Abstract
The native extracellular matrix (ECM) serves as a unique platform for tissue engineering because it provides an organ-specific scaffold in terms of both matrix composition and tissue architecture. However, efficacious cell-seeding techniques for recellularizing the ECM constructs with appropriate cell types to restore biological function remain under development. In this study, the impact of spraying as a seeding technique for repopulation of decellularized small intestine was investigated. In a series of experiments, CaCo-2 cells were first used to investigate the effect of spray device type and pressure on cell viability and to optimize parameters for seeding intestinal epithelial cells. High cell viability and a homogeneous cell distribution were obtained when cell suspensions were sprayed through an airbrush at low pressure. Next, the effect of seeding method and spray pressure on the size and dispersal of intestinal organoids, a more complex and clinically relevant intestinal stem cell population, was evaluated. The feasibility of seeding intestinal epithelial cells onto decellularized scaffolds was next studied using sprayed CaCo-2 cells, which survived the spray-seeding process and formed a monolayer on the scaffold. Finally, airbrush seeding was used to spray intestinal organoids onto the scaffolds, with cell survival and tissue architecture evaluated after 1 week of culture. Organoids seeded through pipetting onto the decellularized scaffold survived, but demonstrated aggregation, with cells organized around multiple small lumens. In contrast, organoids airbrush spray seeded at 0.35 bar onto the decellularized scaffold not only engrafted but also demonstrated formation of an epithelial monolayer that resembled the absorptive surface found on intestinal villi. The results suggest that seeding cells through airbrush spraying holds great potential for use in tissue engineering, especially for large-scale tubular organ recellularization.
Keywords: : organoids, decellularization, spray, airbrush, intestine, tubular scaffolds
Introduction
Patients with intestinal failure are not able to absorb enteral nutrients at a rate that allows for health maintenance and growth. Short bowel syndrome (SBS), the most common cause of intestinal failure, has an incidence rate of 3–5 per 100,000 births per year1 and affects both the pediatric and adult patient populations. Adults develop SBS after surgical bowel resection, often after mesenteric ischemia, volvulus, Crohn's disease, oncological resection, or trauma.2 In children, the most common causes are intestinal atresia, necrotizing enterocolitis, gastroschisis, midgut volvulus, and extensive aganglionosis.3 Despite advances in surgical therapy to enhance intestinal function, 41% of SBS patients depend on total parenteral nutrition (TPN) to maintain fluid, nutrient, and electrolyte balance.4 TPN is complicated by high rates of liver failure, sepsis, and venous thrombosis. Intestinal transplantation remains an option to restore nutritional autonomy to SBS patients; however, even in patients transplanted after the year 2000, graft survival at 5 years remains only 50%.5 Patients with a successfully transplanted allograft still require life-long immunosuppression, which confers increased risk of opportunistic infections and malignancies.
One solution to this clinical problem would be to build a patient-specific organ on a transplantable scaffold repopulated with autologous cells. Initial work in this field used digested primary intestinal tissue, termed “organoid units,” both to generate neomucosa on denuded segments of native bowel6 and to generate intestinal epithelium on synthetic scaffolds for transplantation in rats after massive small bowel resection.7 In addition, immortalized CaCo-2 cells and mouse intestinal crypts, both renewable intestinal epithelial cell populations, have been shown to be capable of generating intestinal mucosa on synthetic scaffolds.8
Synthetic scaffolds are limited by their lack of native vasculature, and recent research has demonstrated that biologically compatible scaffolds, complete with intact vasculature and preserved anatomic architecture, can be generated using perfusion decellularization of whole native organs.9 These scaffolds, comprising the native extracellular matrix (ECM), have been shown in models of lung, heart, and kidney to support organ-specific cell types that can reconstitute function.10–12 Recently, this technique has been applied to generate intestinal scaffolds.13
The epithelium of the gastrointestinal tract is characterized by a combination of crypt domains containing proliferating intestinal stem cells and villous protrusions containing differentiated cell types, most importantly enterocytes responsible for nutrient absorption. The epithelium has a high turnover rate, so any source of cells to repopulate intestinal scaffolds must contain proliferative cell types. A well-described method for maintaining intestinal epithelium in three-dimensional (3D) culture is the isolation of intestinal crypts. When these isolated intestinal crypts are embedded in Matrigel and exposed to an array of growth factors, they form organoids or “miniguts.”14 These organoids have crypt/villus domains organized around central lumens, but are too small as single units to be useful clinically.
Other groups have attempted to apply these organoids to both synthetic and decellularized organ scaffolds, but methods have been limited to cutting single organoids in half and placing them in appropriate orientation for adherence to the graft.15 An ideal method for seeding intestinal organoids to a scaffold construct would encourage the formation of a monolayer of these cells on the scaffold rather than clusters around small lumens, distribute cells over a broad surface area, and eventually have the potential to be scaled up to recellularize large tubular organs.
The most common method of seeding cells is cell aspiration or pipetting. However, recently direct deposition of cells through spraying has received increasing attention as a tool for site-specific, on-demand application during surgeries, diagnostic procedures,16–18 and construction of tissue-engineered scaffolds.19 This technology enables rapid cell delivery over large or irregularly shaped scaffolds or tissue surfaces. One of the advantages of spraying is its adaptability to endoscopic applications, either to administer cells to hollow organs as cell therapy or to seed tubular constructs.20 Moreover, spraying enables rapid direct deposition of biomaterials, including cell-loaded hydrogels21–24 and nanofibers,16,18 onto biological tissues. Spray seeding using a conventional airbrush has been one of the most studied and promising methods for tissue engineering applications because it requires only a simple apparatus and a high-pressure gas source. The compatibility of airbrush has been proven with a number of cell types, including epithelial cells,16,17 keratinocytes,22 fibroblasts,18,25 mesenchymal stem cells,23,26 and chondrocytes.14 Although there have been many studies to explore spray deposition of cells for wound healing,25–27 cartilage repair,17,22 and coating tissue-engineered implants,19,20 the compatibility of spraying with seeding epithelial progenitor cells onto decellularized intestinal scaffolds has not yet been studied.
The goal of this research is to investigate the effects of spray parameters and techniques on the repopulation of decellularized intestinal scaffolds. Spray seeding is a promising technology that can be easily adapted for flexible endoscopic delivery (Fig. 1A), which would allow for homogeneous delivery of progenitor cells to the decellularized scaffolds without damaging their tubular structure. Here we tested two different types of spray devices: a commercial airbrush (Fig. 1B) and a 3D printed spray device (3D spray; Fig. 1C). The airbrush spray gun contains two nozzles: an inner nozzle that feeds the cell suspension and an outer nozzle that feeds a high velocity stream of compressed air that draws out the inner solution into a conical spray and delivers it to the target surface (Fig. 1B). In contrast, in the 3D spray device, the cell suspension is injected through two syringes to a common central channel through which compressed air is continuously flowing. The primary objective of this study was to evaluate the effects of different seeding techniques and spray parameters on cell viability, distribution, and repopulation of decellularized intestinal scaffolds.
FIG. 1.
Illustration of therapeutic delivery method for recellularization of intestine (A). Spray devices for seeding of organoids included conventional two nozzle airbrush (B) and the three-dimensional (3D) printed spray device (C).
Materials and Methods
Small intestine isolation and decellularization
Surgical isolation of jejunal segments
Scaffolds were generated using jejunal segments procured from male, Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). After induction of anesthesia with inhaled isoflurane, a midline laparotomy was performed to access the small bowel. The superior mesenteric artery (SMA) and superior mesenteric vein (SMV) were isolated both proximal and distal to a 4-cm segment of proximal jejunum, and all small branching vessels were ligated. The SMA and SMV were then ligated just distal to the jejunal segment. After systemic heparinization, the animal was euthanized by exsanguination, the proximal SMA was cannulated with a 20-gauge angiocatheter (Smiths Medical, Minneapolis, MN), and a small incision was made in the SMV for venting. Residual blood was flushed from the isolated bowel with 10 mL of ice-cold phosphate-buffered saline (PBS), and efficient flow through was confirmed with PBS effluent through the incised SMV. The bowel was then transected at the margins of the first and fifth mesenteric branching vessels, and the marginal vessels at each end were ligated. The lumen was flushed with cold PBS and the jejunal segment was excised, taking care not to disrupt the cannulated vascular pedicle. All experiments with animals were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (protocol 2014N000308) and were performed in compliance with the Animal Welfare Act.
Decellularization of small bowel
The angiocathether inserted into the SMA was placed into a decellularization chamber and connected to a three-way stopcock allowing for infusion of decellularization solutions. At 90 mmHg of constant pressure, the bowel underwent serial perfusion of 200 mL of 0.1% sodium dodecyl sulfate (SDS; Thermo Fisher Scientific, Waltham, MA) in deionized water, 200 mL of deionized water, and 1000 mL of 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in deionized water. For sterilization, 100 mL of 1% hydrogen peroxide and 0.08% peracetic acid (Spor-Klenz; STERIS, Mentor, OH) was infused and subsequently flushed out with 2 L of PBS. Before experimental use, scaffolds were stored in PBS containing 1% antibiotic-antimycotic solution (Thermo Fisher Scientific) at 4°C.
DNA quantification
Genomic DNA was extracted from native rat jejunum (n = 3) and decellularized rat jejunum (n = 3) and quantified using a validated published protocol.28 In brief, samples were digested in a 55°C oven for 2 h under constant rotation in a buffer containing 400 mM NaCl, 1% SDS, 20 mM Tris-Cl (pH 8.0), and 5 mM EDTA (pH 8.0) with Proteinase K (200 μg mL−1; Sigma-Aldrich) and RNAse A (10 μg mL−1; Sigma-Aldrich). DNA was then concentrated using a phenol–chloroform extraction followed by ethanol precipitation. Quantity and purity of DNA were assessed based on absorbance at 260, 280, and 320 nm (NanoDrop 1000; Thermo Fisher Scientific).
Cell culture
CaCo-2 cells were cultured in flasks using Dulbecco's modified Eagle medium (DMEM; Life Technologies, Waltham, MA) supplemented with 10% (v/v) fetal bovine serum (Sigma-Aldrich), 1% (v/v) antibiotic antimycotic solution (Sigma-Aldrich), and 1% (v/v) MEM nonessential amino acids (Thermo Fisher Scientific) in a humidified incubator at 37°C and 5% CO2. Cells of low passage were thawed, washed, and plated in tissue culture plates and grown to confluence before use in each experimental repetition. Cells were put into single cell suspension by trypsinization, and were washed, counted, and resuspended at a concentration of 0.5 million cells/mL in DMEM just before each experiment.
Organoid culture and preparation for seeding
Organoid culture
C57Bl/6 mice (Charles River Laboratories) ranging in age from 1 to 3 months were euthanized by CO2 asphyxiation and the small intestine was removed through a midline laparotomy. The lumen was then flushed with ice-cold PBS, the bowel opened longitudinally, and the mucosal surface was gently scraped with a glass slide (Fisher Scientific, Waltham, MA) to remove villi. The intestine was next incubated in PBS+EDTA (10 mM) for 1 h on ice. The tissue was opened flat onto a 60-mm tissue culture plate (Corning, Corning, NY) containing cold PBS, and the mucosal surface was scraped firmly with the edge of a glass slide to remove crypts. The PBS with crypts in suspension was filtered through a 70-μL cell strainer (Fisher Scientific) into a 50-mL centrifuge tube and crypts were counted. Tubes were centrifuged at 750 g for 5 min to form a pellet, and then crypts were resuspended in 400 μL Matrigel (Corning). A 24-well plate was prewarmed and 50-μL drops of Matrigel were plated to the center of each of 8 wells with an ideal plating density of 500 crypts per well.
After incubation of the plate at 37°C for 15 min to polymerize the Matrigel, 300 μL of crypt culture medium was added to each well. Crypt culture medium comprised Advanced DMEM/F12 (Life Technologies) containing supplements N2 (1:100; Life Technologies), serum-free B27 (1:50; Life Technologies), N-acetylcysteine (1 μM; Sigma), GlutaMAX-1 (1:100; Life Technologies), HEPES (10 mM; Life Technologies), penicillin/streptomycin (1:100; Life Technologies), and growth factors murine recombinant epidermal growth factor (EGF) (50 ng/mL; R&D Systems, Minneapolis, MN), Noggin (100 ng/mL; Peprotech, Rocky Hill, NJ), R-spondin1 (600 ng/mL; R&D Systems), CHIR99021 (3 μM; Stemgent, Lexington, MA), and Y-27632 dihydrochloride (10 μM ApexBio, Houston, TX). The medium was changed every 2–3 days and plates were maintained in a 37°C humidified incubator containing 5% CO2.
Organoid preparation for seeding
Organoids from 8 wells of a 24-well plate were used for seeding each scaffold segment. One milliliter of ice-cold Advanced DMEM-F12 (Life Technologies) was added to each well and the plate was incubated on ice for 5 min. The organoids were then pipetted up and down 10 times slowly through a 1000-μL pipette tip to break up the Matrigel. The solution of organoids/Matrigel/medium was then transferred to a 15-mL centrifuge tube and centrifuged at 700 g for 5 min to pellet the organoids. Next, the supernatant and Matrigel were removed. The organoid pellet was then washed in 10 mL of ice-cold PBS and centrifuged to form a pellet again. Finally, PBS was removed and the organoids were resuspended in 200 μL of crypt culture medium for seeding.
Fabrication of 3D printed spray device
In this study, a new spray device (3D Spray) with a nozzle diameter of 1000 μm was designed by SolidWorks and 3D printed through a stereolithography-type FormLabs Form 1+ (Somerville, MA) printer. Stereolithography-type 3D printing was chosen to create strong prints with fine detail from a cured resin. A translucent photopolymer resin (GPCL02; FormLabs, Somerville, MA) was used for compatibility with ultraviolet light sterilization.
Delivery of cells through pipetting, airbrush, and 3D spray device
To evaluate the effect of seeding techniques on cell viability, the CaCo-2 cell suspension (0.5 million cells/mL) was either pipetted or sprayed into a single well of a six-well tissue culture plate (Corning). For spraying experiments, the cell suspension was delivered either through a standard airbrush or through the 3D spray device (nozzle diameter: 1000 μm) at varied pressures of 0.35, 0.5 and 1 bar. A Badger Model 150 airbrush (Badger Air-Brush Company, Franklin Park, IL), a handheld, dual-action device with a 750 μm nozzle diameter, was used. An oil-free air compressor (TC908 Aspire Compressor; Badger Air-Brush Company) was used as the air source, with an inline air filter (McMaster-Carr, Elmhurst, IL) attached to the air pressure regulator. The angle between the spray devices and the surface was kept constant at 45° and the delivery height was kept at 5 cm. The nozzle was purged with 1 mL of medium between experiments to verify that no cells were remaining from a previous treatment.
Cell viability and cluster size
CaCo-2 cells (0.5 million cells/mL) were chosen for the viability tests, and organoids (1 million cells/mL) were chosen for measuring cluster size as a function of seeding technique and pressure. After delivery of cells either through pipetting or through spraying, cells were immediately fluorescently labeled using Live/Dead Cell Imaging Kit (488/570) (Life Technologies), as described by the manufacturer's protocol. Cellular viability and distribution were evaluated with fluorescence microscopy (Zeiss Observer Z1, Oberkochen, Germany) using a 10× objective. Cell viability was quantified by counting live and dead cells using ImageJ software. Percentage viability was calculated as the number of live cells divided by the total number of observed cells. The longest length of clusters was defined as the farthest distance between two outside edges of each of the organoid clusters, and data were compiled from seven different fluorescent microscopy images for each sample using ImageJ software.
Seeding CaCo-2 cells and organoids onto decellularized scaffolds
Preparation of scaffolds for seeding
Scaffolds were removed from PBS, and the bowel was separated from the mesentery. The bowel lumen was then opened along the mesenteric side and cut into 0.5 cm2 squares. Scaffold pieces were incubated in DMEM-F12 medium for 4 h at 37°C before use in experiments. Thirty-five millimeter tissue culture dishes were coated with a 0.5 cm2 deep layer of boiled 1.5% agarose in deionized water, which was then allowed to cool and solidify. Scaffold pieces were positioned with the mucosal side facing upward on this agarose surface and secured in place with 0.1-mm minutien pins (Fine Science Tools; North Vancouver, BC, Canada).
Seeding scaffolds with cells
For spray seeding, a 200 μL solution of medium containing either CaCo-2 cells (0.5 million cells/mL) or a combined solution from 8 wells of organoids (each seeded initially at 500 crypts/well) was sprayed onto the scaffold segments using a standard airbrush (nozzle diameter: 750 μm) at a pressure of 0.35 bar from a height of 5 cm. The angle between the spray device and the surface was kept constant at 45°. Between each cell suspension delivery, the nozzle was purged with 1 mL of medium. For suspension seeding, the 200 μL solution of medium containing either CaCo-2 cells (0.5 million cells/mL) or organoids (8 combined wells, each seeded initially at 500 crypts/well) was carefully layered onto the scaffolds using a 200 μL pipette. The scaffold with newly seeded cells was placed in a 37°C incubator for 3 h, after which 2 mL of the appropriate medium for each cell type was added and changed every 2–3 days. Scaffolds seeded with CaCo-2 cells were cultured for 5 days and scaffolds seeded with organoids were cultured for 7 days before being fixed for analysis.
Histological processing
Scaffolds were fixed in 4% paraformaldehyde in PBS overnight at 4°C, embedded in HistoGel (Richard-Allan Scientific, San Diego, CA) for orientation, dehydrated, embedded in paraffin blocks, and 5-μm sections were then made for staining. For hematoxylin and eosin (H&E) staining, sections were deparaffinized, rehydrated, stained with hematoxylin QS (Vector Laboratories, Burlingame, CA) and eosin Y (Thermo Fisher Scientific), and mounted with Permount (Thermo Fisher Scientific) after dehydration. All images were taken using a Nikon Eclipse TE200 microscope and NIS-Elements imaging software (Nikon Instruments, Melville, NY).
Statistical analysis
All results are expressed as mean ± standard deviation. Data analysis was conducted using OriginPro statistical software (OriginLab; Graphic and Analysis, Northampton, MA). For significance, a minimum p-value of <0.05 was used. For comparison of three or more means, a one-way analysis of variance was performed using Tukey's post hoc test.
Results and Discussion
Effect of seeding technique on CaCo-2 cell viability
CaCo-2 cells were selected for initial experiments with seeding techniques and scaffold recellularization because they are representative of the intestinal enterocyte phenotype and rapidly expand in culture. These neoplastic CaCo-2 cells were useful for optimizing seeding parameters, but do not have any clinical application. The seeding techniques compared were pipetting the cell suspension, airbrush spraying the cells, and spraying the cells with the 3D spray device. Each spray technique was evaluated at multiple pressures. Effects of seeding technique and spray pressure on viability and distribution of CaCo-2 cells were analyzed with Live/Dead staining, which was imaged by fluorescence microscopy.
The percentage of viable cells in each condition was compared (Fig. 2). Because cells were exposed to higher shear and elongational forces during spraying, the highest cell survival rates were obtained in pipette-seeded controls (96% ± 1%) and the lowest cell survival rates were seen when high pressures (0.5 and 1 bar) were used during spraying. Moreover, large cell clusters in the range of 200–300 μm were observed when cells were delivered either through pipetting (Fig. 2A) or through spraying with the 3D spray device at 0.35 and 0.5 bar of pressure (Fig. 2B, C). Cells sprayed with the 3D device at 1 bar showed reduced clustering but also had much lower cell viability. In contrast, an even distribution of cells was observed when cells were delivered through airbrush spraying even at low pressure (0.35 bar; Fig. 2E). Image analysis results showed that there is not a statistically significant difference between the viability of cells delivered through airbrush versus those delivered with the 3D spray device at each pressure.
FIG. 2.
Fluorescence microscopy images of Live/Dead-treated CaCo-2 cells in Dulbecco's modified Eagle medium pipetted (A), sprayed with a 3D spray device at 0.35, 0.75, and 1 bar (B–D, respectively), and sprayed with an airbrush at 0.35 bar (E). Green staining indicates live cells, red staining indicates dead cells, and arrows indicate cell clusters. Images were taken at 10× magnification. The graph shows viability of CaCo-2 cells compared by seeding technique and pressure. #Indicates a statistical difference between pipetting versus all other conditions. *Indicates a statistically significant difference versus all other pressures within a technique, as determined by one-way analysis of variance; p < 0.05.
Suspension seeding resulted in clusters of cells on the tissue culture plate because there was not enough shear force to break the cells apart. The cell clustering seen at lower pressures with the 3D spray device was due to the low mixing rate between the cell suspension and air. Cells were simply carried forward with the air out of the device, and reduced clustering was only seen when higher pressure created a greater shear force on cells (Fig. 2B–D). However, this shear force required to break up the cell clusters was associated with lower cell viability.
The ability of the airbrush to evenly distribute cells even at low pressure can be attributed to its two-nozzle system. The air from the outer nozzle surrounded the cell suspension flowing from the inner channel and created turbulent airflow that mixed the air evenly with the cells and broke up any cell clusters. This phenomenon resulted in delivery of a homogeneous distribution of cells at low pressures, preserving cell viability.
Taken together, these results showed that seeding technique, nozzle diameter, and pressure have strong effects on cell viability. Previous studies demonstrated that low air pressures and large nozzle diameters suppress the elongational and shear stresses on cells and increase their survival.21,29,30 Veazey et al. reported that the fibroblast survival rate reached its maximum value (94%), when the cells were sprayed at the lowest pressure (0.41 bar) and the largest nozzle combination (746 μm).29 Owing to greater homogeneity of cell distribution and high cell viability, the airbrush spray technique was chosen for subsequent studies on recellularization of small intestine.
Effect of seeding technique and spray pressure on organoid cluster size
Organoids are a valuable source of cells for repopulating intestinal tissue-engineered constructs because they contain intestinal stem cells that can generate renewable epithelium. However, organoids are challenging to work with because they form large aggregations of cells in suspension, and these clusters must be broken up before seeding onto scaffolds to cover large areas. Therefore, the two-nozzle airbrush spray technique was compared with the conventional pipetting method of seeding organoids to evaluate the effect of seeding technique and pressure on the length and number of organoid clusters (Fig. 3).
FIG. 3.
Effect of the method of cell delivery and spray pressure on the length of clusters was evaluated after cell pipetting (A) and airbrush spraying at 0.35 bar (B) and 0.5 bar (C). Fluorescence microscopy images of Live/Dead-treated organoids pipetted and sprayed at different pressures are shown in the insets. Green staining indicates live cells and red staining indicates dead cells. Images were taken at 10× magnification. The scale bar represents 100 μm. The number and size of clusters in each condition were then compared (D).
The length of clusters ranged from 157 ± 132 μm (pipetting) to 46 ± 26 μm (airbrush spraying, 1 bar). The number of clusters ranged from 56 (pipetting) to 124 (airbrush spraying, 1 bar). Pipetting clearly resulted in large organoids that had not been subjected to sufficient shear force to break them apart. With airbrush spraying, the shear force acting on the organoids became greater as spray pressure increased, which resulted in disaggregation of clusters and a more homogeneous cell distribution. Since cell viability also decreased as pressure increased, 0.35 bar was chosen as an optimum pressure for seeding organoids.
Generation of decellularized intestinal constructs
Whole segments of rat jejunum subjected to perfusion decellularization with SDS, deionized water, Triton X-100, and PBS became grossly transparent as the cellular material was removed but maintained their overall structure of a tubular organ with intact vasculature (Fig. 4A, B). On histological examination with H&E staining, the bowel showed loss of all nuclear and cellular material but maintained its structural crypt-villus architecture (Fig. 4C, D). Total DNA content of the decellularized scaffolds was analyzed and found to be reduced by 97% in comparison with that of native small intestine (Fig. 4 graph). This decellularization method resulted in intestinal scaffolds comprising only the ECM, which had preserved anatomic structure and patent vasculature, two necessary qualities that would allow organs engineered on this platform to be eventually transplantable. These scaffolds were then opened along the mesenteric border in preparation for seeding experiments (Fig. 4E).
FIG. 4.
Gross images of native (A) and decellularized (B) intestinal scaffolds. H&E staining shows native intestinal architecture (C) and complete loss of cellular and nuclear material after decellularization (D). DNA content was reduced to <3% of cadaveric bowel (graph). Experimental setup for seeding decellularized scaffolds through airbrush spraying (E). H&E, hematoxylin and eosin.
CaCo-2 cell seeding onto decellularized scaffolds
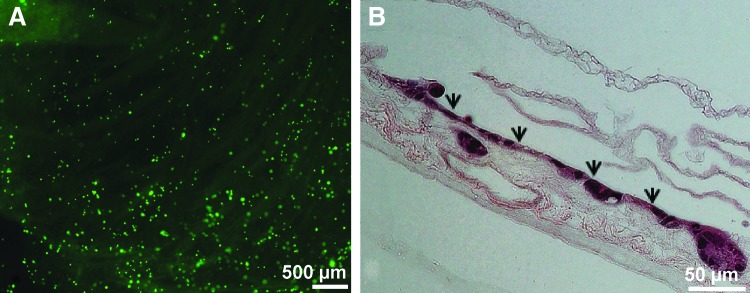
To evaluate cell viability and distribution of cells seeded onto decellularized intestinal scaffolds, a suspension of CaCo-2 cells (0.5 million cells/mL) was sprayed onto scaffold segments (n = 3) at a pressure of 0.35 bar from a height of 5 cm, and cells were evaluated immediately after seeding with Live/Dead staining. Imaging of the seeded scaffolds demonstrated an even distribution of viable cells over the entirety of the 0.5 cm2 area (Fig. 5A). To determine the ability of the scaffold to support longer term survival of the airbrush-sprayed CaCo-2 cells, separate seeded scaffolds (n = 3) were carried out to 5 days of culture before fixing for histology. Cross-sectioning and H&E staining of these scaffolds demonstrated that the cells not only survived but also formed a monolayer of epithelium similar to that found on intestinal villi (Fig. 5B).
FIG. 5.
Fluorescent microscopy images of Live/Dead-treated CaCo-2 cells on a decellularized scaffold after spraying (A). H&E staining of CaCo-2 cells airbrush spray seeded onto the decellularized scaffold after 5 days of culture (B) Arrowheads highlight the cellular monolayer.
Recellularization of scaffolds with intestinal organoids
After determining that the decellularized scaffolds could support CaCo-2 cells, scaffolds were recellularized with intestinal organoids as a source of not only differentiated cells but also intestinal stem cells. Crypts were isolated from C57Bl/6 mice, matured into organoids in Matrigel, and expanded in culture (Fig. 6A). These organoids were then seeded onto decellularized scaffolds either with pipetting (n = 2) or by spraying the organoid suspension through airbrush at 0.35 bar (n = 2). These organoid-seeded scaffolds were cultured for 7 days and fixed for histology.
FIG. 6.
Light microscopy image of mouse intestinal organoids cultured in Matrigel (A) and stained with H&E in section (A, inset). Organoids pipette seeded onto the decellularized scaffold and cultured for 7 days (B, arrowheads mark multiple lumens). Organoids airbrush spray seeded onto the decellularized scaffold and cultured for 7 days (C). Inset in (C) shows monolayer formation at high magnification.
H&E staining of scaffolds that had been pipette seeded (Fig. 6B) demonstrated survival of the organoids on the scaffold with an architecture resembling a conglomerate of 3D spherical structures similar to the configuration of organoids within Matrigel (Fig. 6A inset). Many of the cells within these intact whole organoids did not make contact with the scaffold construct and were instead organized around multiple smaller lumens. In contrast, H&E staining of the scaffolds onto which organoids had been seeded using the airbrush (Fig. 6C) showed dispersion of organoids along the scaffold, leading to formation of a monolayer with all of the cells in direct contact with decellularized ECM. This monolayer of intestinal epithelium spread along the scaffold with one continuous apical surface resembling the absorptive surface found on the villi of native bowel.
Conclusion
This study describes a novel method of seeding intestinal organoids, which contain both differentiated epithelial cells and stem cells, to form epithelium on decellularized organ scaffolds. We demonstrated that the airbrush spray seeding method can be applied to CaCo-2 cells, a continuous cell line of human epithelial colorectal adenocarcinoma cells that express enterocytic differentiation. CaCo-2 cells showed pressure and technique-dependent viability and formed monolayers on decellularized intestinal ECM scaffolds. Airbrush spraying of intestinal organoids at low pressure resulted in high cell survival rates and broad coverage of the decellularized constructs. More importantly, spray seeding also transformed the cluster-like architecture of organoids, which was seen in suspension seeding, into a monolayer of epithelium with a common basal surface along the scaffold. This configuration is more favorable for development of an absorptive surface that could be useful in a clinical setting.
The use of decellularized native ECM scaffolds has the potential to transform intestinal engineering away from tissue scale constructs and toward transplantable whole organs. As constructs become large and more architecturally complex, new seeding methods such as spraying will become increasingly useful, as they can be scaled up to cover large areas and adapted to efficiently seed various hollow viscous organs. Future investigations will focus on modifying the airbrush device to be compatible with colonoscopic delivery, which will facilitate its use in tissue engineering, as well as in in vivo cell therapy applications.
Acknowledgments
The authors thank Prof. Abigail Koppes for access to the fluorescence microscope, Theodore Lutkus for fabrication of 3D spray devices, Kentaro Kitano for assistance in developing decellularization protocols, and Marissa Puzan for assistance with organoid culture. This research was supported by the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award 2T32DK007754-16A1 from the National Institute of Diabetes and Digestive and Kidney Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Squires R.H., Duggan C., Teitelbaum D.H., Wales P.W., Balint J., Venick R., Rhee S., Sudan D., Mercer D., and Martinez J.A. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 161, 723, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLegge M., Alsolaiman M.M., Barbour E., Bassas S., Siddiqi M.F., and Moore N.M. Short bowel syndrome: parenteral nutrition versus intestinal transplantation. Where are we today? Dig Dis Sci 52, 876, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hess R.A., Welch K.B., Brown P.I., and Teitelbaum D.H. Survival outcomes of pediatric intestinal failure patients: analysis of factors contributing to improved survival over the past two decades. J Surg Res 170, 27, 2011 [DOI] [PubMed] [Google Scholar]
- 4.O'keefe S.J., Buchman A.L., Fishbein T.M., Jeejeebhoy K.N., Jeppesen P.B., and Shaffer J. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol 4, 6, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Grant D., Abu‐Elmagd K., Mazariegos G., Vianna R., Langnas A., Mangus R., Farmer D., Lacaille F., Iyer K., and Fishbein T. Intestinal transplant registry report: global activity and trends. Am J Transplant 15, 210, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Avansino J.R., Chen D.C., Hoagland V.D., Woolman J.D., and Stelzner M. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery 140, 423, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Grikscheit T.C., Siddique A., Ochoa E.R., Srinivasan A., Alsberg E., Hodin R.A., and Vacanti J.P. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg 240, 748, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costello C.M., Hongpeng J., Shaffiey S., Yu J., Jain N.K., Hackam D., and March J.C. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng 111, 1222, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyette J.P., Gilpin S.E., Charest J.M., Tapias L.F., Ren X., and Ott H.C. Perfusion decellularization of whole organs. Nat Protoc 9, 1451, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Ott H.C., Matthiesen T.S., Goh S.-K., Black L.D., Kren S.M., Netoff T.I., and Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., and Vacanti J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16, 927, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., and Ott H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19, 646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Totonelli G., Maghsoudlou P., Garriboli M., Riegler J., Orlando G., Burns A.J., Sebire N.J., Smith V.V., Fishman J.M., and Ghionzoli M. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 33, 3401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T., Vries R.G., Snippert H.J., Van de Wetering M., Barker N., Stange D.E., Van Es J.H., Abo A., Kujala P., and Peters P.J. Single Lgr5 stem cells build crypt villus structures in vitro without a mesenchymal niche. Nature 459, 262, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Finkbeiner S.R., Freeman J.J., Wieck M.M., El-Nachef W., Altheim C.H., Tsai Y.-H., Huang S., Dyal R., White E.S., and Grikscheit T.C. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open 4, 1462, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens A.M., Casey B.J., Sikorski M.J., Wu K.L., Tutak W., Sandler A.D., and Kofinas P. In situ deposition of PLGA nanofibers via solution blow spinning. ACS Macro Lett 3, 249, 2014 [DOI] [PubMed] [Google Scholar]
- 17.De Windt T., Vonk L., Buskermolen J., Visser J., Karperien M., Bleys R., Dhert W., and Saris D. Arthroscopic airbrush assisted cell implantation for cartilage repair in the knee: a controlled laboratory and human cadaveric study. Osteoarthritis Cartilage 23, 143, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Behrens A.M., Lee N.G., Casey B.J., Srinivasan P., Sikorski M.J., Daristotle J.L., Sandler A.D., and Kofinas P. Biodegradable‐polymer‐blend‐based surgical sealant with body‐temperature‐mediated adhesion. Adv Mater 27, 8056, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiebes A.L., Albers S., Klopsch C., Jockenhoevel S., and Cornelissen C.G. Spraying respiratory epithelial cells to coat tissue-engineered constructs. Biores Open Access 4, 278, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiebes A.L., Reddemann M.A., Palmer J., Kneer R., Jockenhoevel S., and Cornelissen C.G. Flexible endoscopic spray application of respiratory epithelial cells as platform technology to apply cells in tubular organs. Tissue Eng Part C Methods 22, 322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pehlivaner Kara M.O., and Ekenseair A.K. In situ spray deposition of cell‐loaded, thermally and chemically gelling hydrogel coatings for tissue regeneration. J Biomed Mater Res Part A 104, 2383, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Tritz J., Rahouadj R., de Isla N., Charif N., Pinzano A., Mainard D., Bensoussan D., Netter P., Stoltz J.-F., and Benkirane-Jessel N. Designing a three-dimensional alginate hydrogel by spraying method for cartilage tissue engineering. Soft Matter 6, 5165, 2010 [Google Scholar]
- 23.Roberts A., Wyslouzil B.E., and Bonassar L. Aerosol delivery of mammalian cells for tissue engineering. Biotechnol Bioeng 91, 801, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Pehlivaner Kara M.O., and Ekenseair A.K. Free epoxide content mediates encapsulated cell viability and activity through protein interactions in a thermoresponsive, in situ forming hydrogel. Biomacromolecules 18, 1473, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Falanga V., Iwamoto S., Chartier M., Yufit T., Butmarc J., Kouttab N., Shrayer D., and Carson P. Autologous bone marrow—derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13, 1299, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Bahoric A., Harrop A., Clarke H., and Zuker R. Aerosol vehicle for delivery of epidermal cells—an in vitro study. Plast Surg 5, 153, 1997 [Google Scholar]
- 27.Kirsner R.S., Marston W.A., Snyder R.J., Lee T.D., Cargill D.I., and Slade H.B. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet 380, 977, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J., and David W.R. Molecular Cloning: A Laboratory Manual, 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2001 [Google Scholar]
- 29.Veazey W., Anusavice K.J., and Moore K. Mammalian cell delivery via aerosol deposition. J Biomed Mater Res Part B Appl Biomater 72, 334, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hendriks J., Visser C.W., Henke S., Leijten J., Saris D.B., Sun C., Lohse D., and Karperien M. Optimizing cell viability in droplet-based cell deposition. Sci Rep 5, 11304, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]