicity as grades III and IV.
2.1. Statistical analysis
The distribution of each variable was checked cross-sectionally at baseline and follow-up. All unusual values were verified. Mean values and standard deviations were tabulated for normally distributed variables; the median and 25th and 75th percentiles were tabulated for skewed variables. We compared baseline liver enzymes and various baseline demographic and clinical characteristics between treatment groups using the Student’s t-test or the Wilcoxon rank-sum test, as appropriate. Changes in liver enzymes during both the early and late stage of treatment were compared with baseline levels using repeated measures analyses, with skewed variables transformed to attain normality. We also compared changes in liver enzymes between treatment groups using the Mann–Whitney U-test. We compared the proportion of patients with liver enzyme abnormalities at baseline vs. at 12 months using the McNemar test for correlated proportions and the Chi-square test to compare between the two treatment groups. All tests used α = 0.05 as the cut-off for statistical significance. Analyses were performed using SPSS software version 14.0 (SPSS Inc., Chicago, IL, USA).
The study was approved by the ethics committee of the National Institute for Research in Tuberculosis, and informed written consent was obtained from all patients prior to study enrollment.
3. Results
Of the 179 patients enrolled into the once-daily ART study, 168 patients (132 men, 36 women) were found eligible for the current analysis (11 were not randomized – early drop-outs). Follow-up data were available for 157 patients at 4 months, 151 at 6 months, and 140 patients at 12 months (15 patients died, 11 were lost to follow-up, and two missed study visits) (Figure 1).
3.1. Patient characteristics
At baseline, the 168 HIV-infected patients with TB (79% men) had a mean age of 36 years, mean body weight of 42 kg, a median CD4 count of 93 cells/mm3, and HIV viral load of 242 000 copies/ml. One hundred and four patients were randomized to the EFV arm and 64 to the NVP arm (the data safety monitoring board stopped the intake of patients into the NVP arm after the first interim analysis, which explains the unequal numbers). At baseline, patients did not differ significantly between the two treatment arms with respect to body weight, CD4 cell count, or viral load (Table 1). Forty-four percent of patients consumed alcohol (often or habitually) and 2.4% had a hepatitis co-infection (hepatitis B in three patients, hepatitis C in one patient). None of the patients in our study population had a hepatitis B and C co-infection. Drug intake was directly observed 3 days a week and overall adherence was >90%. The majority of subjects had an undetectable HIV load after 12 months of ART.
Table 1.
Baseline characteristics of the study populations of the two treatment groups
| Variables at baseline | Patients on EFV-based regimen (n = 104) | Patients on NVP-based regimen (n = 64) | p-Value |
|---|---|---|---|
| Age (years) | 35 (6.9) | 38 (1.7) | 0.06 |
| Weight (kg) | 41.9 (7.9) | 43 (7.7) | 0.89 |
| Male gender | 81 (78%) | 51 (80%) | 0.78 |
| Alcohol consumption (often) | 43 (41%) | 31 (48%) | 0.14 |
| CD4 count (cells/mm3) | 90 (53–130) | 75 (34–130) | 0.14 |
| Viral load (copies/ml) | 259 000 (81 200–531 000) | 203 000 (87 900–370 000) | 0.79 |
| AST (U/l) | 43 (29–65) | 47 (29–52) | 0.93 |
| ALT (U/l) | 26 (16–42) | 31 (19–41) | 0.94 |
| SAP (U/l) | 127 (91–232) | 211 (74–260) | 0.78 |
| Serum bilirubin (mg/dl) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.26 |
EFV, efavirenz; NVP, nevirapine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; SAP, serum alkaline phosphatase; SD, standard deviation; IQR, interquartile range.
Results are mean (SD), n (%), or median (IQR). Comparison between groups: t-test for means, and Wilcoxon signed rank test for medians.
3.2. Baseline liver enzymes
Baseline liver enzymes were comparable in the two treatment groups. AST and ALT were significantly higher among patients with CD4 counts <90 cells/mm3 (54 vs. 41 mg/dl, p < 0.002, and 35 vs. 27 mg/dl, p = 0.01, respectively), while SAP was higher among patients with a viral load >300 000 (249 vs. 171 mg/dl, p = 0.04). These cut-offs were based on the median levels at baseline. At baseline, ALT was >100 mg/dl in three patients (2%), AST was 100 mg/dl in seven patients (6%), and SAP was >350 mg/dl in 18 patients (12%); these patients were excluded from the analysis.
3.3. Longitudinal liver enzymes
3.3.1. Effect of ATT alone
After 2 months of ATT, there was an increase in the median values of liver enzymes, although this was not statistically significant (ALT 26 vs. 27 mg/dl, p = 0.71, and AST 41 vs. 42, p = 0.69). These rises were minimal (less than two times ULN), and ART was initiated.
3.3.2. Effect of ART and ATT together
At 2 weeks of ART, ALT increased from 26 to 32 mg/dl (p = 0.03) and at 6 weeks AST from 41 to 46 mg/dl (p = 0.02). These increases remained within the ULN range and ART was continued. Even after 4 months of ATT given along with ART, a statistically significant rise in ALT was noticed (31 vs. 26 mg/dl, p = 0.001), but this was also within normal limits. The increase in SAP at 8 weeks was to a level slightly above normal and was also statistically significant (166 vs. 131 mg/dl, p = 0.001) (Table 2).
Table 2.
Pattern of change in liver enzymes on co-administration of once-daily NNRTI-based ART with rifampin-based ATTa
| Liver enzymes (normal range) | Baseline (n = 168) (No ATT/ART) | 0 week (n = 168) (2 months ATT + no ART) | 2 weeks (n = 157) (ATT + ART) | 1 month (n = 161) (3 months ATT + 1 month ART) | 6 weeks Median (IQR) (n = 133) | 2 months (n = 159) (4 months ATT + 2 months ART) | 4 months (n = 157) (6 months ATT + 4 months ART) | 6 months (n = 151) (no ATT + 6 months ART) | 12 months (n = 140) (no ATT + 12 months ART) |
|---|---|---|---|---|---|---|---|---|---|
| ALT (U/l) (5–41 U/l) | 26 (17–41) | 27 (21–36) | 32b (23–44) | 32 (24–53) | 33 (24–51) | 31 (24–43) | 31 (23–40) | 30 (22–46) | 29 (20–40) |
| AST (U/l) (5–40 U/l) | 41 (29–60) | 42 (33–54) | 43 (33–57) | 43 (34–60) | 46b (36–60) | 42 (32–54) | 39 (32–53) | 40 (30–52) | 34 (27–46) |
| SAP (U/l) (35–140 U/l) | 131 (85–238) | 131 (99–203) | 150 (98–250) | 172 (111–297) | 169 (118–275) | 166 (119–269) | 157 (118–226) | 171b (124–233) | 143 (107–176) |
| Serum bilirubin (mg/dl) (0.2–1.0 mg/dl) | 0.4 (0.3–0.6) | 0.3 (0.3–0.4) | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.3 (0.3–0.4) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) |
NNRTI, non-nucleoside reverse transcriptase inhibitor; ART, antiretroviral therapy; ATT, anti-tuberculosis therapy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAP, serum alkaline phosphatase; IQR, interquartile range.
All values are median (IQR). Comparison between time-points was done by repeated measure analysis.
p <0.005 was considered significant.
There were two cases of grade III hepatotoxicity (liver enzyme elevation along with clinical jaundice) in the EFV arm, 1 month after initiating ART (third month of ATT). ATT was changed to non-hepatotoxic drugs and ART was temporarily withheld. The patients were managed symptomatically with liver supportive supplements (Liv.52 tablets), and after hepatotoxicity had subsided, ART was reintroduced without further toxicity.
After 6 months, ATT was stopped (i.e., month 4 of ART) and only ART continued. At this point, there was no significant change in liver enzymes from before.
At month 12 of ART (6 months after stopping ATT), AST and SAP declined significantly as compared to their month 4 levels (34 vs. 39, p = 0.01, and 143 vs. 157, p = 0.001, respectively). The ALT levels were comparable (29 vs. 31, p = 0.44) (Table 2).
At 12 months, there was a significant reduction in the proportion of patients showing SAP >350 mg/dl. Repeated measure analysis for 140 patients did not show any significant change in AST or ALT levels over 12 months (data not shown).
3.4. Changes in liver enzyme levels by type of ART regimen
During the early stage of treatment, there was a significant change in AST level in the EFV arm when ATT was given along with ART, while in the NVP arm the change was noted both in AST and ALT later, by 6 months (Table 3). Subsequently, the levels showed a trend to decline. The proportion of patients with ALT or AST >100 mg/dl at 12 months was not different between the two arms (3% vs. 2%).
Table 3.
Pattern of change in serum liver enzymes during treatment, categorized by the type of NNRTI-based ART regimen
| Liver enzyme | Change in liver enzymes at different time-points in the study – regimen wise, mean (SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Levels at 0 week (mg/dl) | Change at 2 weeks | Change at 1 month | Change at 6 weeks | Change at 2 months | Change at 4 months | Change at 6 months | Change at 12 months | ||
| AST | EFV (n = 100) | 41 | 6.7 (32)a | 1.0 (29) | 3.0 (23) | −0.3 (27) | −5.2 (36) | −6.8 (35) | −0.3 (26) |
| NVP (n = 60) | 43 | −2.3 (26) | 10.9 (89) | 5.7 (37) | −0.6 (29) | 3.1 (25) | 7 (31)a | −0.6 (29) | |
| p-Value | - | 0.05 | 0.84 | 0.42 | 0.56 | 0.10 | 0.01 | 0.56 | |
| ALT | EFV (n = 100) | 27 | 9.5 (30) | 9.8 (28) | 9.0 (26) | 5.9 (28) | 2.6 (29) | 2.8 (31) | 1.8 (32) |
| NVP (n = 60) | 28 | 4.2 (19) | 17.9 (99) | 12.9 (39) | 4.4 (23) | 8.5 (30) | 14.3 (33)a | 13.6 (37)a | |
| p-Value | - | 0.12 | 0.26 | 0.85 | 0.56 | 0.24 | 0.01 | 0.04 | |
| SAP | EFV (n = 100) | 134 | 45 (107) | 56 (119) | 70 (123) | 55 (145) | 17 (148) | 31 (196) | −15 (118) |
| NVP (n = 60) | 126 | 8.5 (46) | 39 (116) | 48 (144) | 4.5 (130) | 43 (108) | 40 (104) | 3 (84) | |
| p-Value | - | 0.01 | 0.40 | 0.55 | 0.85 | 0.48 | 0.97 | 0.93 | |
NNRTI, non-nucleoside reverse transcriptase inhibitor; ART, antiretroviral therapy; SD, standard deviation; EFV, efavirenz; NVP, nevirapine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; SAP, serum alkaline phosphatase.
p <0.05, Mann–Whitney U-test, used for comparisons between regimens at the same time-point, i.e. AST at 2 weeks in the EFZ arm was significantly higher than in the NVP arm. Similarly, AST and ALT at 6 months in the NVP arm were significantly different from enzymes at 6 months in the EFZ arm.
Discussion
In this study, the initiation of ATT led to an expected but not significant increase in liver enzyme levels. Co-administration of ART with ATT was associated with elevated ALT during the early phase of treatment; however these elevations were mild, not associated with severe or rate-limiting toxicity, and decreased over time. Similar results were also observed among the Home-Based AIDS Care cohort of Uganda, where modestly elevated hepatic aminotransferases were common before starting ART and during co-administration of ART with TB treatment when measured at 3 months; however these elevations were not associated with severe toxicity and no patients were excluded from the study on the basis of severely elevated aminotransferases.12 Moreover, similar to the findings of our group, the prevalence of elevated hepatic aminotransferases in this cohort also declined at periodic examinations during 2 years of treatment with ART. The association of liver enzyme elevation and hepatic toxicity with ATT when co-administered with ART is not unexpected, since drug-induced hepatitis is a common complication of TB therapy and may be due to the initial induction of liver enzymes by rifampin. However, previous studies have also reported low rates of hepatotoxicity when ART is administered along with rifampin.8,13 Moses et al. reported 1.3% of grade II and <1% of grade III ALT elevation at 1 and 2 months after starting TB treatment (incidence rate 4.2/10 years of follow-up).13 In the Botswana cohort of 155 patients on ART, while more hepatotoxic events occurred in the group exposed to TB treatment than in those not exposed (9% vs. 3%, p = 0.05), there was no difference between patients treated with NVP and those treated with EFV.8 Failure to see a significant change in liver enzymes when ATT was co-administered with NVP-based ART is a positive finding of our study. In fact, the proportion of patients with grade II hepatotoxicity was no different between the EFV and NVP arms. This is an important finding for resource-limited countries where there is high prevalence of TB and HIV, laboratory facilities are limited, and NVP-based ART regimens are the most economical. The data from this study are reassuring in that both the baseline and ‘on treatment’ incidence of ALT elevations was very low and did not complicate patient management or safety. Our findings are the first from India and also the first using a once-daily NVP regimen with concomitant rifampin-based ATT. A Spanish study on the co-administration of rifampin and NVP in HIV–TB co-infected patients also showed that the development of liver toxicity or skin rash was no higher for the combination than when the drugs were given separately.14 However, as reported previously, the virological efficacy of once-daily NVP-based ART given along with ATT was significantly lower in our trial and therefore this combination is not a preferred option.15 The magnitude and rate of transaminitis that we observed in our cohort appears low compared with that of other published reports.16–18 However direct comparisons are difficult to make since definitions of elevated aminotransferases vary, ranging from >2.0 to >5.0 times ULN. The Uganda study, using the same level of aminotransferases as in our study (>2.5 times ULN) had a greater number of patients with elevated aminotransferases at baseline (38 vs. 7). However, by the end of 12 months the number of patients with abnormal aminotransferases was almost the same as in our study (grade II: 8 vs. 6).12 Earlier studies have suggested a greater risk of the development of hepatotoxicity among patients with low CD4 cell counts, high viral loads, and older age. 19–21 In our group, patients with advanced immunosuppression had modestly elevated aminotransferases at baseline. Although the number of patients in this group was small, we observed that the development of hepatotoxicity had a statistically significant association with immunosuppression of the patients as measured by CD4 cell counts. One reason could be that patients with low CD4 cell counts are more prone to acquire opportunistic infections, necessitating consumption of different drugs, leading to subclinical liver damage and thereby increasing susceptibility to hepatotoxicity. However since this was a sub-study to a drug efficacy trial, patients with elevated aminotransferases (>2.5 times ULN) were excluded from enrollment, hence the patient population was a selected one. Our findings should be interpreted in the context of a few limitations. Firstly patients had to fulfill several inclusion and exclusion criteria to be eligible for the trial. Hence baseline liver function was mostly in the normal range. Patients with severe pre-existing liver or renal disease were excluded. Also, we did not have a comparison group of patients with only HIV infection on ART, which would have helped us to determine the additive effect of ATT on liver enzymes when co-administered with ART. Further, the median CD4 cell count of the participants in the present analysis was well below the values that have been associated with an increased risk of NVP-associated hepatotoxicity in developed countries. Since NVP is a common cause of transaminitis that may resolve even with continuation of antiretrovirals, any further rise after the initial close monitoring may have gone unnoticed.22 Last, we did not look for an interaction with alcohol at the individual level and hepatitis B/C co-infection was rare. In previous cohorts from the same region, the prevalence of HIV–hepatitis B virus co-infection varied from 6% to 9%, and of HIV–hepatitis C virus co-infection from 2% to 3%.23,24 So these co-infections are rare in this population. In summary, HIV–TB patients treated with an intermittent anti- TB regimen and once-daily NNRTI-based ART regimen did not experience significant changes in liver function over 12 months. Minimal elevation of aminotransferases occurred, but was transient, improved after completion/discontinuation of ATT, and was not worse with NVP than EFV. Hepatotoxicity was rare in this group of patients with normal liver function at baseline and low rates of hepatitis B/C co-infection.
Acknowledgments
We thank the staff of the departments of clinical research (in Madurai, Vellore, and Chennai), biochemistry, and statistics, and the HIV section of the National Institute for Research in Tuberculosis for their support and cooperation. We wish to thank the ART medical officers at Madurai, Vellore, and Chennai ART clinics, the patients who participated in the study, and Ms D. Kalaivani for her continuous and excellent secretarial assistance. We also wish to thank the faculty of the Master’s Program in Clinical Research at the Sackler School of Graduate Biomedical Education, Tufts University, Boston, USA.
Footnotes
Conflict of interest: All authors – no conflicts.
Ethical approval: The study was approved by the ethics committee of the National Institute for Research in Tuberculosis (formerly the Tuberculosis Research Centre), and informed written consent was obtained from all patients prior to enrollment in the primary randomized controlled trial. Financial support: National AIDS Control Organization (NACO), India, National Institutes of Health (NIH) (Fogarty grant 2D43TW000237-17 to CP), NIH/National Institute of Allergy and Infectious Diseases (CFAR grant 1P30AI42353-12 to CP), and Center for Research Resources (grant UL1 RR025752).
References
- 1.World Health Organization. Global tuberculosis control 2010. Geneva: WHO; 2010. [accessed on May 9, 2011]. Available at: http://whqlibdoc.who.int/publications/2010/9789241564069_eng.pdf. [Google Scholar]
- 2.World Health Organization. Recommendation for a public health approach. Geneva: WHO; 2010. [accessed on September 9, 2010]. Antiretroviral therapy for HIV infection in adults and adolescents. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed] [Google Scholar]
- 3.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan FA, Minion J, Pai M, Royce S, Burman W, Harries AD, et al. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–1299. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Treatment of tuberculosis. 4. Geneva: WHO; 2010. [accessed September 9, 2010]. Available at: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf. [Google Scholar]
- 6.Manosuthi W, Sungkarnuparph S, Thakkinstian A, Vibhagool A, Kiertiburanakul S, Rattanasiri S, et al. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS. 2005;19:1481–1486. doi: 10.1097/01.aids.0000183630.27665.30. [DOI] [PubMed] [Google Scholar]
- 7.Friedland, Khoos S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–1302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 8.Shipton LK, Wester CW, Stock S, Ndwapi N, Gaolathe T, Thior I, et al. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB–HIV coinfection in Botswana. Int J Tuberc Lung Dis. 2009;13:360–366. [PMC free article] [PubMed] [Google Scholar]
- 9.Parthasarathy R, Sarma GR, Janardhanam B, Ramachandran P, Shantha T, Sivasubramanian S, et al. Hepatic toxicity in South Indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle. 1986;67:99–108. doi: 10.1016/0041-3879(86)90003-6. Article PDF (893KB. [DOI] [PubMed] [Google Scholar]
- 10.Padmapriyadarsini C, Ramesh Kumar S, Terrin N, Narendran G, Menon PA, Ramachandran G, et al. Dyslipidemia among HIV infected patients with tuberculosis taking once-daily nonnucleoside reverse transcriptase inhibitor based antiretroviral therapy in India. Clin Infect Dis. 2011;52:540–546. doi: 10.1093/cid/ciq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DAIDS Table for grading the severity of adult and pediatric adverse events. Bethesda, MD: Division of AIDS, National Institute of Allergy and Infectious Diseases; 2004. [accessed on 23rd July 2013]. Available at: http://www.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/Documents/daidsaegradingtable.pdf. [Google Scholar]
- 12.Weidle PJ, Moore D, Mermin J, Buchacz K, Were W, Downing R, et al. Liver enzymes improve over twenty-four months of first-line non-nucleoside reverse transcriptase inhibitor-based therapy in rural Uganda. AIDS Patient Care STDS. 2008;22:787–79. doi: 10.1089/apc.2008.0020. [DOI] [PubMed] [Google Scholar]
- 13.Moses M, Zachariah R, Tayler-Smith K, Misinde D, Foncha C, Manzi M, et al. Outcomes and safety of concomitant nevirapine and rifampicin treatment under programme conditions in Malawi. Int J Tuberc Lung Dis. 2010;14:197–202. [PubMed] [Google Scholar]
- 14.Olivia J, Moreno S, Sanz J, Ribera E, Molina JA, Rubio R, et al. Co-administration of rifampin and nevirapine in HIV-infected patients with tuberculosis. J AIDS. 2003;17:637–638. doi: 10.1097/00002030-200303070-00024. [DOI] [PubMed] [Google Scholar]
- 15.Swaminathan S, Padmapriyadarsini C, Venkatean P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Efficacy and safety of once-daily nevirapine or efavirenz based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis. 2011;53:716–724. doi: 10.1093/cid/cir447. [DOI] [PubMed] [Google Scholar]
- 16.Manfredi R, Calza L, Chicod F. Prospective, open-label comparative study of liver toxicity in an unselected population of HIV-infected patients treated for the first time with efavirenz or nevirapine HIV. Clin Trials. 2005;6:302–311. doi: 10.1310/EWWC-YLJ6-8LHE-054A. [DOI] [PubMed] [Google Scholar]
- 17.Kim AA, Wanjiku L, Macharia DK, Wangai M, Isavwa A, Abdi H, et al. Adverse events in HIV-infected persons receiving antiretroviral drug regimens in a large urban slum in Nairobi, Kenya, 2003–2005. J Int Assoc Physician AIDS Care (Chic) 2007;6:206–209. doi: 10.1177/1545109707304494. [DOI] [PubMed] [Google Scholar]
- 18.Patel, Patel K, Patel J, Shah N, Patel B, Rani S. Safety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral naïve patients in India who are coinfected with tuberculosis and HIV-1. J Acquir Immune Defic Syndr. 2004;37:1166–1169. doi: 10.1097/01.qai.0000135956.96166.f0. [DOI] [PubMed] [Google Scholar]
- 19.Manosuthi W, Mankatitham W, Lueangniyomkul A, Chimsuntorn S. Sungkanuparph Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 2008;9:294–299. doi: 10.1111/j.1468-1293.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- 20.Pedral-Sampaio DB, Alves CR, Netto EM, Brites C, Oliveira AS, Badaro R. Efficacy and safety of efavirenz in HIV patients on rifampin for tuberculosis. Braz J Infect Dis. 2004;8:211–216. doi: 10.1590/s1413-86702004000300004. [DOI] [PubMed] [Google Scholar]
- 21.Towner WJ, Xu L, Leyden WA, Horberg MA, Chao CR, Tang B, et al. The effect of HIV infection, immunodeficiency and antiretroviral therapy on the risk of hepatic dysfunction. J Acquir Immune Defic Syndr. 2012;60:321–327. doi: 10.1097/QAI.0b013e31824e9ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):s21–s33. doi: 10.1097/00126334-200309011-00005. [DOI] [PubMed] [Google Scholar]
- 23.Padmapriyadarsini C, Chandrabose J, Victor L, Hanna LE, Arunkumar N, Swaminathan S. Hepatitis B or hepatitis C co-infection in individuals infected with human immunodeficiency virus and effect of anti-tuberculosis drugs on liver function. J Postgrad Med. 2006;52:92–96. [PubMed] [Google Scholar]
- 24.Saravanan S, Velu V, Kumarasamy N, Nandakumar S, Murugavel KG, Balakrishnan P, et al. Coinfection of hepatitis B and hepatitis C virus in HIV-infected patients in south India World. J Gastroenterol. 2007;13:5015–5020. doi: 10.3748/wjg.v13.i37.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]