Abstract
Background
The role of T-cell responses against Mycobacterium tuberculosis antigens in tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) is unclear.
Methods
Peripheral blood mononuclear cells from 45 HIV patients with treated TB, of whom 12 developed TB-IRIS, were collected at weeks 0, 2, and 6 of antiretroviral therapy (ART). Production of interferon-γ (IFN-[γ]) and interleukin-2 by T cells after stimulation with purified protein derivative (PPD) or early secretory antigenic target-6 (ESAT-6) and T-cell expressions of CCR5 and CXCR3 were assessed by flow cytometry. IFN-γ and CXCL10 were assayed by enzyme-linked immunosorbent assay.
Results
TB-IRIS patients had higher proportions of PPD- and ESAT-6–reactive IFN-γ+CD4+ and CD3+CD4- T cells at weeks 0, 2, and 6. IFN-γ levels were also higher in peripheral blood mononuclear cell culture supernatants at all times with PPD but only at weeks 2 and 6 with ESAT-6. There were few differences for interleukin-2. CXCL10 levels in supernatants after PPD and ESAT-6 stimulation were only higher at week 6. CXCR3+/CCR5+CD4+ T cells were higher at week 2, and CCR5+CD4+ T cells were higher at week 6.
Conclusions
TB-IRIS is associated with Th1 responses against M. tuberculosis antigens by CD4+ and CD3+CD4- T cells that are present before ART and amplified afterward. It is unclear if these cause immunopathology or reflect a high pathogen load.
Keywords: HIV/AIDS, IRIS, tuberculosis, TB-IRIS
Introduction
Over 33 million people are living with HIV infection worldwide, and India is home to about 2.4 million of these people.1 There were 8.7 million new tuberculosis (TB) cases reported worldwide in 2011 and that includes about 1.1 million cases among people with HIV infection. India and China alone account for about 38% of these TB cases.2 TB is reported to be the most common opportunistic infection among persons with HIV in India and is associated with 3.5 times faster progression to death as compared with HIV-infected persons without TB.3 Although the advent of combination antiretroviral therapy (ART) has led to a substantial decline in the morbidity and mortality associated with TB in HIV-infected patients,4,5 between 8% and 43% of HIV patients with treated TB who initiate ART experience TB-associated immune reconstitution inflammatory syndrome (TB-IRIS).6
TB-IRIS is a form of immune restoration disease that presents at a median time of 10–14 days after ART is commenced, especially in patients with severe CD4+ T-cell deficiency.7–9 Although it is generally acknowledged that TB-IRIS is a consequence of restoring an immune response against antigens of live or dead mycobacteria, it is unclear why this causes immunopathology in only some patients and which components of that immunopathology are responsible for the inflammatory disease.
A substantial amount evidence indicates that a high pathogen load is a major determinant of an immune response against Mycobacterium tuberculosis that causes immunopathology in patients with TB-IRIS. Indirect evidence is provided by observations that disseminated TB,10 a shorter period of TB therapy before ART is commenced,7–9 and drug-resistant M. tuberculosis,11 all increase the risk of TB-IRIS. Direct evidence is provided by a study of HIV patients with tuberculous meningitis who developed meningitis as a manifestation of TB-IRIS, in whom positive cerebrospinal fluid cultures for M. tuberculosis were more common than in patients who did not develop TB-IRIS.12 Similarly, Conesa-Botella et al 13 demonstrated that urine levels of mycobacterial lipoaribomannan before ART were higher in patients who developed TB-IRIS compared with patients who did not.
Whereas the role of pathogen load in the pathogenesis of TB-IRIS is becoming clearer, the role played by the immune system is not. Bourgarit et al 14,15 demonstrated that patients with TB-IRIS exhibit higher proportions of circulating tuberculin-activated interferon-γ (IFN-[γ])+ T cells than controls using IFN-γ ELISpot assays and argued that Th1 responses were central to the pathogenesis of TB-IRIS. Similar findings were subsequently reported from studies on small numbers of patients using IFN-γ ELISpot assays 16 or polychromatic intracellular flow cytometry.17 A role for Th1 cells has been clearly shown in a mouse model of immune restoration disease associated with Mycobacterium avium infection, but it was also apparent that activation of myeloid cells contributed to the immunopathology.18 Meintjes et al 19 not only confirmed an association of TB-IRIS with increased proportions of IFN-[γ]+ T cells reacting with several M. tuberculosis antigens using IFN-γ ELISpot assays but also observed that patients who did not develop TB-IRIS exhibited increased proportions of such cells and questioned whether they caused the immunopathology. In support of this view, 2 studies conducted in large numbers of HIV patients with treated TB from South East Asia using whole-blood IFN-γ release assays (IGRAs) demonstrated that tuberculin-activated IFN-γ production increased after commencing ART in all patients and, although responses were higher in TB-IRIS patients, the difference between patient groups was not statistically significant at the time of the TB-IRIS.20,21
To clarify the role of Th1 responses in the pathogenesis of TB-IRIS, we examined peripheral blood mononuclear cells (PBMCs) from HIV patients with treated TB before and during the first 6 weeks of ART for production of Th1 cytokines [IFN-γ and interleukin-2 (IL-2)] within, and the expression of Th1-associated chemokine receptors (CCR5 and CXCR3) upon, T cells and also assessed production of Th1 cytokines (IFN-[γ]) and chemokines (CXCL10) in cultures. We report that patients who develop TB-IRIS have higher IFN-γ responses to M. tuberculosis antigens before ART, which increase after ART, compared with patients who do not develop TB-IRIS. Although Th1 responses to M. tuberculosis antigens may drive inflammation in TB-IRIS, it is also possible that they are a marker of a higher pathogen load.
Methods
Patient Enrolment and Study Design
We performed a prospective cohort study at the YR Gaitonde Centre for AIDS Research and Education (YRG CARE) Medical Center, a tertiary referral HIV care center in Chennai, southern India, between January 2007 and July 2010. One hundred seventeen consecutive untreated HIV/TB coinfected patients were prospectively included and followed longitudinally for up to 1 year or until they developed TB-IRIS. Inclusion criteria were as follows: more than 18 years of age, HIV-1 infection, no previous ART, recent diagnosis of TB, and indications for ART. Diagnosis of TB was based on clinical, radiological, and/or bacteriological findings. The study patients were treated for TB following the guidelines of the National AIDS Control Organization, India, 2007, with the standard regimen of 4 drugs (including Isoniazid, Rifampin, Pyrazinamide, and Ethambutol) in the intensive phase and 2 drugs (Isoniazid and Rifampin) in the continuation phase for a minimum period of 6 months.22 Of the study population, 12 patients (10%) developed TB-IRIS and were compared with 33 patients who did not develop TB-IRIS for the immunology studies. Patients were defined as having paradoxical TB-IRIS if they fulfilled the criteria of the International Network for the Study of HIV-associated IRIS.6 The study was approved by the institutional review board, and all patients provided written informed consent.
Intracellular Cytokine Staining for Measuring CD4+ and CD3+CD4- T-Cell Responses Against Mycobacterial Antigens
Cryopreserved PBMCs were thawed in RPMI 1640 (Sigma–Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum, and the cells were rested overnight at 37°C with 5% CO2. The PBMCs were stimulated with the mycobacterial antigens PPD or ESAT-6 (Statens Serum Institut, Copenhagen, Denmark). RPMI medium alone served as a negative control, and SEB (Sigma–Aldrich) was used as a positive control. After culture for 2 hours, the cells were treated with Golgi Plug (BD Biosciences, San Jose, CA) and further incubated for an additional 4 hours. Cells were surface stained with anti-CD4 ECD (Beckman Coulter Inc, Fullerton, CA). After washing with 2% fetal bovine serum in phosphate-buffered saline (PBS), the cells were permeabilized using FACS PERM (BD Biosciences) and then intracellularly stained with anti-IFN-γ FITC (BD Biosciences), anti-IL-2 PE (BD Biosciences), and anti-CD3 PerCP Cy5.5 (BD Biosciences). Cells were washed, and the cell pellet was resuspended in 1% paraformaldehyde in PBS and acquired in the Beckman Coulter Epics XL/MCL Flow Cytometer (Beckman Coulter Inc, Miami, FL). Data were analyzed using FlowJo version 7.6.3.
Measurement of IFN-γ and CXCL10 Levels in PBMC Culture Supernatants
PBMCs were stimulated with PPD or ESAT-6 by culturing at 37°C with 5% CO2, and after 24 hours, the supernatant was harvested and the cytokines were measured using human BD OptEIA enzyme-linked immunosorbent assay kits (BD Biosciences Pharmingen, San Diego, CA). Briefly, the wells were coated with capture antibody, diluted in coating buffer, and incubated overnight at 4°C. Samples were pipetted into appropriate wells and incubated for 2 hours at room temperature. Detection antibody with avidin–horseradish peroxidase was added to plates for color development, and the plates were incubated for 30 minutes. A stop solution was then added, and absorbance was read on a plate reader (Dynex MRXIIRevelation Enzyme-Linked Immunosorbent Assay Reader; Dynex Technologies Inc, Chantilly, VA) at 450 nm.
Assessment of CCR5 and CXCR3 Expressions on CD4+ and CD3+CD4- T Cells
Thawed PBMCs were rested overnight at 37°C with 5% CO2 and surface stained with the antibody cocktail of anti-CCR5 FITC (BD Biosciences), anti-CXCR3 PE (BD Biosciences), anti-CD4 ECD (Beckman Coulter Inc), and anti-CD3 PerCp Cy5.5. After incubation in the dark at room temperature (25 ± 5°C) for 20 minutes, the cells were washed and the cell pellet was resuspended in 1% paraformaldehyde in PBS. The stained cells were acquired in the Beckman Coulter Epics XL/MCL Flow Cytometer (Beckman Coulter Inc). Data were analyzed using FlowJo version 7.6.3.
Statistical Analysis
Baseline and follow-up comparisons between patients who did and who did not develop TB-IRIS were made using Mann–Whitney U test and the Wilcoxon rank sum test/Kruskal–Wallis for continuous variables within the groups. All statistical analyses were performed using the software GraphPad Prism, version 5.0. Statistical significance was defined at a confidence level of 95% (P < 0.05).
Results
Specimens from the 45 study participants who were followed up at baseline, 2 weeks, and 6 weeks after initiation of ART were included for the immunological assays. Of these, 33 (73.33%) belonged to the non-TB-IRIS group of patients and 12 (26.77%) to the TB-IRIS group. PBMCs were available for only 7 of the 12 TB-IRIS patients at week 6.The baseline characteristics of the study population are represented in Table 1.
Table 1. Baseline Demographic Characteristics Of the Study Population.
| Characteristics | TB-IRIS Group (n = 12) | Non-TB-IRIS Group (n = 33) |
|---|---|---|
| Male sex—no of patients (%) | 12 (100) | 28 (84.8) |
| Age—median (IQR) | 34 (30, 39) | 34 (31, 39.5) |
| CD4+ T-cell count—cells per microliter median (IQR) | 68 (54, 139) | 120 (69, 232) |
IQR, interquartile range.
TB-IRIS Patients Had Higher Proportions of IFN-[γ]+ PPD- and ESAT-6–Reactive T Cells Before and After ART
In both the TB-IRIS and non-TB-IRIS groups, the proportions of IFN-[γ]+CD4+ T cells reactive with PPD increased compared with baseline over the 6 weeks of the study (P = 0.0008 among TB-IRIS group, P < 0.0001 among non-TB-IRIS group) (Fig. 1). There was also an increase in the proportion of IFN-[γ]+CD3+CD4- T cells reactive with PPD among the TB-IRIS group (P = 0.0049) but not the non-TB-IRIS group over the 6 weeks of the study. At weeks 0, 2, and 6, the proportions of IFN-[γ]+CD4+ T cells reactive with PPD were higher in patients who developed TB-IRIS compared with patients who did not (P = 0.0207, P < 0.0001, and P < 0.0003, respectively) (Fig. 1A). There were also increased proportions of PPD-reactive IFN-[γ]+CD3+CD4- T cells in TB-IRIS patients when compared with non-TB-IRIS patients at baseline, week 2, and week 6 visits (P = 0.0045, P = 0.001, and P < 0.0001, respectively) (Fig. 1B).
Figure 1.
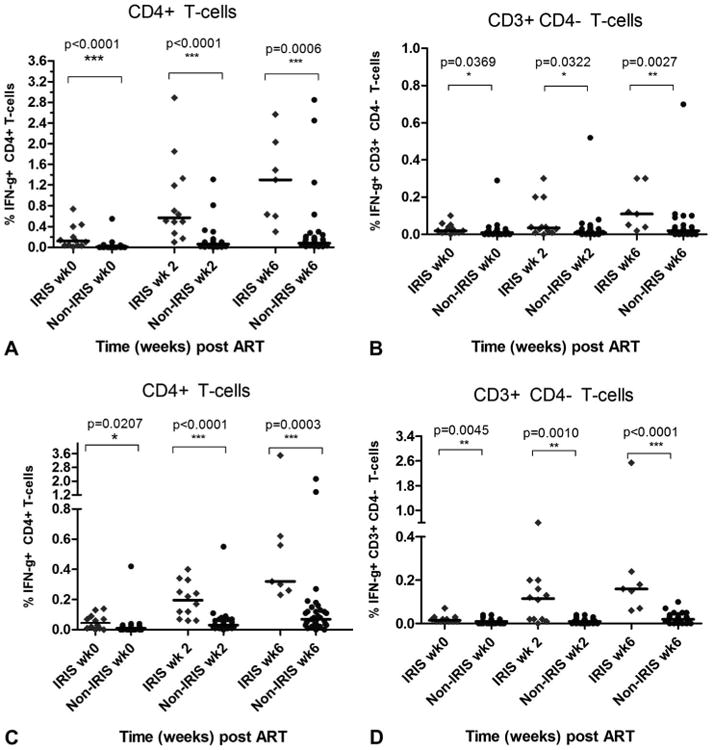
Antigen-reactive IFN-γ+ T cells in TB-IRIS and non-TB-IRIS patients. TB-IRIS patients had higher proportions of IFN-γ+ PPD-reactive CD4+ T cells (A) and CD3+CD4− T cells (B), and ESAT-6–reactive CD4+ T cells (C) and CD3+CD4− T cells (D) than non-TB-IRIS patients before and after ART.
When stimulated with ESAT-6 antigen, the proportions of IFN-[γ]+CD4+ T cells in TB-IRIS patients were higher than in non-TB-IRIS patients at weeks 0, 2, and 6 (P < 0.0001, 0.0001, and 0.0006, respectively) (Fig. 1C). At weeks 0, 2, and 6, proportions of IFN-[γ]+CD3+CD4- T cells were higher in TB-IRIS patients compared with non-TB-IRIS patients (P = 0.0369, 0.0322, and 0.0027, respectively) (Fig. 1D).
TB-IRIS Patients Do Not Demonstrate Major Differences in IL-2+ PPD-Reactive T Cells Before and After ART
The proportions of IL-2+CD4+ and CD3+CD4- T cells after incubation of PBMCs with PPD did not significantly increase during the study visits among both the TB-IRIS and non-TB-IRIS groups. However, the proportion of IL-2+CD4+ T cells was significantly higher in the TB-IRIS group at baseline (P = 0.0043), and the proportion of IL-2+CD3+CD4- T cells was higher at week 6 (P = 0.0046) (data not shown).
TB-IRIS Patients Exhibit Higher Levels of IFN- γ in Supernatants of PBMCs Cultured With PPD or ESAT-6 Before and After ART
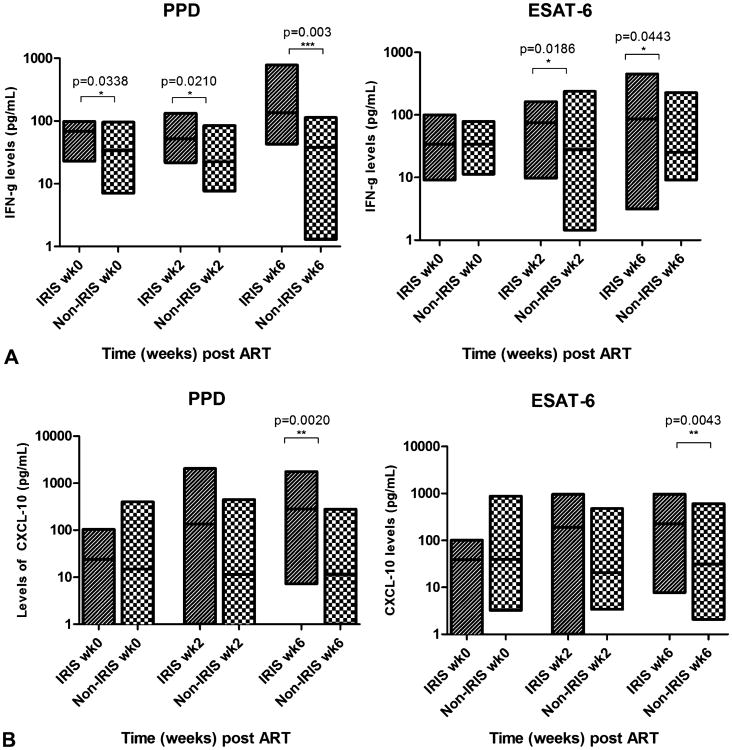
Levels of IFN-γ in supernatants from PBMCs cultured with PPD were significantly elevated within the TB-IRIS group at all study visits compared with baseline (P = 0.006), but there were no significant differences in the levels observed within the non-TB-IRIS group (Fig. 2A). Comparison of IFN γ levels in PBMC culture supernatants between the TB-IRIS and non-TB-IRIS groups at baseline, week 2, and week 6 also demonstrated higher levels in the TB-IRIS group (P = 0.0338, 0.0210, and 0.003, respectively) (Fig. 2A).
Figure 2.
Levels of IFN-γ or CXCL10 in supernatants of PBMCs cultured with PPD or ESAT-6 from TB-IRIS or non-TB-IRIS patients. TB-IRIS patients exhibited higher levels of IFN-γ before and after ART when PBMCs were cultured with PPD but only after ART when cultured with ESAT-6 (A). TB-IRIS patients exhibited higher levels of CXCL10 at only the 6-week time point after ART when PBMCs were cultured with both PPD and ESAT-6 (B).
When PBMCs were cultured with ESAT-6, the levels of IFN-γ in culture supernatants did not change over the 6 weeks within either the TB-IRIS or the non-TB-IRIS groups (Fig. 2A). When levels of IFN-γ in culture supernatants were compared between the TB-IRIS and non-TB-IRIS groups, the levels were not significantly different at baseline but were higher at the week 2 and week 6 visits (P = 0.0186 and 0.0443, respectively) (Fig. 2A).
TB-IRIS Patients Exhibit Higher Levels of CXCL10 in Supernatants of PBMC Cultures Stimulated With PPD or ESAT-6 After 6 Weeks of ART
Levels of CXCL10 in the culture supernatants from PBMCs cultured with PPD increased over the 6 weeks within the TB-IRIS group (P = 0.0363), but not within the non-TB-IRIS group (Fig. 2B). In addition, levels of CXCL10 in the culture supernatants were higher in the TB-IRIS group compared with the non-TB-IRIS group at week 6 (P = 0.0020), but not at baseline or week 2 (Fig. 2B).
Upon culture of PBMC with ESAT-6, levels of CXCL10 in culture supernatants increased over the 3 study visits in the TB-IRIS group (P = 0.0215), but not in the non-TB-IRIS group. In addition, CXCL10 levels were higher in the TB-IRIS group compared with the non-TB-IRIS group at the week 6 visit (P = 0.0043) but did not differ at baseline or week 2 (Fig. 2B).
CCR5+ and CCR5+/CXCR3+CD4+ T Cells May Be Higher in Patients With TB-IRIS
Proportions of CCR5+CD4+ T cells within both the TB-IRIS and non-TB-IRIS groups increased over the study period from baseline to week 6, but the changes were not statistically significant (Fig. 3A). However, the proportions of CCR5+CD4+ T cells were significantly higher in the TB-IRIS than in the non-TB-IRIS group at week 6 (P = 0.0088). In contrast, proportions of CCR5+CD3+CD4- T cells did not change in either group (Fig. 3A). The proportion of CD4+ T cells co-expressing CCR5+ and CXCR3+ was higher in the TB-IRIS group than in the non-TB-IRIS group at week 2 (P = 0.0035), but not at other time points (Fig. 3B). There were no differences in the proportions of CD3+CD4- T cells co-expressing CCR5+ and CXCR3+ between the TB-IRIS and non-TB-IRIS groups during the study period (Fig. 3B).
Figure 3.
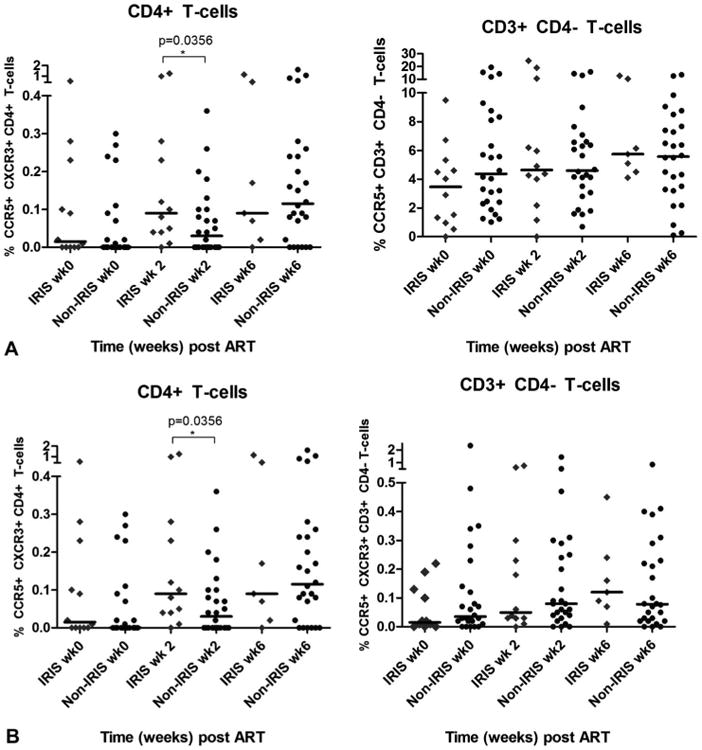
CCR5+ and CCR5+/ CXCR3+ T cells in TB-IRIS and non-TB-IRIS patients before and after ART. TB-IRIS patients had higher proportions of CCR5+CD4+ T cells, but not CD3+CD4− T cells, than non-TB-IRIS patients after ART (A). The difference between the groups is significant at week 6. TB-IRIS patients had higher proportions of CCR5+/CXCR3+CD4+ T cells, but not CD3+CD4− T cells, 2 weeks after commencing ART (B).
Discussion
Using intracellular flow cytometry to detect IFN-[γ]+ antigen-specific T cells, we demonstrated higher proportions of CD4+ and CD3+CD4- T cells reactive with both PPD and ESAT-6 in HIV-infected patients with treated TB who develop TB-IRIS during the first 6 weeks of ART. Notably, these differences were present before ART was commenced and were amplified after ART. In addition, the response among CD4+ T cells was greater than that among CD3+CD4- T cells. The findings in flow cytometry investigations were confirmed in the analyses of supernatants from antigen-stimulated PBMC cultures where IFN-γ+ production after stimulation with PPD, and to a lesser extent with ESAT-6, was higher in patients with TB-IRIS. Furthermore, production of CXCL10, an interferon-inducible chemokine, was also higher in patients with TB-IRIS, though only after 6 weeks of ART. In contrast, PPD-specific cell populations secreting IL-2 were much less frequent than the IFN-γ secreting T-cell populations. Whereas there have been reports of increased IL-2 production by HIV-specific T cells of patients commencing ART when compared with ART-naive patients,23,24 other studies have shown that TB-specific IL-2 responses are delayed and minimal confirming our findings.25
Several previous studies compared T-cell IFN-γ responses to M. tuberculosis antigens in HIV-infected patients with treated TB who did or did not develop TB-IRIS after commencing ART. Bourgarit et al 14,15 reported that patients with TB-IRIS have higher proportions of IFN-γ+ T cells using ELISpot assays. With the same methodology, Meintjes et al 19 demonstrated higher proportions of M. tuberculosis–reactive T cells in patients with TB-IRIS in a cross-sectional analysis but could not show an association in a longitudinal analysis. The same group also reported that TB-IRIS patients had increased levels of IFN-γ in supernatants from cultures of PBMCs with heat-killed M. tuberculosis, along with increased production of other pro-inflammatory cytokines.26 In contrast, using whole-blood IGRAs, neither Elliott et al nor Tieu et al 20,21 demonstrated significant differences in IFN-γ responses between TB-IRIS patients and controls, though the former study found that TB-IRIS patients had significantly higher responses to PPD at week 24, which was many weeks after the median time of TB-IRIS presentation at 10 days. However, both these studies demonstrated that IFN-γ responses to PPD increased after ART in both TB-IRIS patients and controls, as demonstrated in the study reported here. Assaying CXCL10 in plasma from whole-blood IGRAs demonstrates larger T-cell responses to RD1 antigens before and 4 weeks after ART in patients who develop TB-IRIS, but the difference is small and not as clear as in patients who develop TB after commencing ART.27 Taken together, the findings of this and previous studies suggest that IFN-γ responses to PPD and, to a lesser extent RD1 antigens, increase in all HIV patients with treated TB after ART is commenced but that the increase is larger in patients with TB-IRIS and is detectable by ELISpot assays, 14–16,19 intracellular flow cytometry (this study), or assay of IFN-γ in supernatants from cultures of antigen-stimulated PBMCs (this study), but not IGRAs.20,21 In support of this view are the findings of Mahnke et al,17 who used intracellular flow cytometry to demonstrate an increase in polyfunctional T cells responding to pathogen-specific antigens in HIV-infected patients with IRIS associated with a variety of pathogens.
CXCL10 is a member of the α-chemokine subfamily that directs the migration and stimulates the adhesion of activated T cells and natural killer cells by binding to CXCR3, a G protein–coupled receptor. Expression of CXCL10 is seen in many Th1-type inflammatory diseases, where it is thought to play an important role in recruiting activated T cells into sites with tissue inflammation. Recent data support a role for CXCL10 in M tuberculosis infection, and elevated levels of CXCL10 have been found in plasma and at infection foci in individuals infected with M tuberculosis.28,29 Furthermore, high levels of CXCL10 have been found in the delayed-type hypersensitivity reaction to PPD in lymph nodes and lung tuberculous granulomas.30 CXCL10 has recently been found to be as sensitive as IFN-γ in detecting individuals infected with M tuberculosis.29 In the present study, it was observed that, after ART initiation, PPD-specific CXCL10 secretion was elevated only in the TB-IRIS group of patients. This is consistent with the increased levels of IFN-γ observed in the same patient group. CXCL10 secretion for PPD and ESAT-6 did not increase over the 6 weeks within the TB-IRIS group but was elevated in the TB-IRIS group at week 6 after ART initiation (Fig. 2). This presumably reflects the fact that CXCL10 secretion follows IFN-γ production.28
Our findings provide the evidence that HIV-infected patients who develop TB-IRIS have higher Th1 responses to M. tuberculosis antigens before they commence ART and that these responses are amplified after ART is commenced. There are several possible explanations for this. First, the higher mycobacterial load that has been demonstrated in patients who develop TB-IRIS 13 might lead to a larger number of Th1 cells. Second, there might be an inherent or acquired susceptibility to produce higher Th1 responses. An acquired susceptibility might be related to perturbations of innate immune responses that have been associated with TB-IRIS,31 including increased production of IL-18, which is a potent stimulator of Th1 responses,32 and CXCL10, which is a chemoattractant for Th1 cells.28 The findings reported here and by others 14,15,19,26 that IFN-[γ]+ T cells responding to M. tuberculosis antigens are increased in TB-IRIS patients are a likely explanation for the observations that serum levels of IFN-γ are increased in patients with TB-IRIS.26,33 However, this has not been demonstrated in all studies of patients with TB-IRIS.34
In contrast to earlier reports,14 in the present study, ESAT-6 was observed to be a significant antigenic target in patients with TB-IRIS, which is in concordance with the results of a study from South Africa.19 These differences in observation might relate to differences in ethnicity, disease status, or length of anti-TB therapy because the response to ESAT-6 has been observed to decline during successful treatment.35
The immunophenotype of T cells can in part be defined by patterns of cell surface chemokine receptor expression. Th1 lymphocytes express CCR5+ and CXCR3+, whereas Th2 lymphocytes express CCR3+, CCR4+, and CCR8+.36 To further investigate the role of Th1 responses in TB-IRIS, we assessed the CCR5 and CXCR3 expressions on blood T cells before and after ART and demonstrated that TB-IRIS patients had higher proportions of CCR5+CD4+ T cells at week 6. There was also an increase in the proportion of CD4+ T cells co-expressing CCR5+ and CXCR3+ in the TB-IRIS group compared with the non-TB-IRIS group at the week 2 visit after ART initiation, but this did not persist. It has been previously shown that M. tuberculosis infection of monocyte-derived macrophages increases CCR5+expression on T cells when compared with the CXCR3+ expression and that CCR5+CD4+ T cells accumulate in the lung at higher rates during the course of active TB.37 Given the association of CCR5+ T cells with M. tuberculosis disease, and our finding of increased CCR5+CD4+ T cells in TB-IRIS, we suggest that CCR5 inhibitor therapy should be considered as a therapeutic strategy to prevent TB-IRIS in HIV-infected patients with treated TB who commence ART.
In summary, TB-IRIS patients exhibited higher proportions of CD4+ and CD3+CD4- T cells producing IFN-γ and increased production of IFN-γ and CXCL10 in culture after stimulation of PBMC with M. tuberculosis antigens. The proportion of CCR5+CD4+ T cells was also higher in the patients with TB-IRIS. Higher IFN-γ responses to M. tuberculosis antigens before ART in patients who subsequently developed TB-IRIS might indicate an increased mycobacterial load or an inherent or acquired susceptibility to higher Th1 responses. Whatever the cause, CCR5 inhibitor therapy might be used to modulate the migration of CCR5+CD4+ T cells in HIV-infected patients with treated TB when given ART.
Acknowledgments
The authors are grateful to the laboratory and clinical research staff at YRG CARE Medical Center, Chennai, India, for their facilitation of study and all the study participants. The authors are grateful to the Research and Education in HIV/AIDS for Resource-Poor Countries (REACH) Initiative for supporting the standardization and setting up of laboratory assays.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Presented at the 23rd Annual Australasian Society for HIV Medicine Conference, September 26–28, 2011, Canberra, Australia, Paper 323; and 19th Conference on Retroviruses and Opportunistic Infections, March 5–8, 2012, Seattle, WA, Poster 940.
References
- 1.National AIDS Control Organization (NACO) New Delhi, India: 2010. [Accessed March 4, 2013]. HIV declining in India; new infections reduced by 50% from 2000-2009; sustained focus on prevention required. Available at: http://www.nacoonline.org/upload/HomePage/NACO%20Press%20Release%20on%20HIV%20Estimates.pdf. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report. 2012 Available at: http://www.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
- 3.Kumarasamy N, Solomon S, Flanigan TP, et al. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 4.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumarasamy N, Solomon S, Chaguturu SK, et al. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525–1528. doi: 10.1086/497267. [DOI] [PubMed] [Google Scholar]
- 6.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naidoo K, Yende-Zuma N, Padayatchi N, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burman W, Weis S, Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11:1282–1289. [PubMed] [Google Scholar]
- 11.Meintjes G, Rangaka MX, Maartens G, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48:667–676. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marais S, Meintjes G, Pepper DJ, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2013;56:450–460. doi: 10.1093/cid/cis899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conesa-Botella A, Loembé MM, Manabe YC, et al. Urinary lipoarabinomannan as predictor for the tuberculosis immune reconstitution inflammatory syndrome. J Acquir Immune Defic Syndr. 2011;58:463–468. doi: 10.1097/QAI.0b013e31823801de. [DOI] [PubMed] [Google Scholar]
- 14.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 15.Bourgarit A, Carcelain G, Samri A, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gamma delta T cells. J Immunol. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 16.Tan DBA, Yong YK, Tan HY, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9:307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 17.Mahnke YD, Greenwald JH, DerSimonian R, et al. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber DL, Mayer-Barber KD, Antonelli LRV, et al. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116:3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 21.Tieu HV, Ananworanich J, Avihingsanon A, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25:1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National AIDS Control Organization. New Delhi, India: 2007. [Accessed February 6, 2013]. Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. Available at: http://www.nacoonline.org/upload/Policies%20&%20Guidelines/1.%20Antiretroviral%20Therapy%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents%20Including%20Post-exposure.pdf. [Google Scholar]
- 23.Kapogiannis BG, Henderson SL, Nigam P, et al. Defective IL-2 production by HIV-1-specific CD4 and CD8 T cells in an adolescent/young adult cohort. AIDS Res Hum Retroviruses. 2006;22:272–282. doi: 10.1089/aid.2006.22.272. [DOI] [PubMed] [Google Scholar]
- 24.Kannanganat S, Kapogiannis BG, Ibegbu C, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007;81:12071–12076. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122:597–602. doi: 10.1378/chest.122.2.597. [DOI] [PubMed] [Google Scholar]
- 26.Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver BG, Elliott JH, Price P, et al. Tuberculosis after commencing antiretroviral therapy for HIV infection is associated with elevated CXCL9 and CXCL10 responses to Mycobacterium tuberculosis antigens. Acquir Immune Defic Syndr. 2012;61:287–292. doi: 10.1097/QAI.0b013e31826445ef. [DOI] [PubMed] [Google Scholar]
- 28.Azzurri A, Sow OY, Amedei A, et al. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Ruhwald M, Bodmer T, Maier C, et al. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J. 2008;32:1607–1615. doi: 10.1183/09031936.00055508. Find It Bibliographic Links [Context Link] [DOI] [PubMed] [Google Scholar]
- 30.Ferrero E, Biswas P, Vettoretto K, et al. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leukocyte subpopulations: focus on gamma delta cells. Immunology. 2003;108:365–374. doi: 10.1046/j.1365-2567.2003.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver BG, Elliott JH, Price P, et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis. 2010;202:1728–1737. doi: 10.1086/657082. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell JR, Yadav R, Rossi RJ, et al. IL-18 bridges innate and adaptive immunity through IFN-gamma and the CD134 pathway. J Immunol. 2006;177:234–245. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 33.Worsley CM, Suchard MS, Stevens WS, et al. Multi-analyte profiling of ten cytokines in South African HIV-infected patients with immune reconstitution inflammatory syndrome (IRIS) AIDS Res Ther. 2010;7:36. doi: 10.1186/1742-6405-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddow LJ, Dibben O, Moosa MYS, et al. Circulating inflammatory biomarkers can predict and characterize tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2011;25:1163–1174. doi: 10.1097/QAD.0b013e3283477d67. [DOI] [PubMed] [Google Scholar]
- 35.Nicol MP, Pienaar D, Wood K, et al. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis. 2005;40:1301–1308. doi: 10.1086/429245. [DOI] [PubMed] [Google Scholar]
- 36.Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol. 1999;21:263–285. doi: 10.1007/BF00812257. [DOI] [PubMed] [Google Scholar]
- 37.Santucci MB, Bocchino M, Garg SK, et al. Expansion of CCR5+ CD4+ T-lymphocytes in the course of active pulmonary tuberculosis. Eur Respir J. 2004;24:638–643. doi: 10.1183/09031936.04.000105403. [DOI] [PubMed] [Google Scholar]