Abstract
Objective
Epidemiologically there is a strong relationship between body mass index (BMI) and blood pressure (BP) levels. We prospectively examined randomization to first-step chlorthalidone, a thiazide-type diuretic; amlodipine, a calcium-channel blocker; and lisinopril, an angiotensin-converting-enzyme inhibitor, on BP control and cardiovascular outcomes in a hypertensive cohort stratified by baseline BMI (normal weight [BMI<25], overweight [BMI=25–29·9], and obese [BMI≥30]).
Methods
33,357 hypertensive participants, age ≥55 years were followed for an average of 4·9 years in a randomized, double-blind, practice-based Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), for primary outcome of fatal coronary heart disease (CHD) or nonfatal myocardial infarction and secondary outcomes of stroke, heart failure, combined cardiovascular disease, mortality, and renal failure.
Results
Of participants, 37·9% were overweight and 42·1% were obese at randomization. For each medication, BP control (<140/90 mmHg) was equivalent in each BMI stratum. At year five, 66·1%, 66·5%, and 65·1% of normal weight, overweight, and obese participants, respectively, were controlled. Those randomized to chlorthalidone had highest BP control (67·2%, 68·3%, and 68·4%, respectively) and lisinopril the lowest (60·4%, 63·2%, and 59·6%, respectively) in each BMI stratum. A significant interaction (p=0·004) suggests a lower CHD risk in the obese for lisinopril vs. chlorthalidone (hazard ratio [HR]=0·85, 95% confidence interval [CI]=[0·74–0·98]) and a significant interaction (p=0·011) suggests a higher risk of end-stage renal disease for amlodipine vs. chlorthalidone in obese participants (HR [95% CI]=1·49[1·06–2·08]). However, these results were not consistent among other outcomes.
Conclusions
BMI status does not modify the effects of antihypertensive medications on BP control or cardiovascular disease outcomes.
Keywords: hypertension, blood pressure control, overweight, obesity
INTRODUCTION
A positive linear relationship between body mass index (BMI; kg/m2) and blood pressure (BP) levels has been demonstrated in many epidemiological studies [1]. Given the current high prevalence of overweight and obesity, most hypertension today occurs in people with excess weight [2]. The mean BMI of participants in several large scale hypertension treatment and prevention trials of the past decade has been 28–31 [3–7] and the frequent association between obesity and treatment resistant hypertension is well known [8–10]. It is uncertain if overweight and obese individuals have similar responses to antihypertensive medications compared to normal weight individuals. There are few recommendations regarding which, if any, medications are preferred for the management of hypertension in overweight and obese individuals [8, 11, 12].
The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) was the largest hypertension trial ever undertaken. It enrolled 42,418 individuals with hypertension and at least one additional risk factor for coronary heart disease (CHD) [13–15]. For this study, we stratified participants by baseline BMI as normal weight, overweight, and obese to examine BP control, defined as BP at the goal of <140 mmHg systolic and <90 mmHg diastolic, and cardiovascular outcomes in each BMI stratum and compared the diuretic (chlorthalidone) arm to the two other classes of antihypertensive medications used in the trial: a calcium channel blocker (amlodipine) and an ACE inhibitor (lisinopril). There were no specific hypotheses as to how these comparisons might differ across randomized treatment groups.
METHODS
ALLHAT participants were hypertensive men and women, aged 55 years and older, with at least one additional risk factor for CHD. Of the 42,418 subjects, 33,357 were randomly assigned to therapy with chlorthalidone (n=15,255), amlodipine (n=9,048), or lisinopril (n=9,054) [16]. A fourth arm of the study, which included 9,061 participants assigned to doxazosin, was terminated early [17] and is not considered here. All participants gave written informed consent, and all centers obtained institutional review board approval for the trial.
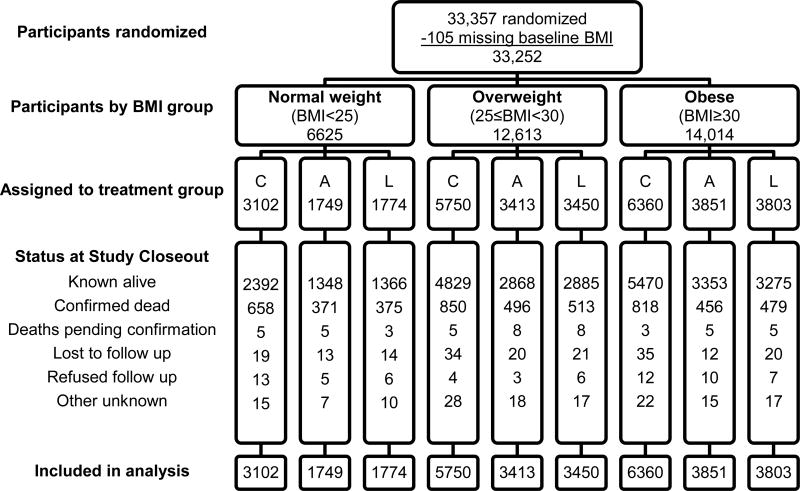
Standard body mass index (BMI) measure was used to determine weight stratification. Participants with BMI values <25 kg/m2 were categorized as “normal weight;” BMI ≥25 and <30 as “overweight;” and those with BMI ≥30 as “obese.” All non-doxazosin participants who had baseline BMI data (n=33,252 of 33,357, 99·7%) were included in these analyses (Figure 1). The ALLHAT study did not obtain data on waist circumference so measures of central obesity could not be included in analyses [18, 19].
Figure 1.
Randomization and follow-up of ALLHAT participants by BMI group
Abbreviations: A, amlodipine; C, chlorthalidone; L, lisinopril
Study medications were identically appearing chlorthalidone, amlodipine, or lisinopril capsules. BP lowering was achieved by titrating the dose of the blinded study drug and adding open label step two (atenolol, clonidine, or reserpine) or step three (hydralazine) agents as necessary to obtain a BP goal of <140/90 mm Hg; each BP result was the average of two seated measurements taken by trained observers using standardized techniques.
Follow-up visits were conducted at one, three, six, nine, and twelve months and every four months thereafter. The primary outcome was a composite of fatal CHD or nonfatal myocardial infarction (MI). Pre-specified secondary outcomes included all-cause mortality, fatal and nonfatal stroke, combined CHD (primary outcome, coronary revascularization, or hospitalized angina), and combined cardiovascular disease (CVD) (combined CHD, stroke, treated angina, heart failure [fatal, hospitalized, or treated nonhospitalized], or peripheral arterial disease). Each component of combined CVD was also prespecified and examined. End-stage renal disease (ESRD) was defined as death due to kidney disease, kidney transplantation, or start of long-term renal dialysis as reported by the clinical sites. Standardized procedures used for reporting and validating study outcomes have been published previously [17]. In addition, all hospitalized heart failure (HF) events were centrally adjudicated in a blinded manner [20] and confirmed the results of the ALLHAT pre-specified HF endpoint of hospitalized, treated without hospitalization or fatal HF.
Data are summarized as means and standard deviations for continuous variables and number of subjects and percentage for categorical variables. Baseline characteristics were compared in participants across BMI strata, and BP and laboratory data during follow-up were compared in participants across BMI and treatment strata. Glomerular filtration rate (GFR) was estimated using the simplified Modification of Diet in Renal Disease (MDRD) Study equation [21]. Significance testing was performed using the t-test and chi-square contingency table analyses for continuous and categorical covariates, respectively. Outcomes were analyzed using an intention-to-treat approach. The Cox proportional hazards model was used to determine time-to-event hazard ratios and 95% confidence intervals (CI). Adjusted Cox models included independent variables associated with cardiovascular outcomes: age, male gender, black race, diabetes history, and current smoker. Since unadjusted analyses of outcomes were similar to the adjusted analyses, only adjusted analyses are presented. Heterogeneity of treatment effects on BP control and on the use of secondary medications across BMI strata were examined by testing for treatment-BMI interaction in logistic models using a p value <0·05. Similarly, treatment-BMI interactions for outcomes were examined in Cox models. Given the many subgroup and interaction analyses performed, statistical significance at the p<0·05 level should be interpreted with caution. All statistical analyses were carried out using STATA version 12·0.
RESULTS
In the cohort, 37·9% were overweight and 42·1% were obese. Baseline characteristics of the cohort, categorized by BMI, are shown in Table 1. Compared to normal weight participants, obese and overweight participants were younger, less likely to be current smokers, more likely to have been on antihypertensive treatment for more than two months prior to entering the trial, and less likely to have prevalent atherosclerotic cardiovascular disease and/or LVH reported at baseline. They were also more educated and more likely to have diabetes mellitus, had higher total and low-density lipoprotein cholesterol and lower high-density lipoprotein levels, had higher fasting glucose levels, and had slightly lower systolic BP and higher diastolic BP at study entry. Percent of those with BP at goal did not differ across BMI strata. Obese patients were more likely to be African American, female, and to have more ST-T wave changes on electrocardiogram (ECG) and less likely to have a history of MI/stroke or a history of CHD than normal weight participants. Overweight individuals were more likely to be male and to have a history of CHD.
Table 1.
Baseline characteristics by BMI category
| BMI<25 | 25≤BMI<30 | p* | BMI≥30 | p† | |
|---|---|---|---|---|---|
| Number randomized | 6625 | 12613 | 14014 | ||
| Age, mean (sd) years, n (%) | 69·5 ( 8·4) | 67·4 (7·6) | <0·001 | 65·2 (7·0) | <0·001 |
| 55–59 | 843 (12·7) | 2127 (16·9) | <0·001 | 3374 (24·1) | <0·001 |
| 60–69 | 2617 (39·5) | 5685 (45·1) | 6867 (49·0) | ||
| 70–79 | 2295 (34·6) | 3960 (31·4) | 3323 (23·7) | ||
| 80+ | 870 (13·1) | 841 ( 6·7) | 450 ( 3·2) | ||
| Black, n (%) | 2186 (33·0) | 4031 (32·0) | <0.144 | 5540 (39·5) | <0·001 |
| Women, n (%) | 3166 (47·8) | 5005 (39·7) | <0·001 | 7426 (53·0) | <0·001 |
| Education, mean (sd) years | 10·8 (4·2) | 11·1 (4·1) | <0·001 | 11·0 (3·9) | 0·007 |
| Cigarette smoking, n (%) | |||||
| Current | 2170 (32·8) | 2700 (21·4) | <0·001 | 2407 (17·2) | <0·001 |
| Past | 2203 (33·3) | 5401 (42·8) | 5810 (41·5) | ||
| Never | 2252 (34·0) | 4512 (35·8) | 5797 (41·4) | ||
| Weight, mean (sd) lbs. | 140·4 (22·0) | 172·9 (22·6) | <0·001 | 212·8 (35·7) | <0·001 |
| Body mass index, mean (sd) Kg/M2 | 22·5 (2·1) | 27·5 (1·4) | <0·001 | 35·3 (5·3) | <0·001 |
| Antihypertensive treatment, n (%) | |||||
| Treated | |||||
| ≥2 months | 5575 (84·2) | 10898 (86·4) | <0·001 | 12395 (88·5) | <0·001 |
| <2 months | 223 (3·4) | 427 (3·4) | 479 (3·4) | ||
| Untreated | 827 (12·5) | 1288 (10·2) | 1140 (8·1) | ||
| ASCVD‡, n (%) | 3734 (56·4) | 6767 (53·7) | <0·001 | 6642 (47·4) | <0·001 |
| ST-T Wave, n (%) | 633 (9·6) | 1301 (10·4) | 0·082 | 1477 (10·7) | 0·023 |
| Type 2 diabetes, n (%) | 1548 (23·4) | 4248 (33·7) | <0·001 | 6220 (44·4) | <0·001 |
| HDL-C<35mg/dl, n (%) | 531 (8·0) | 1609 (12·8) | <0·001 | 1729 (12·3) | <0·001 |
| LVH by ECG or Echo, n (%) | 1635 (24·7) | 2678 (21·2) | <0·001 | 2656 (19·0) | <0·001 |
| Visit 1 blood pressures: | |||||
| SBP, mean (sd) mm Hg | 145·3 (14·6) | 144·9 (13·9) | 0·061 | 144·5 (13·7) | <0·001 |
| DBP, mean (sd) mm Hg | 82·4 (10·2) | 83·3 (9·8) | <0·001 | 83·8 (9·8) | <0·001 |
| BP <140/90, n (%) | 1818 (27·4) | 3415 (27·1) | 0·588 | 3900 (27·8) | 0·561 |
| Visit 2 blood pressures: | |||||
| SBP, mean (sd) mm Hg | 146·8 (16·1) | 146·3 (15·7) | 0·072 | 146·0 (15·4) | 0·001 |
| DBP, mean (sd) mm Hg | 83·0 (10·3) | 84·0 (9·9) | <0·001 | 84·5 (10·0) | <0·001 |
| BP <140/90, n (%) | 1784 (26·9) | 3369 (26·7) | 0·746 | 3857 (27·5) | 0·371 |
| Serum potassium, mean (sd) mmol/l | 4·4 (0·7) | 4·4 (0·7) | 0·492 | 4·3 (0·7) | 0·001 |
| Fasting serum glucose, mean (sd) mg/dl | 108·9 (50·2) | 120·7 (54·4) | <0·001 | 132·4 (61·5) | <0·001 |
| Serum cholesterol, mean (sd) mg/dl | 213·1 (43·0) | 215·7 (43·7) | <0·001 | 217·9 (43·5) | <0·001 |
| LDL-cholesterol, mean (sd) mg/dl | 132·4 (36·8) | 135·9 (37·0) | <0·001 | 137·5 (37·4) | <0·001 |
| HDL-cholesterol, mean (sd) mg/dl | 52·2 (17·1) | 45·9 (14·1) | <0·001 | 45·3 (13·5) | <0·001 |
| Fasting triglycerides, mean (sd) mg/dl | 144·0 (106·4) | 175·5 (143·8) | <0·001 | 184·3 (136·4) | <0·001 |
| Serum creatinine, mean (sd) mg/dl | 1·0 (0·3) | 1·0 (0·3) | 0·168 | 1·0 (0·3) | <0·001 |
| Treatment groups, n (%) | |||||
| Chlorthalidone | 3102 (46·8) | 5750 (45·6) | 0·263 | 6360 (45·4) | 0·124 |
| Amlodipine | 1749 (26·4) | 3413 (27·1) | 3851 (27·5) | ||
| Lisinopril | 1774 (26·8) | 3450 (27·4) | 3803 (27·1) | ||
| History of MI or Stroke, n (%) | 1684 (25·4) | 3100 (24·6) | 0·200 | 2924 (20·9) | <0·001 |
| History of CHD, n (%) | 1702 (25·8) | 3480 (27·8) | 0·003 | 3204 (23·1) | <0·001 |
Abbreviations: ASCVD, arteriosclerotic cardiovascular disease; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; DBP, diastolic blood pressure; ECHO, echocardiogram; ECG, electrocardiography; HDL-C, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation; ST-T Wave, segment of the ECG.
For trial eligibility, participants had to have at least 1 other risk factor in addition to hypertension. Thus the indicated risk factors are not mutually exclusive or exhaustive, and may not represent prevalence.
Comparison of overweight to normal weight subjects
Comparison of obese to normal weight subjects.
History of MI or stroke, history of coronary revascularization, major ST segment depression or T-wave inversion on any electrocardiogram in the past two years, other atherosclerotic CVD (history of angina pectoris; history of intermittent claudication, gangrene, or ischemic ulcers; history of transient ischemic attack; coronary, peripheral vascular, or carotid stenosis ≥50% documented by angiography or Doppler studies; ischemic heart disease documented by reversible or fixed ischemia on stress thalium or dipyridamole thalium, ST depression ≥1 mm for ≥1 minute on exercise testing or Holter monitoring; reversible wall motion abnormality on stress echocardiogram; ankle-arm index <0·9; abdominal aortic aneurysm detected by ultrasonography, computed tomography scan, or radiograph; carotid or femoral bruits).
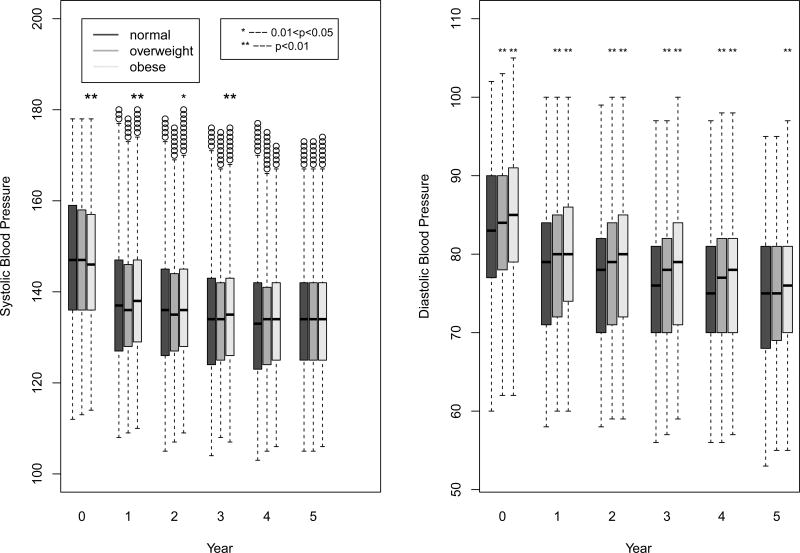
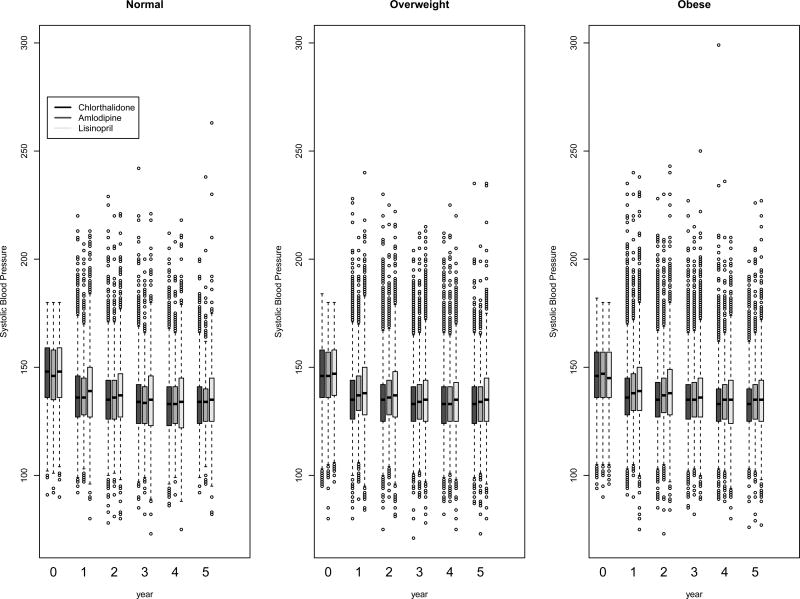
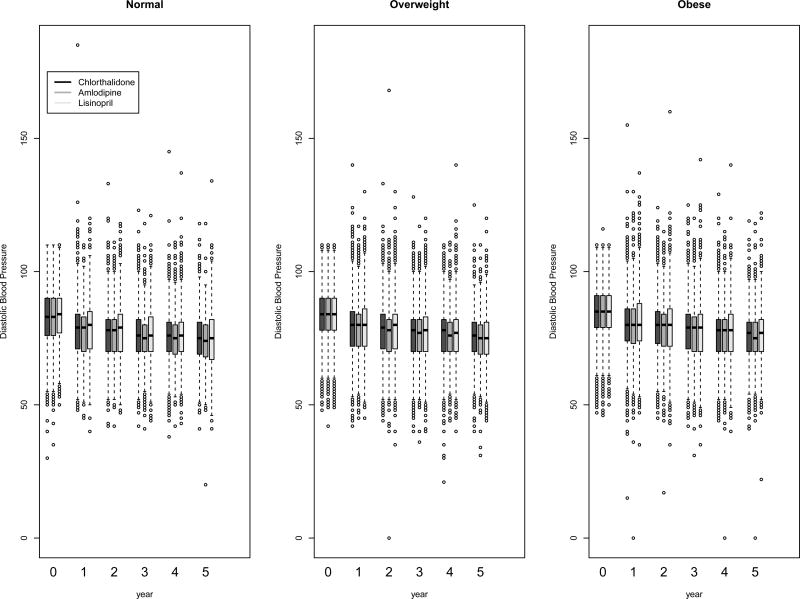
Systolic and diastolic BP levels at baseline and on follow-up, categorized by BMI, are presented in Figure 2. Although there were statistically significant differences due to the large sample size, systolic and diastolic BP levels were generally similar in the three BMI groups at baseline and on follow-up. At baseline, 26·9%, 26·7%, 27·5%, and at year five, 66·1%, 66·5%, and 65·1% of the normal weight, overweight, and obese participants, respectively, had systolic BP controlled to <140 mmHg and diastolic BP controlled to <90 mmHg. The BP control percentages were not significantly different from each other among the BMI subgroups at either time point.
Figure 2.
Box-and-whisker plots of systolic and diastolic blood pressure at baseline and follow-up by BMI group. To make the graph more interpretable, the plotted data was Winsorized at the upper and lowest 1% of the distribution, where extreme values below or above the 1st and 99th percentiles were set equal to the 1st and 99th percentiles, respectively
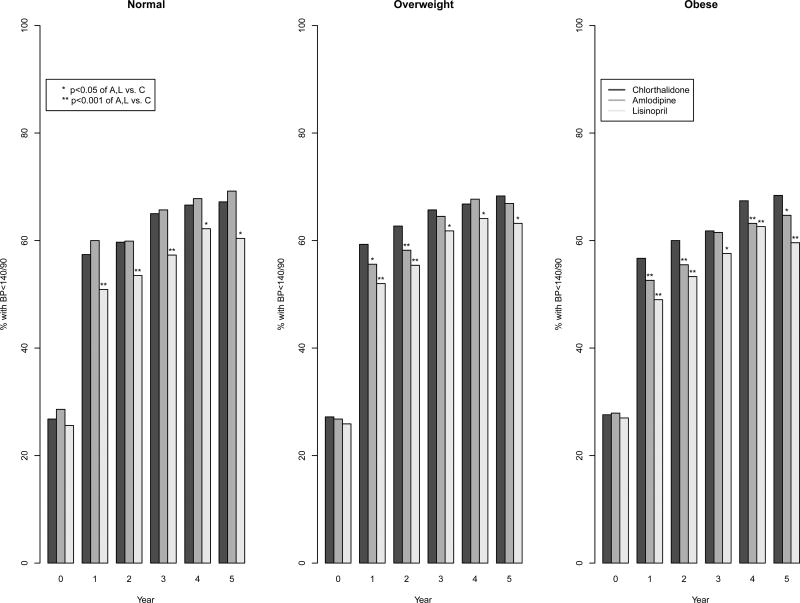
BP control over follow-up by primary medication (Figures 2–5)
Figure 5.
Bar plots of blood pressure control by BMI and treatment group
At baseline, the percentage of individuals in each of the three BMI groups with BP <140/90 mmHg was similar in those assigned to treatment with chlorthalidone, amlodipine, and lisinopril. At year one of follow-up, mean systolic BP was similar in the three BMI subgroups with the use of chlorthalidone (137·2 mm Hg in normal, 136·4 mm Hg in the overweight, and 137·1 mm Hg in the obese). In participants randomized to amlodipine, systolic BP was slightly higher in the overweight and obese groups compared to the normal weight group (137·1 mm Hg in the normal, 138·2 mm Hg in the overweight, and 139·3 mm Hg in the obese). In those treated with lisinopril, there was little difference in systolic BP between the three BMI groups (139·9 mm Hg in normal, 139·3 mm Hg in overweight, and 140·7 mm Hg in the obese). Diastolic BP was slightly higher in the obese group compare to the normal group in all three treatment arms at year one (overall, 78·1 mm Hg in the normal, 79·0 mm Hg in the overweight, and 80·2 mm Hg in the obese). In all three BMI groups, the lowest percent of BP at goal (<140/90 mm Hg) was on lisinopril. Amlodipine and chlorthalidone were equivalent for BP at goal in normal weight group (57·4% on chlorthalidone and 60·0% on amlodipine had BP at goal) but chlorthalidone was superior in the overweight (59·3% on chlorthalidone and 55·6% on amlodipine) and obese groups (56·7% on chlorthalidone and 52·6% on amlodipine) at year one (interaction p-value =0·002 for overweight and 0·001 for obese compared to normal). A similar interaction was detected at year two. However, by year five of follow-up there were no statistically significant interactions between randomization drug and BMI group. Participants randomized to lisinopril had nearly similar percentage of those at BP goal in the three BMI groups (60·4%, 63·2%, and 59·6%, respectively), and the lowest percent of achieving goal BP compared to chlorthalidone or amlodipine in all three BMI groups. Similar BP control differences were found when stratifying by race (Supplemental Digital Content 2).
The number of medications needed to get BP to goal in the three BMI groups over the follow-up period was also examined (Table 2). Participants who were normal weight required fewer medications (mean=1·73) than those who were overweight (mean=1∙88) and obese (mean=1·96). Participants assigned to lisinopril in all three BMI groups required a greater number of medications (mean=2·01) to control BP than those assigned to amlodipine (mean=1·89) or chlorthalidone (mean=1·81). There were significant interaction terms for amlodipine×overweight (p=0.04) and for amlodipine×obese (p=0.03) at year one for the outcome of whether a participant was on 2+ medications versus none or one medication. However, no significant interactions were observed at year five.
Table 2.
Blood pressure control and medications by treatment group and BMI
| Normal BMI <25 |
Overweight 25≤BMI<30 |
Obese BMI≥30 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| C | A | L | C | A | L | C | A | L | |
| 1 year | |||||||||
| N | 2533 | 1433 | 1443 | 4833 | 2836 | 2864 | 5394 | 3264 | 3148 |
| On rz drug, n (%) | 2228 (88·0) | 1286 (89·7) | 1220 (84·6)* | 4342 (89·8) | 2543 (89·7) | 2429 (84·8)† | 4868 (90·3) | 2919 (89·4) | 2640 (83·9)† |
| BP controlled, n (%)‖ | 1307 (58·7) | 780 (60·7) | 628 (51·5)† | 2603 (60·0) | 1443 (56·7)* | 1285 (52·9)† | 2829 (58·1) | 1567 (53·7)* | 1318 (49·9)† |
| Not on rz drug, n (%) | 305 (12·0) | 147 (10·3) | 223 (15·5) | 491 (10·2) | 293 (10·3) | 435 (15·2) | 526 (9·8) | 345 (10·6) | 508 (16·1) |
| BP controlled, n (%) | 145 (47·5) | 81 (55·1) | 108 (48·4) | 267 (54·4) | 140 (47·8) | 206 (47·4) | 238 (45·3) | 149 (43·2) | 228 (44·9) |
| On 0 drugs, n (%) | 77 (3·0) | 26 (1·8) | 55 (3·8) | 108 (2·2) | 60 (2·1) | 77 (2·7) | 103 (1·9) | 64 (2·0) | 79 (2·5) |
| BP controlled, n (%)** | 41 (53·3) | 17 (65·4) | 22 (40·0) | 63 (58·3) | 27 (45·0) | 40 (52·0) | 49 (47·6) | 23 (35·9) | 41 (51·9) |
| On 1 drug, n (%) | 1762 (69·6) | 1073 (74·9) | 930 (64·4) | 3270 (67·7) | 1919 (67·7) | 1792 (62·6) | 3611 (66·9) | 2102 (64·4) | 1893 (60·1) |
| BP controlled, n (%)†† | 1084 (61·5) | 672 (62·6) | 538 (57·9) | 2064 (63·1) | 1099 (57·3) † | 1073 (59·9)* | 2172 (60·2) | 1148 (54·6)† | 1083 (57·2)* |
| On 2 drugs, n (%) | 594 (23·5) | 279 (19·5) | 325 (22·5) | 1213 (25·1) | 707 (24·9) | 727 (25·4) | 1385 (25·7) | 879 (26·9) | 844 (26·8) |
| BP controlled, n (%) | 296 (49·8) | 148 (53·1) | 139 (42·8)* | 646 (53·3) | 391 (55·3) | 285 (39·2) † | 730 (52·7) | 446 (50·7) | 330 (39·1)† |
| On 3+ drugs, n (%) | 100 (3·9) | 55 (3·8) | 133 (9·2) | 242 (5·0) | 150 (5·3) | 268 (9·4) | 295 (5·5) | 219 (6·7) | 332 (10·5) |
| BP controlled, n (%) | 31 (31·0) | 24 (43·6) | 37 (27·8) | 97 (40·1) | 66 (44·0) | 93 (34·7) | 116 (39·3) | 99 (45·2) | 92 (27·7)* |
| p-value (on # drugs) ‡ | 0·003 | <0·001 | 0·937 | <0·001 | 0·036 | <0·001 | |||
| 5 years | |||||||||
| N | 981 | 555 | 501 | 1972 | 1169 | 1182 | 2273 | 1416 | 1235 |
| On rz drug, n (%) | 791 (80·6) | 470 (84·7)* | 373 (74·5)* | 1593 (80·8) | 986 (84·3)* | 883 (74·7)† | 1942 (85·4) | 1181 (83·4) | 911 (73·8)† |
| BP controlled, n (%) | 552 (69·8) | 331 (70·4) | 233 (62·5)* | 1137 (71·4) | 688 (69·8) | 572 (64·8)* | 1364 (70·2) | 784 (66·4) | 567 (62·2)† |
| Not on rz drug, n (%) | 190 (19·4) | 85 (15·3) | 128 (25·6) | 379 (19·2) | 183 (15·7) | 299 (25·3) | 331 (14·6) | 235 (16·6) | 324 (26·2) |
| BP controlled, n (%) | 107 (56·3) | 56 (65·9) | 72 (56·3) | 216 (57·0) | 99 (54·1) | 175 (58·5) | 197 (59·5) | 139 (59·2) | 173 (53·4)* |
| On 0 drugs, n (%) | 32 (3·3) | 8 (1·4) | 12 (2·4) | 36 (1·8) | 20 (1·7) | 29 (2·5) | 25 (1·1) | 17 (1·2) | 18 (1·5) |
| BP controlled, n (%) | 18 (56·3) | 7 (87·5)§ | 8 (66·7)§ | 20 (55·6) | 9 (45·0) | 16 (55·2) | 19 (76·0) | 11 (64·7)§ | 13 (72·2)§ |
| On 1 drug, n (%) | 413 (42·1) | 244 (44·0) | 192 (38·3) | 754 (38·2) | 420 (35·9) | 382 (32·3) | 833 (36·7) | 440 (31·1) | 391 (31·7) |
| BP controlled, n (%) | 309 (74·8) | 172 (70·5) | 136 (70·8) | 557 (73·9) | 305 (72·6) | 282 (73·8) | 600 (72·0) | 309 (70·2) | 284 (72·6) |
| On 2 drugs, n (%) | 353 (36·0) | 215 (38·7) | 144 (28·7) | 737 (37·4) | 457 (39·1) | 379 (32·1) | 862 (37·9) | 512 (36·2) | 376 (30·5) |
| BP controlled, n (%) | 229 (64·9) | 153 (71·2) | 82 (56·9) | 506 (68·7) | 305 (66·7) | 235 (62·0) | 601 (69·7) | 337 (65·8) | 207 (55·1)† |
| On 3+ drugs, n (%) | 183 (18·8) | 88 (15·9) | 153 (30·5) | 445 (22·6) | 272 (23·3) | 392 (33·2) | 553 (24·3) | 447 (31·6) | 450 (36·4) |
| BP controlled, n (%) | 103 (56·3) | 55 (62·5) | 79 (51·6) | 270 (60·7) | 168 (61·8) | 214 (54·6) | 341 (61·7) | 266 (59·5) | 236 (52·4)* |
| p-value (on # drugs)‡ | 0·069 | <0·001 | 0·609 | <0·001 | <0·001 | <0·001 | |||
Abbreviations: A, amlodipine; BP, blood pressure; C, chlorthalidone; L, lisinopril; rz, treatment.
p<0·05, adjusted for the following: age, race, gender, education current smoker, on antihypertensive treatment at baseline, ASCVD, diabetes, HDL<35 mg/dL, baseline LVH, baseline SBP, baseline DBP, baseline potassium, fasting baseline glucose, baseline total cholesterol, baseline LDL, baseline HDL, fasting triglycerides, baseline creatinine, baseline CHD, comparison of A vs. C & L vs. C
p<0·001, adjusted for the following: age, race, gender, education current smoker, on antihypertensive treatment at baseline, ASCVD, diabetes, HDL<35 mg/dL, baseline LVH, baseline SBP, baseline DBP, baseline potassium, fasting baseline glucose, baseline total cholesterol, baseline LDL, baseline HDL, fasting triglycerides, baseline creatinine, baseline CHD, comparison of A vs. C & L vs. C
overall p-values from 4x2 contingency table, comparing A vs. C & L vs. C.
failure to converge due to insufficient number of events
p=0.029 for A/C x overweight/normal interaction and p=0.018 for A/C x obese/normal interaction
p=0.032 for L/C x obese/normal interaction
p=0.005 for A/C x overweight/normal interaction and p=0.009 for A/C x obese/normal interaction
Cardiovascular Outcomes
(Table 3; Figures 6–7: Figure, Supplemental Digital Content 1, which shows cumulative disease and mortality event rates for normal weight, overweight, and obese subgroups, by treatment group)
Table 3.
Cox regression of outcomes by drug treatment group within BMI groups *
| BMI<25 | 25≤BMI<30 | BMI≥30 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCB vs Diuretic | ACE-I vs Diuretic | CCB vs Diuretic | ACE-I vs Diuretic | CCB vs Diuretic | ACE-I vs Diuretic | |||||||
|
| ||||||||||||
| HR | p | HR | p | HR | p | HR | p | HR | P | HR | p | |
| CHD† | 1·04 | 0·669 | 1·19 | 0·058 | 1·04 | 0·568 | 1·00 | 0·971 | 0·91 | 0·192 | 0·85 | 0·024 |
| CCHD | 1·02 | 0·840 | 1·11 | 0·176 | 1·04 | 0·430 | 1·08 | 0·145 | 0·96 | 0·463 | 0·96 | 0·468 |
| CCVD | 1·07 | 0·264 | 1·07 | 0·228 | 1·02 | 0·606 | 1·13 | 0·002 | 1·03 | 0·417 | 1·06 | 0·124 |
| HF | 1·34 | 0·012 | 1·17 | 0·189 | 1·23 | 0·019 | 1·28 | 0·005 | 1·48 | 0·00 | 1·17 | 0·052 |
| Hosp/Fatal HF | 1·31 | 0·032 | 1·11 | 0·413 | 1·20 | 0.056 | 1·19 | 0·071 | 1·46 | 0·000 | 1·05 | 0·606 |
| Stroke | 0·97 | 0·792 | 1·14 | 0·311 | 0·89 | 0·256 | 1·15 | 0·162 | 0·95 | 0·642 | 1·15 | 0·141 |
| Death | 1·00 | 0·969 | 1·01 | 0·845 | 0·98 | 0·661 | 1·01 | 0·918 | 0·90 | 0·088 | 0·97 | 0·633 |
| ESRD‡ | 0·64 | 0·110 | 0·98 | 0·936 | 1·00 | 0·983 | 0·86 | 0·444 | 1·49 | 0·020 | 1·49 | 0·022 |
| Cancer | 1·13 | 0·237 | 1·05 | 0·663 | 1·00 | 0·980 | 1·06 | 0·427 | 0·97 | 0·707 | 0·93 | 0·329 |
| Hosp/Treated Angina | 0·97 | 0·728 | 1·04 | 0·710 | 1·01 | 0·931 | 1·16 | 0·019 | 1·05 | 0·414 | 1·09 | 0·175 |
| Hosp Angina | 0·90 | 0·395 | 1·10 | 0·419 | 1·01 | 0·946 | 1·17 | 0·040 | 0·99 | 0·888 | 1·02 | 0·798 |
| CRVSC | 1·15 | 0·233 | 1·16 | 0·207 | 1·13 | 0·095 | 1·12 | 0·125 | 1·04 | 0·611 | 1·04 | 0·578 |
| PAD | 1·02 | 0·867 | 0·92 | 0·580 | 0·81 | 0·093 | 1·17 | 0·163 | 0·81 | 0·114 | 0·95 | 0·705 |
Abbreviations: ACE-I, angiotensin-converting-enzyme inhibitor; BMI, body mass index; CCB, calcium channel blocker; CCHD, combined coronary heart disease; CCVD, combined cardiovascular disease; CHD, coronary heart disease; CRVSC, coronary revascularization; ESRD, end stage renal disease; HF, heart failure; Hosp., hospital; PAD, peripheral artery disease
The following independent variables were used in the Cox regression: age, male gender, black race, diabetes history, current smoker.
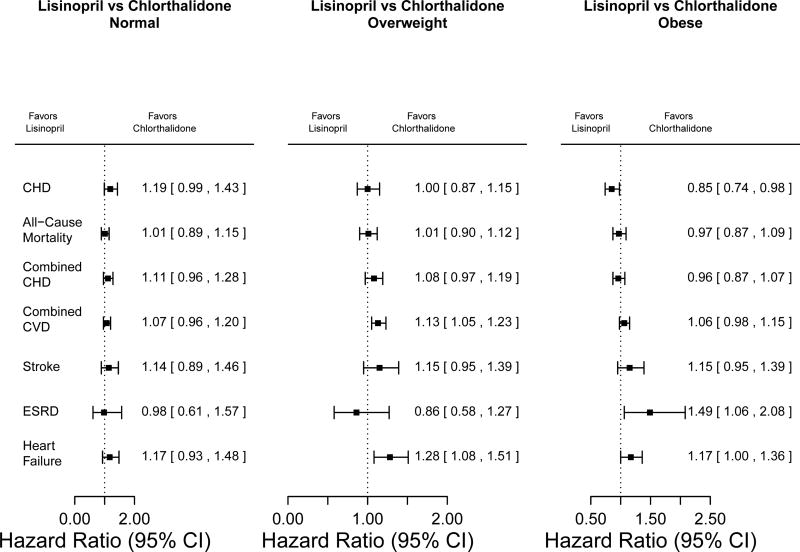
Significant interaction between CHD and BMI for ACEI vs. Diuretic when interaction term included in the Cox regression (p=0·004).
Significant interaction between ESRD and BMI for CCB vs. Diuretic when interaction term included in the Cox regression (p=0·011)
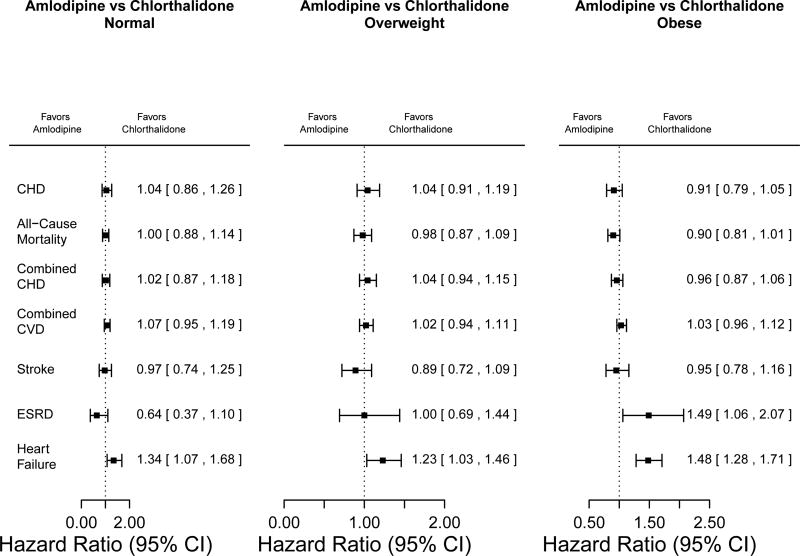
Figure 6.
Hazard ratios for comparisons of amlodipine versus chlorthalidone in normal-weight, overweight, and obese participants, adjusted for age, male sex, black race, diabetes history, and current smoker
Figure 7.
Hazard ratios for comparisons of lisinopril versus chlorthalidone in normal-weight, overweight, and obese participants, adjusted for age, male sex, black race, diabetes history, and current smoker
Amlodipine versus Chlorthalidone
There were no significant differences between amlodipine and chlorthalidone treatment in the three BMI groups for the primary study outcome of fatal and non-fatal CHD events. There were also no significant differences in all-cause mortality, combined CHD, combined CVD, and stroke outcomes between the two medications in the three BMI groups. With regard to heart failure, there were consistent findings in the three BMI groups with amlodipine use being associated with significantly higher risk (HRs =1·34, 1·23, and 1·48 for normal, overweight, and obese participants, respectively). Only for ESRD was there a difference among BMI groups, with a higher risk in the amlodipine group in those who were obese (HRs [95% CI] 0·64 [0·37–1·10], 1·00 [0·69–1·44], and 1·49 [1·06–2·07] for normal weight, overweight, and obese participants, respectively; interaction p-value=0·011).
Lisinopril versus Chlorthalidone
There were no significant differences between lisinopril and chlorthalidone for most cardiovascular disease outcomes among BMI groups. There was, however, a lower risk of CHD events in the obese group for lisinopril (HR [95% CI] = 0·85 [0·74–0·98], interaction p-value = 0·004) as compared to the normal weight and overweight groups (HRs not significantly different from 1). Although significant differences were observed overall in both stroke and HF, not stratified by BMI [17], HF was significant in only one BMI stratum and we did not detect any significant interactions.
DISCUSSION
There are two main outcomes of interest in this study. First, there were no clinically meaningful differences in the percentage of subjects whose BP was at goal across the chlorthalidone, amlodipine, or lisinopril treatment groups by BMI class. In normal weight, overweight, and obese participants, systolic and diastolic BP values were within ~0·5–1·5 mmHg of each other for each medication. The second outcome of interest was that there were few differences in cardiovascular events for any of the three study medications by BMI group. The effect of the medications was the same independent of BMI, suggesting that BMI has no bearing on cardiovascular disease outcomes provided that BP is equally treated in the BMI groups.
Two prior studies have examined the impact of weight on BP control and cardiovascular disease outcomes. The Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) study [6] reported that the combination of hydrochlorothiazide (HCTZ) and benazepril was more effective than the combination of amlodipine and benazepril in preventing the combined end points of CVD death, non-fatal MI, and stroke in participants who were obese or overweight (~85% of the cohort) than in those who had normal weight. The study investigators concluded that HCTZ was a more effective antihypertensive agent in obese or overweight individuals than in normal weight individuals. That conclusion differs from the current findings. This difference could be due to two factors. First, ALLHAT used chlorthalidone, which is approximately 50% stronger and longer acting than HCTZ at similar doses [22]. In addition, the HCTZ dose used in ACCOMPLISH (12·5–25 mg/d) is lower than the doses proven to lower CVD events in outcome trials (minimum dosing of 25–50 mg/d). Second, the rate of the primary outcome in ACCOMPLISH (19·1 per 1000 person-years) as compared to the same type of event rate in ALLHAT (29·4 per 1000 person-years) differs, suggesting fundamental differences in study populations. For example, in the overall cohort, ALLHAT had 35·6% black and 53·2% male, whereas ACCOMPLISH had 11·9% black and 60·7% male. The other study to examine weight, hypertension, and CVD outcomes was the Systolic Hypertension in Elderly Program (SHEP) [23]. In that study, treatment with chlorthalidone compared to placebo was equally effective in reducing mortality and stroke in both overweight and normal weight participants, similar to the current findings.
The position paper of the Obesity Society and of the American Society of Hypertension [11] states that all classes of BP medication are effective for controlling hypertension in obese people. However, ACE inhibitors or angiotensin receptor blockers are selected as first-line treatment because they are not associated with metabolic disturbances to which obese persons are predisposed. The European Society for Hypertension [8] also recommends ACE inhibitors or calcium channel blockers, for similar reasons, for patients with the metabolic syndrome, a pathology generally associated with obesity or overweight. The document also stated that diuretics in low dosage and beta blockers with vasodilating properties should be considered as additional drugs if needed. The Joint Statement of the Association for the Study of Obesity and Hypertension and the European Society of Hypertension [9] underlines the frequent presence of resistant hypertension in the obese population that generally require more than three drugs to obtain hypertension control. They recommend a “flexible” approach for the treatment of hypertension in obese subjects that include the use of diuretics, ACE inhibitors, and beta blockers with vasodilating properties. In such cases they recommended that ACE inhibitors should be “the first line” in the approach for the treatment of hypertension in those patients. Dihydropyridine calcium channel blockers are also considered effective in the treatment of obesity related hypertension [24].
Contrary to these recommendations, in our analyses, the ACE-inhibitor lisinopril was associated with the lowest percentage of participants with BP at goal of all three medications in all three BMI groups. Participants assigned lisinopril were also more likely to require add-on medications, especially if obese. Further, we extensively evaluated major clinical outcomes in ALLHAT participants with metabolic syndrome, both with and without diabetes and by race, and showed that none of the comparator drugs was superior to chlorthalidone (12.5–25 mg/d) in preventing any CV or renal outcome and that chlorthalidone was superior to all in preventing heart failure and stroke (for stroke, compared with doxazosin, and for blacks with lisinopril) – in patients both with and without metabolic syndrome [18–20].
Three other study outcomes of our analyses should be noted. First, a statistically significant interaction was detected for the treatments lisinopril versus chlorthalidone and BMI for outcome CHD. Due to the large number of statistical tests that were conducted in this subgroup analysis (for the main outcomes, 13 tests for an interaction and 78 tests within the strata were conducted) and the lack of consistent results for BP at goal and other cardiovascular outcomes, it is possible that this p-value is statistically significant by chance due to the issue of multiple testing. Second, there was a higher risk of ESRD with lisinopril and amlodipine as compared to chlorthalidone in the obese subgroup. This result should be viewed with caution, given the small number of events. However, the fact that chlorthalidone had the highest percentage of BP control may be an important factor as to why renal disease was less common with chlorthalidone as compared to the other two medications – though our previous analyses did not support a contention that achieved BP levels fully explained chlorthalidone’s superiority in preventing heart failure and stroke [25–27]. Third, in the first three years of follow-up, obese participants were ~3% less likely to have achieved BP at goal compared to normal weight individuals. Only in the last two years of follow-up was BP the same as the other two groups. This lag could be explained by clinical inertia in the treatment of hypertension in obese people. Of the three BMI groups, participants who were obese required the highest number of antihypertensive medications.
This study was based on a randomized, double-blinded, multicenter, practice-based clinical trial, conducted in an ethnically and clinically diverse cohort (most of which was overweight or obese) and had a nearly 100% capture rate of outcomes. Two caveats should be recognized in considering our results. First, since the time that ALLHAT was initiated, it has become common practice to prescribe combinations of medication for the treatment of hypertension [28]. Second, ALLHAT did not measure waist circumference, not allowing us to derive a measure of central obesity as a determinant of BP control and cardiovascular outcomes. The INTERHEART study [29] reported that the waist-hip ratio is a more sensitive predictor of CVD outcomes than is BMI. While this is so, BMI remains a widely-used measure of body weight that is easily obtained. Finally, our current analysis does not examine the “obesity paradox,” that heavier hypertensive people appear to have fewer CVD outcomes than normal weight ones. This will be reported separately.
In conclusion, we found that the relative effectiveness of thiazide-like diuretics, calcium channel blockers, and ACE inhibitors for treating BP to goal and preventing cardiovascular events does not vary by BMI category. Patients who were overweight and obese required more medications to control BP than those who were of normal weight, and subjects treated with lisinopril in the three BMI groups needed more medications to control blood pressure than patients assigned to amlodipine or chlorthalidone.
Supplementary Material
Figure, Supplemental Digital Content 1. Cumulative disease and mortality event rates for normal weight, overweight, and obese subgroups, by treatment group
Figure, Supplemental Digital Content 2a. Bar plots of blood pressure control by BMI and treatment group for Blacks.
Figure, Supplemental Digital Content 2b. Bar plots of blood pressure control by BMI and treatment group for non-Blacks.
Figure 3.
Box-and-whisker plots of systolic blood pressure at baseline and follow-up by BMI and treatment group
Figure 4.
Box-and-whisker plots of diastolic blood pressure at baseline and follow-up by BMI and treatment group
Acknowledgments
We thank Dr. Ellen Breckenridge for her editorial assistance, Mohammad L. Rahman for assistance with the tables, and Glenn W. Schreyer for his programming help.
Role of the funding sources
National Heart, Lung, and Blood Institute contracts NO1-HC-35130 and HHSN268201100036C supported this study. ALLHAT investigators acknowledge medications contributed by Pfizer, Inc., (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
The National Heart, Lung, and Blood Institute was involved in all aspects other than direct operations of the study centers. This included collection, analysis, and interpretation of the data plus the decision to submit the manuscript for publication. Pfizer Inc., AstraZeneca, and Bristol-Myers Squibb had no role in the design and conduct of the study, the collection, analysis, and interpretation of the data; or the preparation or approval of the manuscript.
Barry Davis, Sara Pressel, and Jose-Miguel Yamal had full access to all the data in the study. Barry Davis, Director of the Clinical Trials Center, takes responsibility for the integrity of the data and the accuracy of the data analysis. ALLHAT Steering Committee made the final decision to submit the paper for publication.
Abbreviations
- ACE inhibitor
angiotensin-converting-enzyme inhibitor
- ALLHAT
Antihypertensive and Lipid-Lower Treatment to Prevent Heart Attack Trial
- BMI
body-mass index
- BP
blood pressure
- CI
confidence interval
- CHD
coronary heart disease
- CVD
cardiovascular disease
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- HR
hazard ratio
- LVH
left ventricular hypertrophy
- MDRD
Modification of Diet in Renal Disease
- MI
myocardial infarction
- ECG
electrocardiogram
Footnotes
Clinical trial registration: www.clinicaltrials.gov, NCT00000542
Conflicts of interest
Drs. Reisin, Graves, Yamal, Barzilay, Einhorn, Dart, Retta, and Saklayen, and Ms. Pressel have no conflicts to report. Dr. Davis has received honoraria from Takeda and Amgen.
References
- 1.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, et al. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 2.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;240:1607–1610. doi: 10.1001/jama.240.15.1607. [DOI] [PubMed] [Google Scholar]
- 3.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 4.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 5.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 6.Weber MA, Jamerson K, Bakris GL, Weir MR, Zappe D, Zhang Y, et al. Effects of body size and hypertension treatments on cardiovascular event rates: subanalysis of the ACCOMPLISH randomised controlled trial. Lancet. 2013;381:537–545. doi: 10.1016/S0140-6736(12)61343-9. [DOI] [PubMed] [Google Scholar]
- 7.Weber MA, Bakris GL, Dahlof B, Pitt B, Velazquez E, Gupte J, et al. Baseline characteristics in the Avoiding Cardiovascular events through Combination therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial: a hypertensive population at high cardiovascular risk. Blood Press. 2007;16:13–19. doi: 10.1080/08037050701217643. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 9.Jordan J, Yumuk V, Schlaich M, Nilsson PM, Zahorska-Markiewicz B, Grassi G, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens. 2012;30:1047–1055. doi: 10.1097/HJH.0b013e3283537347. [DOI] [PubMed] [Google Scholar]
- 10.Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17:904–910. doi: 10.1016/j.amjhyper.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 13.Cushman WC, Ford CE, Einhorn PT, Wright JT, Jr, Preston RA, Davis BR, et al. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2008;10:751–760. doi: 10.1111/j.1751-7176.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Accessed April 19, 2012];Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Protocol. Last updated May 2000. Available at https://allhat.sph.uth.tmc.edu/Forms/protocol.pdf.
- 15. [Accessed April 18, 2012];Extension Protocol: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Last updated August 31, 2007. Available at https://allhat.sph.uth.tmc.edu/Forms/ExtensionProtocol.pdf.
- 16.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Am J Hypertens. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 17.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 18.Wright JT, Jr, Harris-Haywood S, Pressel S, Barzilay J, Baimbridge C, Bareis CJ, et al. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2008;168:207–217. doi: 10.1001/archinternmed.2007.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black HR, Davis BR, Barzilay J, Nwachuku C, Baimbridge C, Marginean H, et al. Metabolic and clinical outcomes in nondiabetic individuals with the metabolic syndrome assigned to chlorthalidone, amlodipine, or lisinopril as initial treatment for hypertension: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Diabetes Care. 2008;31:353–360. doi: 10.2337/dc07-1452. [DOI] [PubMed] [Google Scholar]
- 20.Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, et al. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am Heart J. 2007;153:42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. [Accessed July 12, 2013];GFR Calculators: Serum Creatinine and Cystatin C (2012) (With SI Units) Available at http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm.
- 22.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 23.Wassertheil-Smoller S, Fann C, Allman RM, Black HR, Camel GH, Davis B, et al. Relation of low body mass to death and stroke in the systolic hypertension in the elderly program. The SHEP Cooperative Research Group. Arch Intern Med. 2000;160:494–500. doi: 10.1001/archinte.160.4.494. [DOI] [PubMed] [Google Scholar]
- 24.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 25.Wright JT, Jr, Probstfield JL, Cushman WC, Pressel SL, Cutler JA, Davis BR, et al. ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analyses. Arch Intern Med. 2009;169:832–842. doi: 10.1001/archinternmed.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einhorn PT, Davis BR, Wright JT, Jr, Rahman M, Whelton PK, Pressel SL ALLHAT Cooperative Research G. ALLHAT: still providing correct answers after 7 years. Curr Opin Cardiol. 2010;25:355–365. doi: 10.1097/HCO.0b013e32833a8828. [DOI] [PubMed] [Google Scholar]
- 27.Proschan M, Ford CE, Cutler JA, Graumlich JF, Pavlik V, Cushman WC, et al. How much effect of different antihypertensive medications on cardiovascular outcomes is attributable to their effects on blood pressure? Stat Med. 2012;32:884–897. doi: 10.1002/sim.5580. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC-7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 1. Cumulative disease and mortality event rates for normal weight, overweight, and obese subgroups, by treatment group
Figure, Supplemental Digital Content 2a. Bar plots of blood pressure control by BMI and treatment group for Blacks.
Figure, Supplemental Digital Content 2b. Bar plots of blood pressure control by BMI and treatment group for non-Blacks.