Abstract
Introduction
The current study examines the spectrum of malignancies among HIV-infected South Indians enrolled in a clinical care program.
Materials and Methods
We conducted a nested matched case-control study among 42 HIV-infected cases who developed cancer and 82 HIV-infected controls between 1998 and 2008 at a tertiary care HIV care program in South India.
Results
The most common types of cancer included non-Hodgkin’s lymphoma (38.1%), Hodgkin’s lymphoma (16.7%), squamous cell carcinoma (14.3%), and adenocarcinoma (14.3%). The median duration of time from HIV infection to cancer diagnosis was 549 days [interquartile range (IQR): 58–2013]. The nadir CD4 cell count was significantly lower in cases compared to controls (134 cells/μl vs. 169 cells/μl; P = 0.015). Cancer patients were more likely to have a more advanced HIV disease stage at the time of cancer diagnosis compared to control patients (Stage C: 90.5% vs. 49.4%; P<0.0001). Significantly more cancer patients were receiving antiretroviral treatment relative to control patients at the time of cancer diagnosis (92.9% vs. 66.3%; P=0.001).
Conclusions
HIV-infected patients who developed cancer had more advanced immunodeficiency at the time of cancer diagnosis and a lower nadir CD4 cell count. It is possible that with the continued roll-out of highly active antiretroviral therapy in India, the incidence of HIV-associated malignancies will decrease.
Introduction
It is estimated that approximately 2.5 million Indians are currently infected with HIV. [1] In the era of generic highly active antiretroviral therapy (HAART) coupled with improved prophylaxis and treatment for opportunistic infections, the natural history of HIV disease has continued to change in India. [2] The increased survival of HAART-experienced HIV-infected patients may also lead to a rise in chronic comorbid conditions, such as cancer. [3] Data from the developed world have suggested a decrease in AIDS-related malignancies, namely Kaposi sarcoma and non-Hodgkin’s lymphoma, among HAART-experienced patients. [4],[5],[6] However, the spectrum and clinical correlates of malignancies among HIV-infected Indians remain to be fully characterized. [7],[8]
The current case-control study describes the pattern of malignancies among HIV-infected patients at a large tertiary care HIV facility in South India. Data on the spectrum and clinical correlates of developing malignancies can assist in the formulation of targeted primary prevention efforts for HIV-infected Indians in care.
Materials and Methods
Setting
Since 1996, YRG Center for AIDS Research and Education (YRG CARE)-VHS Chennai has provided a continuum of care for over 12,000 HIV-infected individuals. As per World Health Organization (WHO) treatment guidelines, patients were advised to initiate HAART before CD4 cell counts reached <200 cells/μl or when CD4 cell counts ranged between 200 and 350 cells/μl with an AIDS-defining illness. The current study was approved by the free-standing YRG CARE Institutional Review Board (IRB).
0Patients
All patients were HIV infected and >18 years of age at enrollment. Patients who registered with an incident cancer diagnosis following confirmed HIV status between January 1998 and October 2008 were included in the current study. The following analyses were conducted using a nested case-control model in which cases were HIV-infected patients who had been diagnosed with a malignancy following enrollment to care, and controls were HIV-infected patients receiving clinical care. Cases were matched to controls by gender, age, and date of enrollment into clinical care at the end of study follow-up (survival sampling).
Clinical assessment
The following methods were employed for diagnosis of cancer cases: pathological/histological confirmation (88.1%), radiology (97.6%), and clinical (95.2%) diagnosis. All cases of lymphoma were diagnosed using the WHO classification. Further analyses were executed using the YRG CARE Chennai HIV Observational Database. [9] This database collects data on demographics; clinical assessments including data related to the occurrence of new opportunistic infections; current treatment regimens and adverse events (AE); and also laboratory data, including hemoglobin, liver, and renal function tests, CD4 cell counts, and plasma viral load (PVL). In accordance with WHO guidelines, the patient received laboratory monitoring at least every 6 months after initiating therapy. [10]
Statistical analysis
Descriptive statistics were calculated with the mean for variables that were normally distributed, and the median and interquartile range (IQR) for variables influenced by extreme values. To compare proportions, chi-square (χ2) statistics were used, and Student’s t-tests were used to analyze continuous variables. Statistical analyses were performed with SPSS software (version 13.0; SPSS, Chicago, IL, USA). A P-value less than 0.05 was considered statistically significant.
Results
A total of 42 incident cases of cancer were diagnosed among this cohort of South Indian HIV-infected patients following enrollment into clinical care. Two-fifths of cancer cases (41%) were diagnosed after 2004, 27% during 1998–2001, and 31% during 2001–2004. Over two-thirds (69%) of cancer cases were men; the median age at the time of cancer diagnosis was 35 years (IQR: 29.8–46.3). Over 85% of cases and controls had acquired HIV via heterosexual transmission. Cases and controls had the same body mass index (BMI, 20.1 kg/m2). Fewer cases reported alcohol consumption or tobacco use compared to controls [Table 1].
Table 1.
Characteristics of HIV-infected cases and controls (N = 124)
| Characteristic | Cases frequency [mean (%), median (IQR)] | Controls frequency [mean (%), median (IQR)] | P-value* |
|---|---|---|---|
|
| |||
| N= 42 | N = 82 | ||
| Median CD4 cell count (cells/μl) | |||
| At enrollment to care | 199 (88–459) | 233 (111–480) | NS |
| Nadir CD4 cell count | 134 (65–235) | 169 (86–322) | 0.015 |
|
| |||
| Median BMI (kg/m2) | 20.1 (17.9–22.9) | 20.1 (17.3–21.7) | NS |
|
| |||
| Alcohol use | 7.1 | 14.5 | NS |
|
| |||
| Tobacco use | 11.9 | 18.1 | NS |
|
| |||
| HAART experienced | 92.9 | 66.3 | <0.0001 |
|
| |||
| Prior AIDS diagnosis | 54.8 | 48.2 | NS |
|
| |||
| WHO disease stage at the time of cancer diagnosis | |||
| B | 9.5 | 50.6 | <0.0001 |
| C | 90.5 | 49.4 | <0.0001 |
|
| |||
| Median time between enrollment to care and HAART initiation (days) | 81 (8–991) | 28 (1–221) | NS |
Cases and controls matched on gender, age, and year of enrollment into HIV care. NS = not significant,
P-value compares chi-square statistics for categorical variables and Student’s t-tests are for continuous variables.
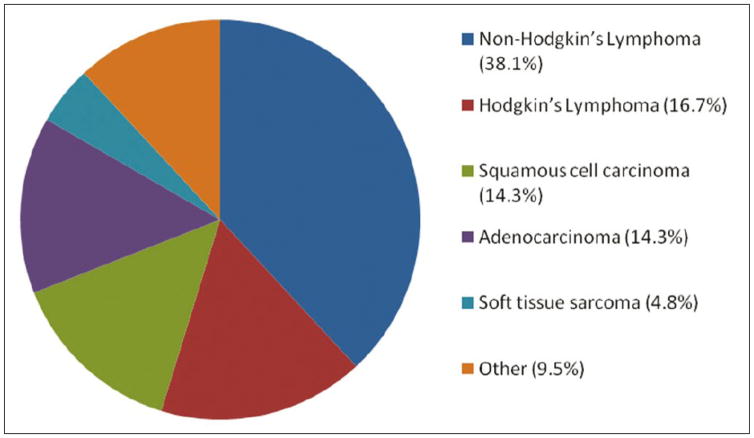
The major types of cancer included non-Hodgkin’s lymphoma (38.1%); Hodgkin’s lymphoma (16.7%); squamous cell carcinoma (14.3%), three in the cervix and one each in the penis, esophagus, and rectum; adenocarcinoma (14.3%), two in the breast and one each in the kidney, lung, trachea, and sinuses; and soft tissue sarcoma (4.8%), one in the bone and the other in the skin. The remaining cancer diagnoses (9.5%) were documented only in a single patient each: extramedullary plasmacytoma, meningioma, multiple myeloma, thyroid follicular carcinoma [Figure 1]. At the time of cancer diagnosis, 7.1% of cases were local and 92.9% were at distant sites. Among the cases of lymphoma, 36% were nodal and 64% were extranodal at the time of diagnosis. [Table 2] presents the morphology of each cancer by the anatomical site of diagnosis.
Figure 1.
Distribution of malignancies (n = 42)
Table 2.
Malignancies categorized by site and morphology (N = 42)
| Site | Morphology | Frequency |
|---|---|---|
| Kidney | Renal cell carcinoma | 1 |
|
| ||
| Lymph node | Non-Hodgkin’s lymphoma | 14 |
| Hodgkin’s lymphoma | 7 | |
|
| ||
| Penis | Squamous cell carcinoma | 1 |
|
| ||
| Bone | Soft tissue sarcoma | 1 |
| Multiple myeloma | 1 | |
|
| ||
| Breast | Adenocarcinoma | 2 |
|
| ||
| Cervix | Squamous cell carcinoma | 3 |
|
| ||
| Esophagus | Squamous cell carcinoma | 1 |
|
| ||
| Lung | Carcinoma | 1 |
|
| ||
| Abdomen | Lymphoma | 1 |
|
| ||
| Trachea | Adenocarcinoma | 1 |
|
| ||
| Colon | Non-Hodgkin’s lymphoma | 1 |
|
| ||
| Meninges | Meningioma | 1 |
|
| ||
| Rectum | Squamous cell carcinoma | 1 |
|
| ||
| Thyroid | Follicular carcinoma | 1 |
|
| ||
| Soft tissue/skin | Soft tissue sarcoma | 1 |
| Extramedullary plasmacytoma | 1 | |
| Erythroplakia | 1 | |
|
| ||
| Sinus | Adenocarcinoma | 1 |
The nadir CD4 cell count was significantly lower in cases compared to controls (134 cells/μl vs. 169 cells/μl; P=0.015). Also, cases had lower CD4 cell counts at the time of enrolling to care and at the time of cancer diagnosis compared to controls. The median CD4 cell count at the time of cancer diagnosis was 164 cells/μl (IQR: 69–333). Cases were more likely to have a more advanced disease stage at the time of cancer diagnosis compared to controls (Stage C: 90.5% vs. 49.4%; P<0.0001). Close to half of the cases (54.8%) and controls (48.2%) had a prior AIDS diagnosis, and the most common AIDS diagnosis was pulmonary tuberculosis among both cases (38.1%) and controls (41.0%).
Significantly more cancer patients were receiving antiretroviral treatment relative to control patients at the time of cancer diagnosis (92.9% vs. 66.3%; P=0.001). Cases had a longer time period between enrollment to care and HAART initiation relative to controls (median number of days: 81 vs. 28). Half of the cancer cases were diagnosed after initiating HAART. The median duration between HAART initiation and cancer diagnosis was 195 days (IQR: 91–814.5). The median duration of time from HIV infection to cancer diagnosis was 549 days (IQR: 58–2013).
A fifth of cases (21.4%) underwent surgery for cancer treatment, 14.3% radiotherapy, and 45.2% chemotherapy. Close to a fifth of cancer cases (19%) died after enrollment to care, and all but one of these patients died as a result of cancer.
Discussion
The current study documents the profile of malignancies among HIV-infected South Indians diagnosed with cancer after enrollment into HIV clinical care. Almost half of the patients (45%) in the current study had developed an AIDS-defining malignancy (non-Hodgkin’s lymphoma or invasive carcinoma of the cervix). This finding is similar to another recent study from Western India where about 43% of patients had developed an AIDS-defining malignancy. [7] The high frequency of non-Hodgkin’s lymphoma is similar to other studies among HIV-infected patients. [7],[11] Data from the developed world have suggested that HAART may decrease the excess risk of developing non-Hodgkin’s lymphoma. [4],[5] A recent study from a North American cohort suggests that non-AIDS-defining cancers represent an increasing proportion of cancers observed in HIV-infected patients, and these patients may be at an increased risk of such cancers compared to the general population. [12] We reported no cases of Kaposi’s sarcoma in this case series, which is in accordance with data from India showing few reported cases of Kaposi’s sarcoma in this region. [13],[14] The profile of cancers among HIV-infected South Indians in the current study appears to differ from the distribution among noninfected South Indians in whom among males cancers of the oral cavity, pharynx, and lungs and among females cancers of the cervix and breast are most common. [15],[16] These differences between HIV-infected and uninfected populations may reflect differing environmental exposures as well as a causative role of HIV-associated immunodeficiency in cancer incidence. As HIV-infected Indians live longer with a growing access to generic HAART, longitudinal studies will need to be conducted to examine the development of malignancies, as well as the further development of regional cancer registries for HIV-infected Indians. [17]
In the current study, patients who developed cancer had more advanced disease at the time of cancer diagnosis and a lower nadir CD4 cell count. This finding is concordant with the high frequency of AIDS-related malignancies, which are more frequent in patients with advanced immunodeficiency defined by a low CD4 cell count. [18] Cancer cases were more likely to initiate HAART after enrollment to care compared to controls, but the time period between enrollment into clinical care and HAART initiation was over three times longer for cases than control patients. The fact that cancer patients were more likely to be receiving HAART relative to controls at the time of cancer diagnosis may reflect heightened detection and screening for malignancies at the time of treatment initiation. Since many patients initiated HAART with advanced immunodeficiency well after they would have met treatment initiation criteria, the association between HAART and cancer may reflect an advanced HIV disease stage. Despite the increasing availability of HAART in India, the clinical reality that patients continue to present to care with advanced immunodeficiency suggests that AIDS-defining malignancies may continue to be prevalent in the HAART era unless further proactive efforts are taken to identify HIV-infected Indians at an earlier stage of HIV infection. It is conceivable that the incidence of AIDS-related malignancies may decrease if HAART is initiated at higher CD4 cell counts. [19]
More than 10% of the neoplasms in this series were likely due to HPV-related oncogenesis (i.e., cervical, anal, and possibly head and neck region squamous cell cancers), which might have been preventable by prior vaccination. Cancer registries from Chennai have recorded a high incidence rate of cervical cancer in India (20.3 per 100,000 person-years), which comprise close to 20% of malignancies in women. [20] Given the limited availability of screening for cervical cancer among the general population in resource-limited settings and the high risk of cervical cancer among HIV-infected individuals, there is a need for further proactive screening of HIV-infected women in care.
A limitation of the current study is that we are unable to comment on the prevalence of malignancies in this population of HIV-infected patients due to the case-control study design and it is possible that we may have underestimated the number of cancer cases. Since HIV-infected patients in the current study were referred to a regional cancer center for treatment and follow-up care following cancer diagnosis, we are unable to provide further details on stage, subtypes, and outcomes of malignancies. The aim of this epidemiological analysis was to provide an overview of the spectrum of malignancies among HIV-infected South Indians, an area with limited available data to date. The strength of the current study was that all cancer diagnoses were made following diagnosis of HIV, though it is possible that carcinogenesis could have begun before enrollment into care.
In light of the increasing availability of HAART and the improved management of HIV-related coinfections, longer survival among HIV-infected Indians will likely increase the importance of cancer in HIV clinical management. [3] Whether AIDS-defining cancers continue to be the primary cancer diagnoses among HIV-infected Indians will likely depend on timely access to HAART. HIV-infected Indians over time who receive HAART may also be at risk for developing other non-HIV-related cancers that are prevalent in the noninfected population, such as cancers of the head and neck and cervix. Continued monitoring of HAART-experienced Indians may reveal further changes in the cancer incidence and provide new strategies for cancer screening and clinical intervention.
Acknowledgments
Source of Support: Brown University’s AIDS International Research and Training Program of the Fogarty International Center at the National Institutes of Health (NIH), USA (grant no. D43TW00237), ACTG-ICTU/ NIH-Chennai site grant for supporting this study.
The authors are grateful to Ms. Rashmi, research nurse; Mr. S. Anand, data manager; Mr. Gurunathan and Mr. Siva, data entry operators; and all the clinical staff at the YRG Centre for AIDS Research and Education, VHS, Chennai, India, for their facilitation of the study. The authors would like to thank.
Footnotes
Conflict of Interest: None
References
- 1.UNAIDS. [Last accessed on 2008 July 9];2.5 million people living with HIV in India. Available from: http://www.unaids.org/en/KnowledgeCentre/Resources/FeatureStories/archive/2007/20070704_India_new_data.asp.
- 2.Kumarasamy N, Solomon S, Chaguturu SK, Cecelia AJ, Vallabhaneni S, Flanigan TP, et al. The changing natural history of HIV disease: Before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41:1525–8. doi: 10.1086/497267. [DOI] [PubMed] [Google Scholar]
- 3.Biggar R, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV/AIDS in India: A review and future directions for research. Infect Agent Cancer. 2009;28:4. doi: 10.1186/1750-9378-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifford G, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: Associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Instit. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 5.Engels E, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 6.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Instit. 2000;92:1823–30. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 7.Dhir AA, Sawant S, Dikshit RP, Parikh P, Srivastava S, Badwe R, et al. Spectrum of HIV/AIDS related cancers in India. Cancer Causes Control. 2008;19:147–53. doi: 10.1007/s10552-007-9080-y. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal B, Ramanathan U, Lokeshwas N, Nair R, Gopal R, Bhatia K, et al. Lymphoid neoplasms in HIV-positive individuals in India. J Acquir Immune Defic Syndr. 2001;29:181–3. doi: 10.1097/00042560-200202010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Cecelia A, Christybai P, Anand S, Jayakumar K, Gurunathan T, Vidya P, et al. Usefulness of an observational database to assess antiretroviral treatment trends in India. Natl Med J India. 2006;19:14–7. [PubMed] [Google Scholar]
- 10.WHO. [Last accessed on 2007 Aug 2];Scaling up antiretroviral therapy in resource-limited settings: Treatment guidelines for a public health approach-2003 revision. Available from: http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf.
- 11.Dal Maso L, Serraino D, Franceschi S. Epidemiology of AIDS-related tumours in developed and developing countries. Eur J Cancer. 2001;37:1188–201. doi: 10.1016/s0959-8049(01)00120-4. [DOI] [PubMed] [Google Scholar]
- 12.Long J, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. 2008;22:489–96. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumarasamy N, Solomon S, Yesudian P, Sugumar P. First report of kaposi’s sarcoma in an AIDS patient from Madras, India. Indian J Dermatol. 1996;41:23–5. [Google Scholar]
- 14.Kumarasamy N, Venkatesh KK, Devaleenol B, Poongulali S, Ahilasamy N. Regression of Kaposi’s sarcoma lesions following highly active antiretroviral therapy in an HIV-infected patient. Int J STD AIDS. 2008;19:786–8. doi: 10.1258/ijsa.2008.008016. [DOI] [PubMed] [Google Scholar]
- 15.Swaminathan R, Selvakumaran R, Esmy PO, Sampath P, Ferlay J, Jissa V, et al. Cancer pattern and survival in a rural district in South India. Cancer epidemiol. 2009;33:325–31. doi: 10.1016/j.canep.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi T, Devesa S, Mangtani P, Mathew A, Cooper N, Kao R, et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2007;37:147–60. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 17.Sankaranarayanan R. Commentary: Cancer incidence among Asian Indians in India and abroad. Int J of Epidemiol. 2008;37:160–1. doi: 10.1093/ije/dym249. [DOI] [PubMed] [Google Scholar]
- 18.Gingues S, Gill MJ. The impact of highly active antiretroviral therapy on the incidence and outcomes of AIDS-defining cancers in Southern Alberta. HIV Med. 2006;7:369–77. doi: 10.1111/j.1468-1293.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. [Last accessed on 2009 July 20];Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents 2009. Available from: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf.
- 20.Nandakumar A, Ramnath T, Chaturvedi M. The magnitude of cancer cervix in India. Indian J Med Res. 2009;130:219–21. [PubMed] [Google Scholar]